false000190710800019071082025-01-132025-01-13
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 13, 2025 |
Lexeo Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-41855 |
85-4012572 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
345 Park Avenue South, Floor 6 |
|
New York, New York |
|
10010 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 212 547-9879 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.0001 par value per share |
|
LXEO |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On January 13, 2025, Lexeo Therapeutics, Inc. (the “Company”) posted on its website an updated corporate presentation (the “Corporate Presentation”). The Corporate Presentation will be used from time to time in meetings with investors and analysts. A copy of the Corporate Presentation is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
Cautionary Note Regarding Forward-Looking Statements
This report contains certain forward-looking statements regarding the business of the Company that are not a description of historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the Company’s expected plans with respect to clinical trials of the Company’s gene therapy candidates and the timing for announcement of data from such trials. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, expectations regarding the initiation, progress, and expected results of the Company’s preclinical studies, clinical trials and research and development programs, the unpredictable relationship between preclinical study results and clinical study results, delays in submission of regulatory filings or failure to receive regulatory approval and liquidity and capital resources. Additional risks and uncertainties that could cause actual results to differ materially from those contemplated by the forward-looking statements are included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, Quarterly Report on Form 10-Q for the quarter ended September 30, 2024 and subsequent future filings the Company may make with the Securities and Exchange Commission from time to time that are available at www.sec.gov.
You are cautioned not to place undue reliance on forward-looking statements which are current only as of the date hereof. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Lexeo Therapeutics, Inc. |
|
|
|
|
Date: |
January 13, 2025 |
By: |
/s/ R. Nolan Townsend |
|
|
|
R. Nolan Townsend, Chief Executive Officer |

Lexeo Therapeutics�Corporate Overview���January 13, 2025

Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, statements regarding Lexeo’s expectations and plans regarding its current product candidates and programs, including statements regarding the timing, progress and results of preclinical and clinical trials of Lexeo’s gene therapy product candidates, the anticipated benefits of its current product candidates, the timing and likelihood of regulatory approval, and expected cash runway. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward looking statements as a result of many factors, including but not limited to: risks and uncertainties related to expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 11, 2024, Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, filed with the SEC on November 13, 2024, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law.
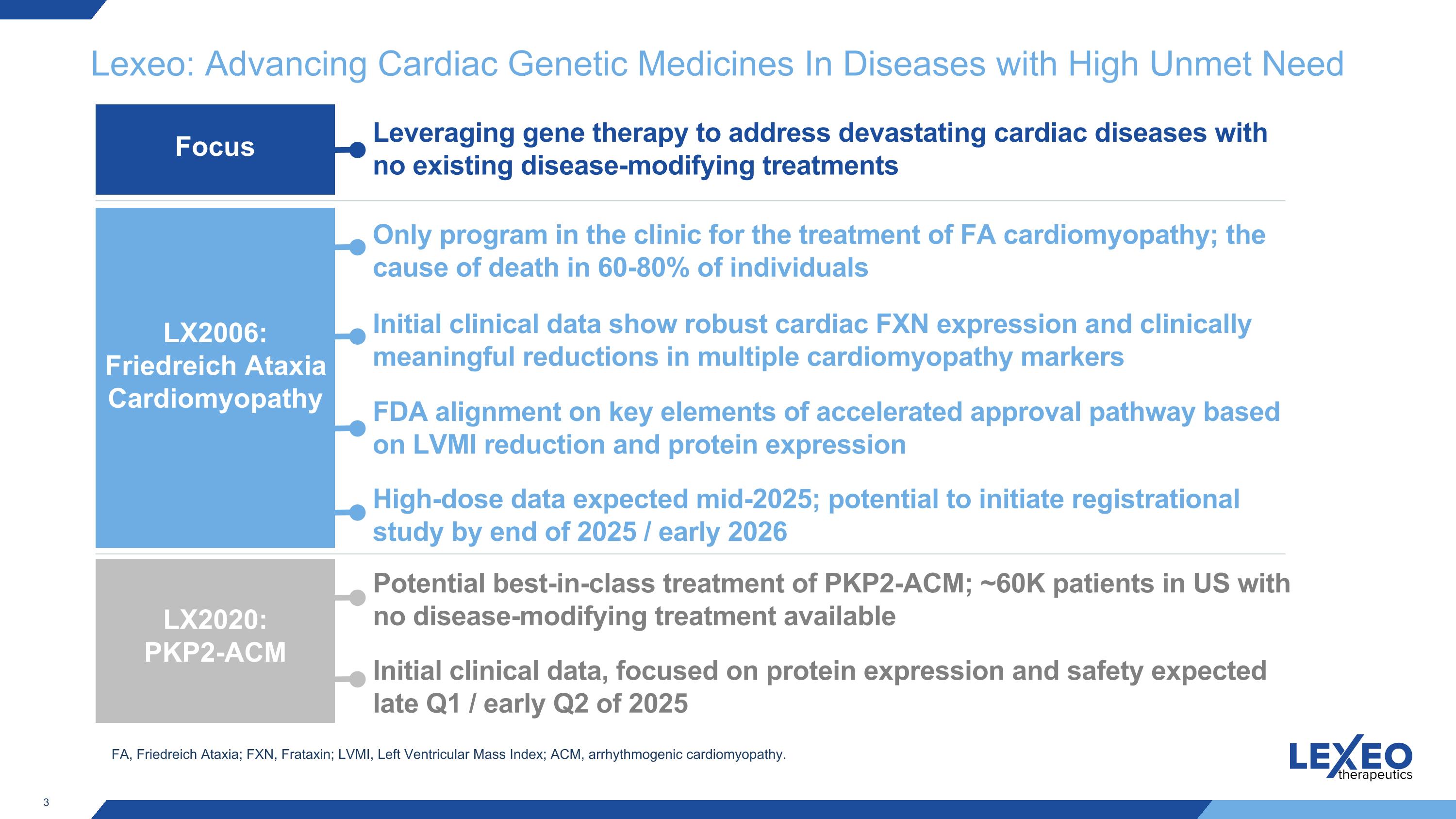
Lexeo: Advancing Cardiac Genetic Medicines In Diseases with High Unmet Need Leveraging gene therapy to address devastating cardiac diseases with no existing disease-modifying treatments Potential best-in-class treatment of PKP2-ACM; ~60K patients in US with no disease-modifying treatment available Initial clinical data, focused on protein expression and safety expected late Q1 / early Q2 of 2025 Only program in the clinic for the treatment of FA cardiomyopathy; the cause of death in 60-80% of individuals Initial clinical data show robust cardiac FXN expression and clinically meaningful reductions in multiple cardiomyopathy markers FDA alignment on key elements of accelerated approval pathway based on LVMI reduction and protein expression LX2006: Friedreich Ataxia Cardiomyopathy LX2020: PKP2-ACM Focus High-dose data expected mid-2025; potential to initiate registrational study by end of 2025 / early 2026 FA, Friedreich Ataxia; FXN, Frataxin; LVMI, Left Ventricular Mass Index; ACM, arrhythmogenic cardiomyopathy.
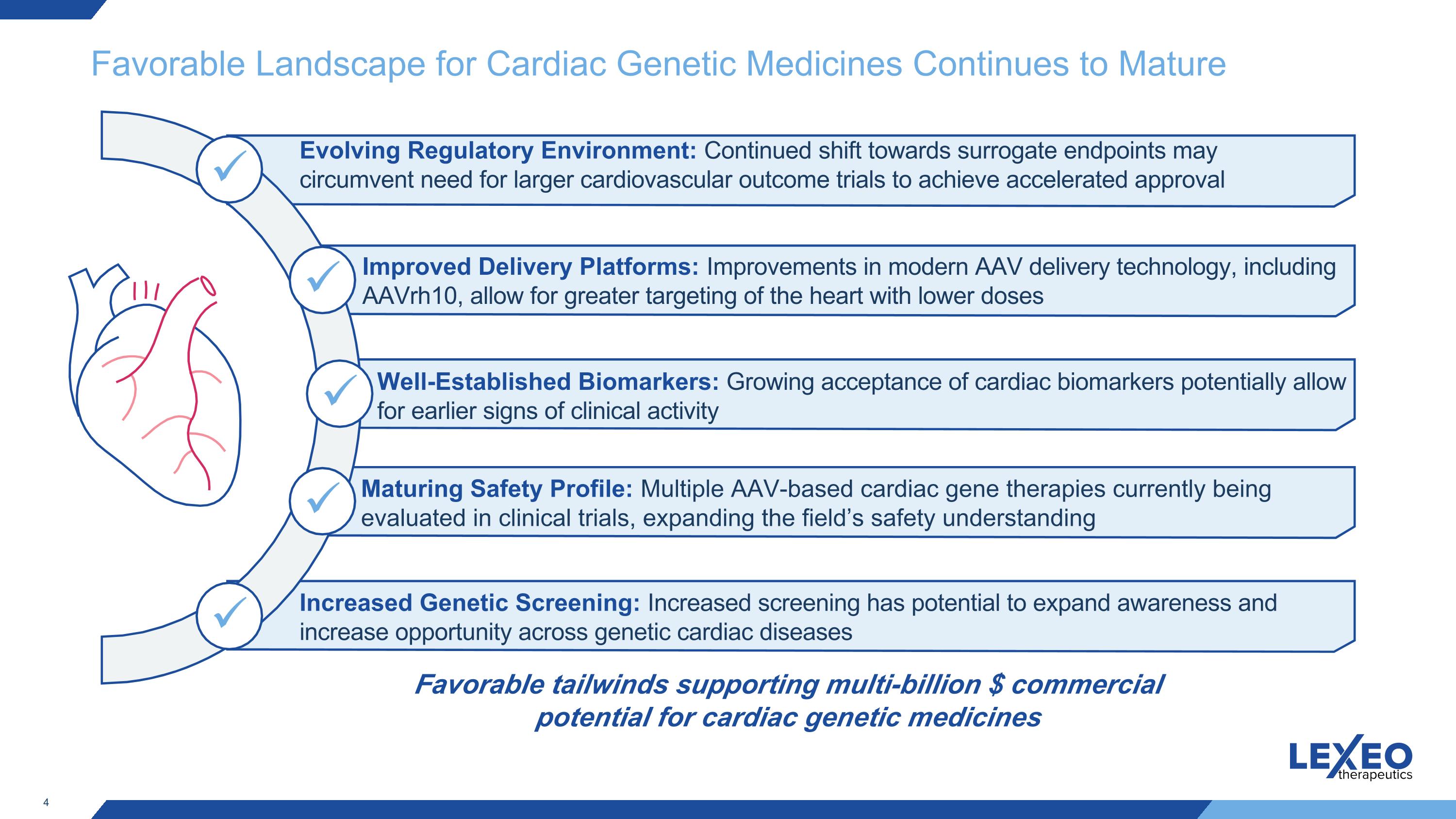
Favorable Landscape for Cardiac Genetic Medicines Continues to Mature Evolving Regulatory Environment: Continued shift towards surrogate endpoints may circumvent need for larger cardiovascular outcome trials to achieve accelerated approval Improved Delivery Platforms: Improvements in modern AAV delivery technology, including AAVrh10, allow for greater targeting of the heart with lower doses Increased Genetic Screening: Increased screening has potential to expand awareness and increase opportunity across genetic cardiac diseases Maturing Safety Profile: Multiple AAV-based cardiac gene therapies currently being evaluated in clinical trials, expanding the field’s safety understanding Well-Established Biomarkers: Growing acceptance of cardiac biomarkers potentially allow for earlier signs of clinical activity Favorable tailwinds supporting multi-billion $ commercial potential for cardiac genetic medicines
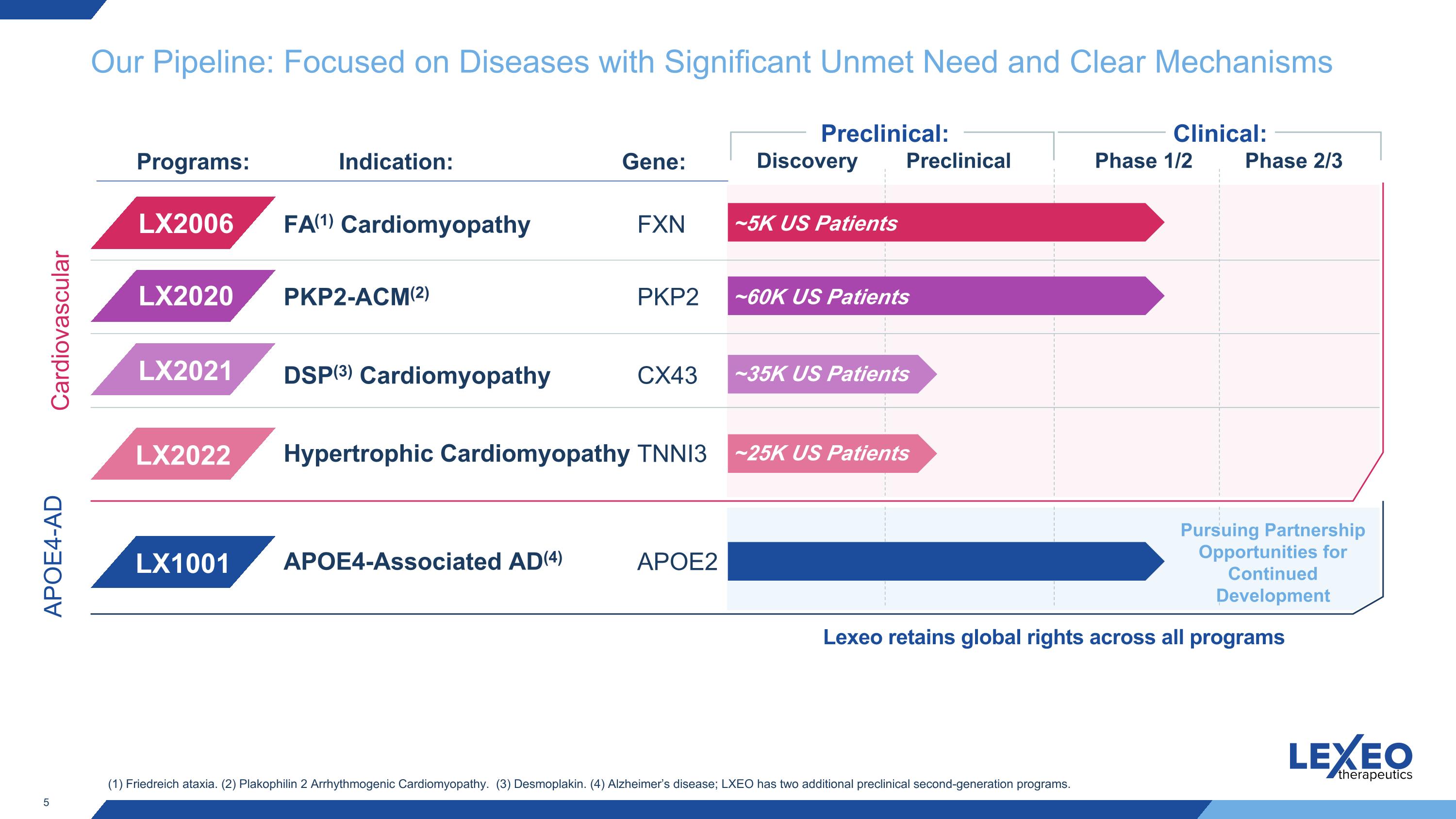
Our Pipeline: Focused on Diseases with Significant Unmet Need and Clear Mechanisms Clinical: Discovery Preclinical Phase 1/2 Phase 2/3 Preclinical: Indication: Programs: FA(1) Cardiomyopathy PKP2-ACM(2) FXN PKP2 Gene: LX2006 LX2020 (1) Friedreich ataxia. (2) Plakophilin 2 Arrhythmogenic Cardiomyopathy. (3) Desmoplakin. (4) Alzheimer’s disease; LXEO has two additional preclinical second-generation programs. CX43 DSP(3) Cardiomyopathy Hypertrophic Cardiomyopathy TNNI3 LX2021 LX2022 APOE4-Associated AD(4) APOE2 LX1001 Cardiovascular APOE4-AD Lexeo retains global rights across all programs Pursuing Partnership Opportunities for Continued Development ~5K US Patients ~60K US Patients ~35K US Patients ~25K US Patients
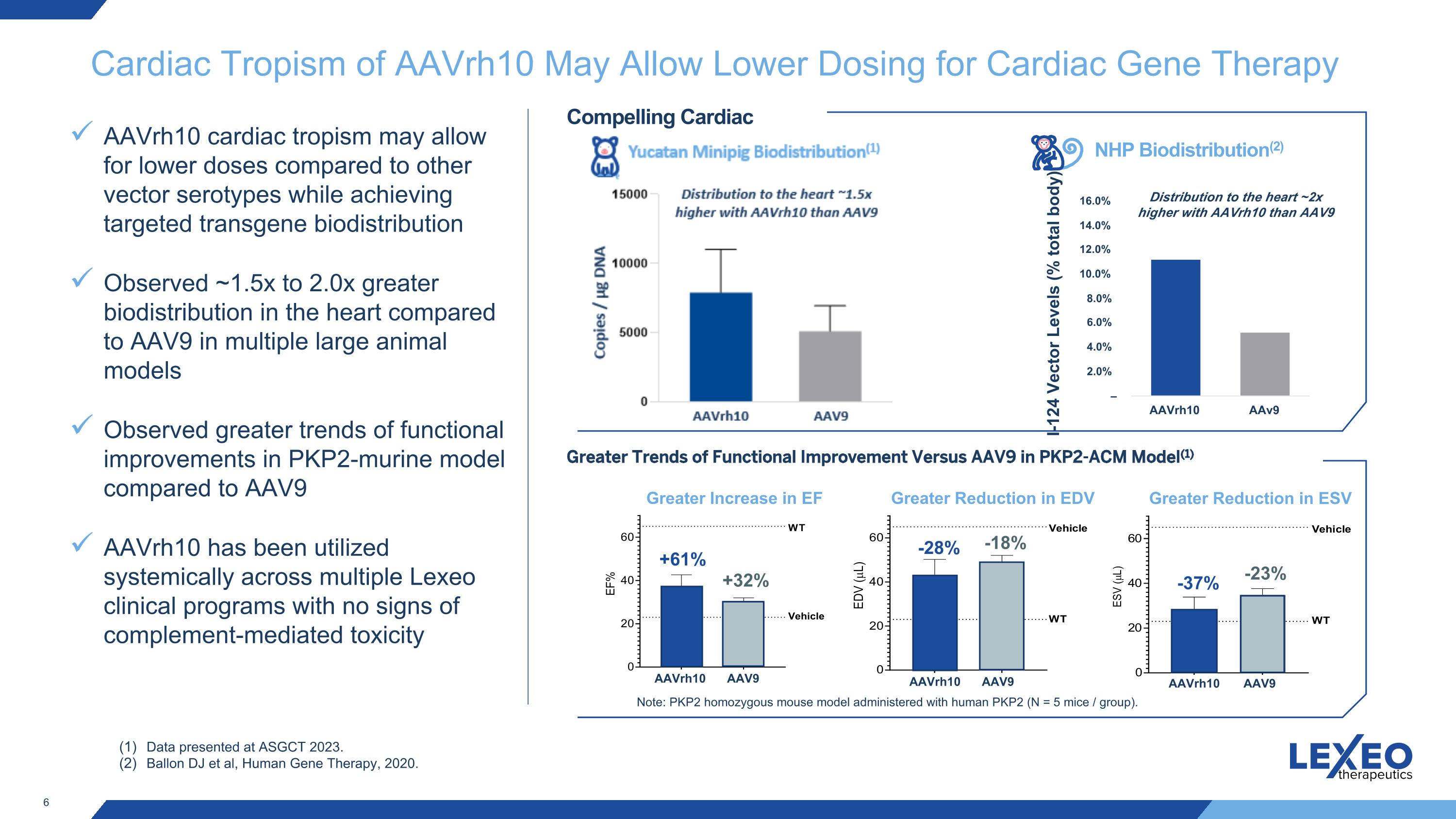
Cardiac Tropism of AAVrh10 May Allow Lower Dosing for Cardiac Gene Therapy AAVrh10 cardiac tropism may allow for lower doses compared to other vector serotypes while achieving targeted transgene biodistribution Observed ~1.5x to 2.0x greater biodistribution in the heart compared to AAV9 in multiple large animal models Observed greater trends of functional improvements in PKP2-murine model compared to AAV9 AAVrh10 has been utilized systemically across multiple Lexeo clinical programs with no signs of complement-mediated toxicity NHP Biodistribution(2) I-124 Vector Levels (% total body) Data presented at ASGCT 2023. Ballon DJ et al, Human Gene Therapy, 2020. Compelling Cardiac Tropism Greater Trends of Functional Improvement Versus AAV9 in PKP2-ACM Model(1) Greater Increase in EF Greater Reduction in EDV Greater Reduction in ESV +61% +32% -28% -18% -37% -23% Note: PKP2 homozygous mouse model administered with human PKP2 (N = 5 mice / group). Distribution to the heart ~2x higher with AAVrh10 than AAV9 AAVrh10 AAV9 AAVrh10 AAV9 AAVrh10 AAV9

LX2006 (FA Cardiomyopathy)
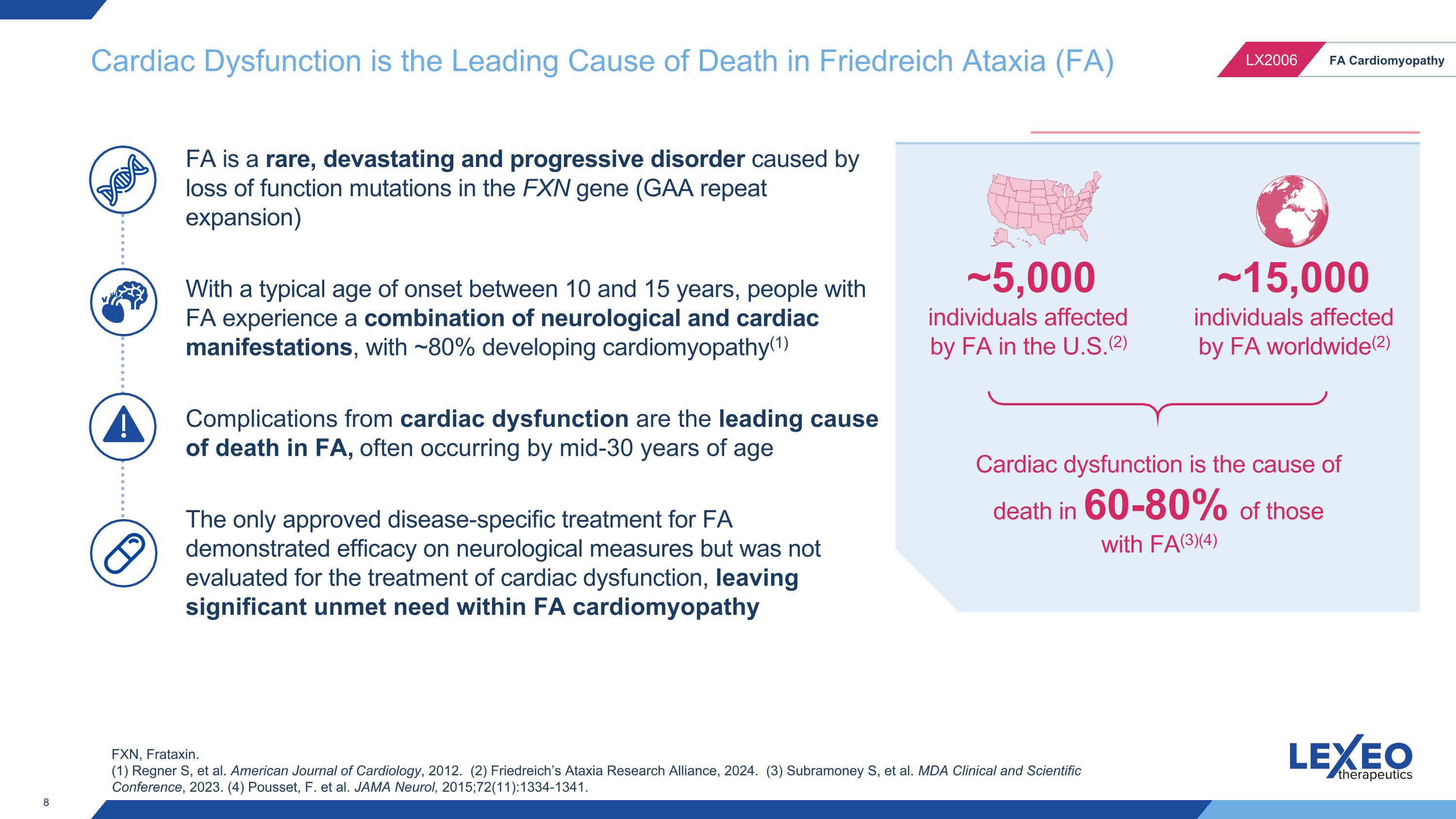
FA is a rare, devastating and progressive disorder caused by loss of function mutations in the FXN gene (GAA repeat expansion) With a typical age of onset between 10 and 15 years, people with FA experience a combination of neurological and cardiac manifestations, with ~80% developing cardiomyopathy(1) Complications from cardiac dysfunction are the leading cause of death in FA, often occurring by mid-30 years of age The only approved disease-specific treatment for FA demonstrated efficacy on neurological measures but was not evaluated for the treatment of cardiac dysfunction, leaving significant unmet need within FA cardiomyopathy Cardiac dysfunction is the cause of death in 60-80% of those with FA(3)(4) Cardiac Dysfunction is the Leading Cause of Death in Friedreich Ataxia (FA) ~15,000 individuals affected by FA worldwide(2) ~5,000 individuals affected by FA in the U.S.(2) FXN, Frataxin. (1) Regner S, et al. American Journal of Cardiology, 2012. (2) Friedreich’s Ataxia Research Alliance, 2024. (3) Subramoney S, et al. MDA Clinical and Scientific Conference, 2023. (4) Pousset, F. et al. JAMA Neurol, 2015;72(11):1334-1341.
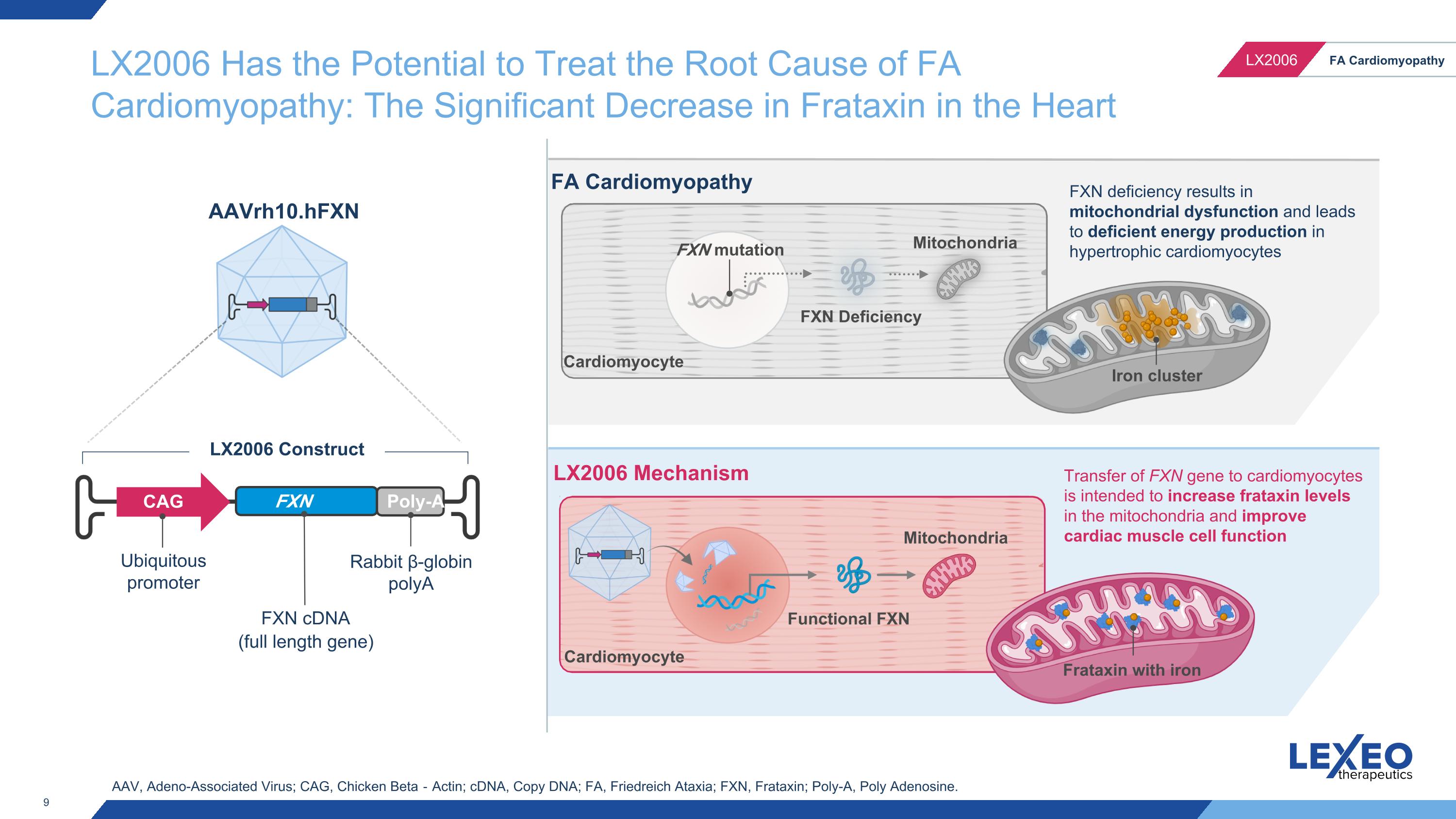
FXN mutation FXN Deficiency Mitochondria Functional FXN FA Cardiomyopathy LX2006 Mechanism Cardiomyocyte Transfer of FXN gene to cardiomyocytes is intended to increase frataxin levels in the mitochondria and improve cardiac muscle cell function FXN deficiency results in mitochondrial dysfunction and leads to deficient energy production in hypertrophic cardiomyocytes Mitochondria Cardiomyocyte AAV, Adeno-Associated Virus; CAG, Chicken Beta‐Actin; cDNA, Copy DNA; FA, Friedreich Ataxia; FXN, Frataxin; Poly-A, Poly Adenosine. LX2006 Has the Potential to Treat the Root Cause of FA Cardiomyopathy: The Significant Decrease in Frataxin in the Heart Ubiquitous promoter FXN cDNA (full length gene) CAG FXN gene Poly-A Rabbit β-globin polyA LX2006 Construct AAVrh10.hFXN Iron cluster Frataxin with iron
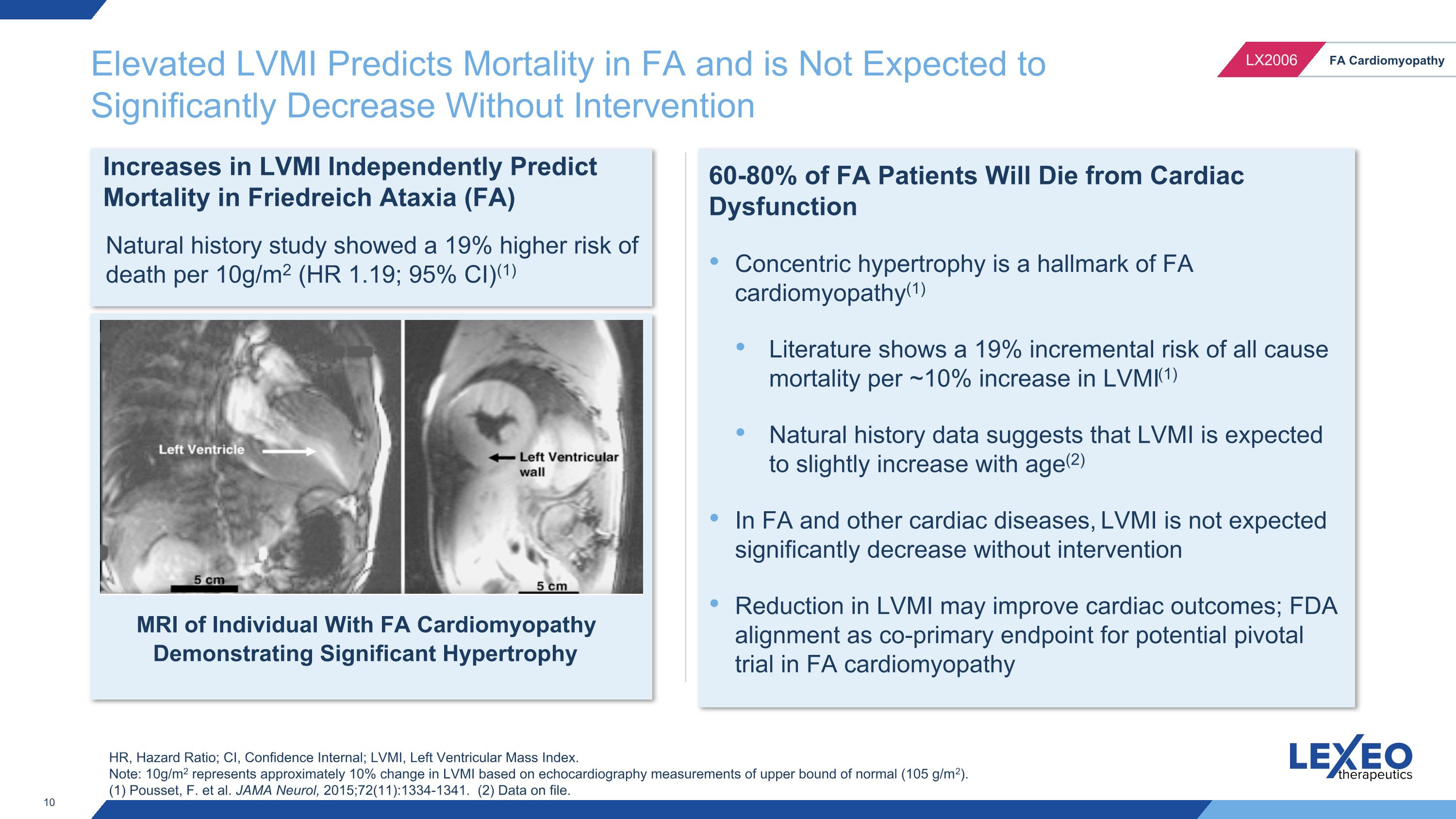
Elevated LVMI Predicts Mortality in FA and is Not Expected to Significantly Decrease Without Intervention Increases in LVMI Independently Predict Mortality in Friedreich Ataxia (FA) Natural history study showed a 19% higher risk of death per 10g/m2 (HR 1.19; 95% CI)(1) HR, Hazard Ratio; CI, Confidence Internal; LVMI, Left Ventricular Mass Index. Note: 10g/m2 represents approximately 10% change in LVMI based on echocardiography measurements of upper bound of normal (105 g/m2). (1) Pousset, F. et al. JAMA Neurol, 2015;72(11):1334-1341. (2) Data on file. 60-80% of FA Patients Will Die from Cardiac Dysfunction Concentric hypertrophy is a hallmark of FA cardiomyopathy(1) Literature shows a 19% incremental risk of all cause mortality per ~10% increase in LVMI(1) Natural history data suggests that LVMI is expected to slightly increase with age(2) In FA and other cardiac diseases, LVMI is not expected significantly decrease without intervention Reduction in LVMI may improve cardiac outcomes; FDA alignment as co-primary endpoint for potential pivotal trial in FA cardiomyopathy MRI of Individual With FA Cardiomyopathy Demonstrating Significant Hypertrophy
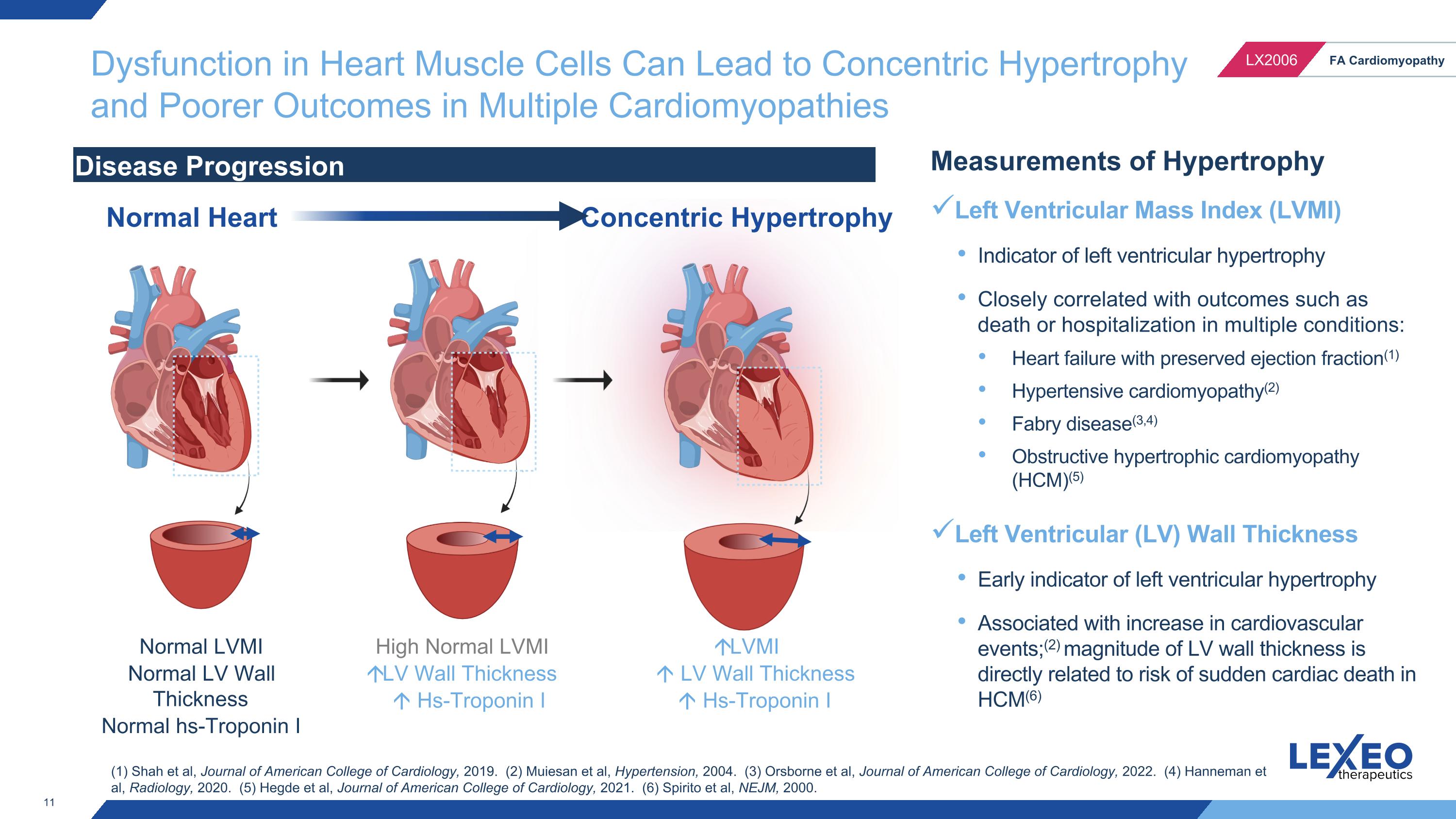
Dysfunction in Heart Muscle Cells Can Lead to Concentric Hypertrophy and Poorer Outcomes in Multiple Cardiomyopathies Normal LVMI Normal LV Wall Thickness Normal hs-Troponin I LVMI LV Wall Thickness Hs-Troponin I Disease Progression Normal Heart Concentric Hypertrophy Measurements of Hypertrophy Left Ventricular Mass Index (LVMI) Indicator of left ventricular hypertrophy Closely correlated with outcomes such as death or hospitalization in multiple conditions: Heart failure with preserved ejection fraction(1) Hypertensive cardiomyopathy(2) Fabry disease(3,4) Obstructive hypertrophic cardiomyopathy (HCM)(5) Left Ventricular (LV) Wall Thickness Early indicator of left ventricular hypertrophy Associated with increase in cardiovascular events;(2) magnitude of LV wall thickness is directly related to risk of sudden cardiac death in HCM(6) High Normal LVMI LV Wall Thickness Hs-Troponin I (1) Shah et al, Journal of American College of Cardiology, 2019. (2) Muiesan et al, Hypertension, 2004. (3) Orsborne et al, Journal of American College of Cardiology, 2022. (4) Hanneman et al, Radiology, 2020. (5) Hegde et al, Journal of American College of Cardiology, 2021. (6) Spirito et al, NEJM, 2000.
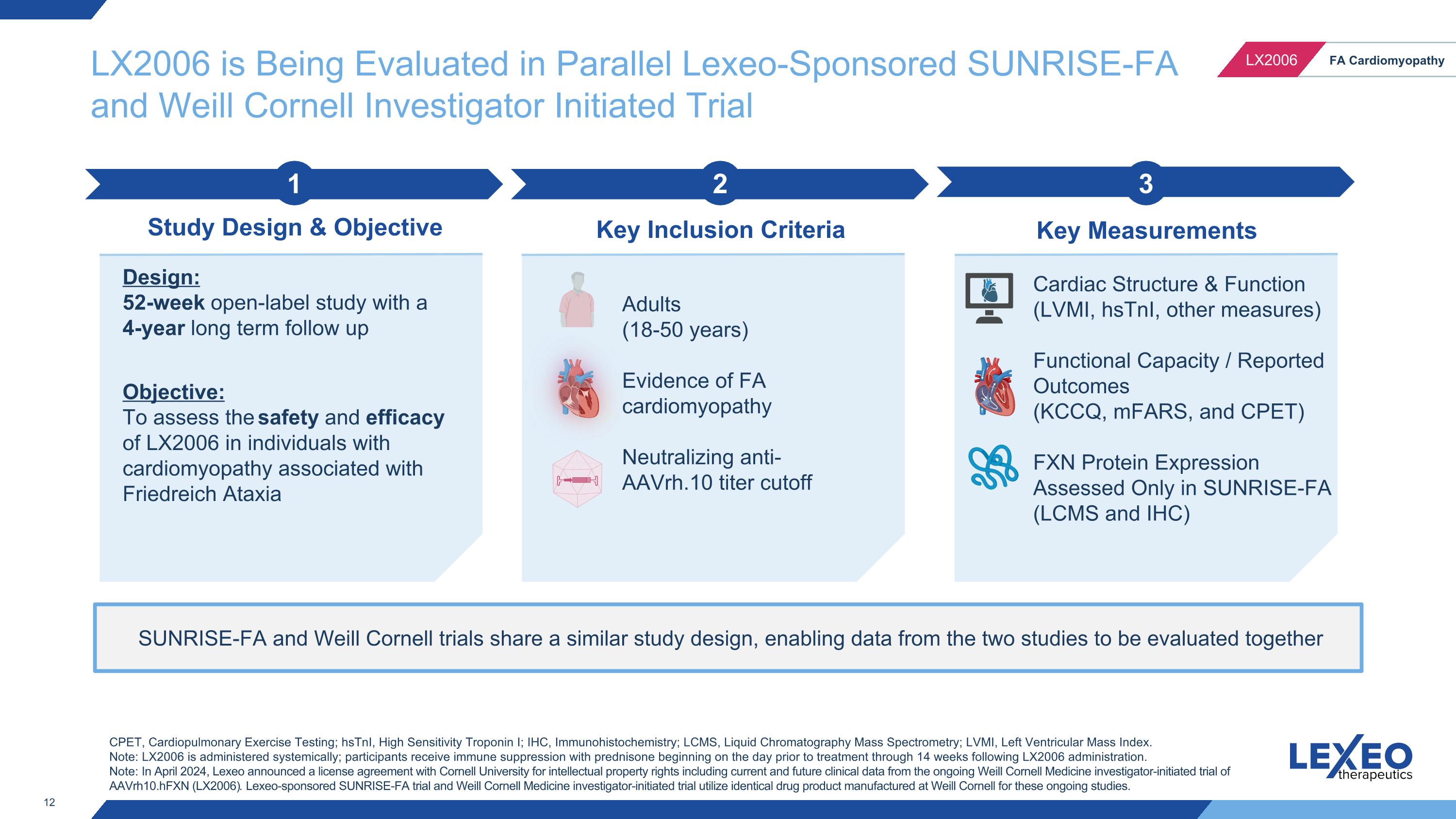
LX2006 is Being Evaluated in Parallel Lexeo-Sponsored SUNRISE-FA and Weill Cornell Investigator Initiated Trial SUNRISE-FA and Weill Cornell trials share a similar study design, enabling data from the two studies to be evaluated together 2 Key Inclusion Criteria 3 Key Measurements 1 Study Design & Objective Design: 52-week open-label study with a 4-year long term follow up Objective: To assess the safety and efficacy of LX2006 in individuals with cardiomyopathy associated with Friedreich Ataxia Adults (18-50 years) Evidence of FA cardiomyopathy Neutralizing anti-AAVrh.10 titer cutoff Cardiac Structure & Function (LVMI, hsTnI, other measures) Functional Capacity / Reported Outcomes (KCCQ, mFARS, and CPET) FXN Protein Expression Assessed Only in SUNRISE-FA (LCMS and IHC) CPET, Cardiopulmonary Exercise Testing; hsTnI, High Sensitivity Troponin I; IHC, Immunohistochemistry; LCMS, Liquid Chromatography Mass Spectrometry; LVMI, Left Ventricular Mass Index. Note: LX2006 is administered systemically; participants receive immune suppression with prednisone beginning on the day prior to treatment through 14 weeks following LX2006 administration. Note: In April 2024, Lexeo announced a license agreement with Cornell University for intellectual property rights including current and future clinical data from the ongoing Weill Cornell Medicine investigator-initiated trial of AAVrh10.hFXN (LX2006). Lexeo-sponsored SUNRISE-FA trial and Weill Cornell Medicine investigator-initiated trial utilize identical drug product manufactured at Weill Cornell for these ongoing studies.
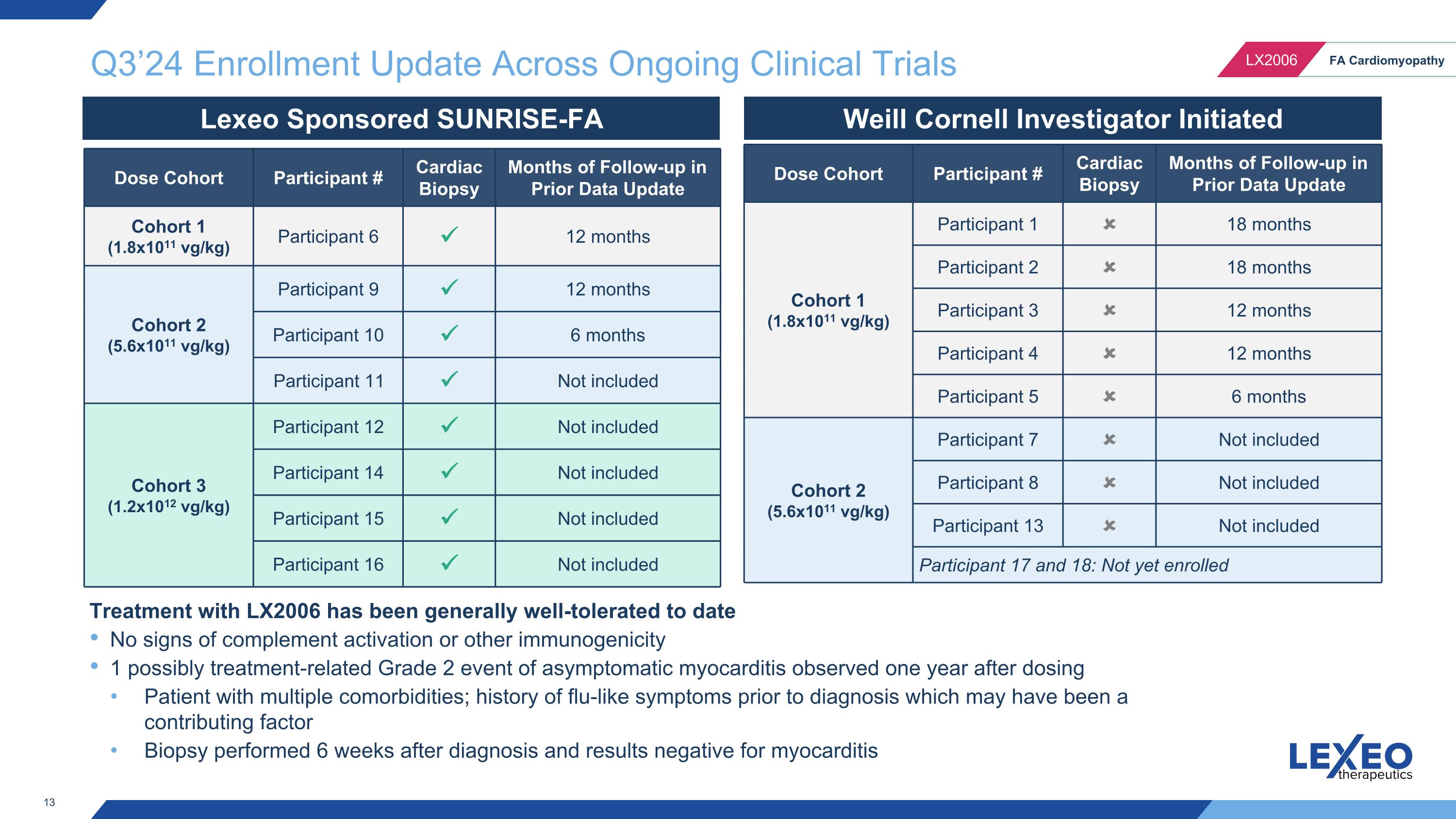
Q3’24 Enrollment Update Across Ongoing Clinical Trials Lexeo Sponsored SUNRISE-FA Weill Cornell Investigator Initiated Dose Cohort Participant # Cardiac Biopsy Months of Follow-up in Prior Data Update Cohort 1 (1.8x1011 vg/kg) Participant 6 12 months Cohort 2 (5.6x1011 vg/kg) Participant 9 12 months Participant 10 6 months Participant 11 Not included Cohort 3 (1.2x1012 vg/kg) Participant 12 Not included Participant 14 Not included Participant 15 Not included Participant 16 Not included Dose Cohort Participant # Cardiac Biopsy Months of Follow-up in Prior Data Update Cohort 1 (1.8x1011 vg/kg) Participant 1 18 months Participant 2 18 months Participant 3 12 months Participant 4 12 months Participant 5 6 months Cohort 2 (5.6x1011 vg/kg) Participant 7 Not included Participant 8 Not included Participant 13 Not included Participant 17 and 18: Not yet enrolled Treatment with LX2006 has been generally well-tolerated to date No signs of complement activation or other immunogenicity 1 possibly treatment-related Grade 2 event of asymptomatic myocarditis observed one year after dosing Patient with multiple comorbidities; history of flu-like symptoms prior to diagnosis which may have been a contributing factor Biopsy performed 6 weeks after diagnosis and results negative for myocarditis
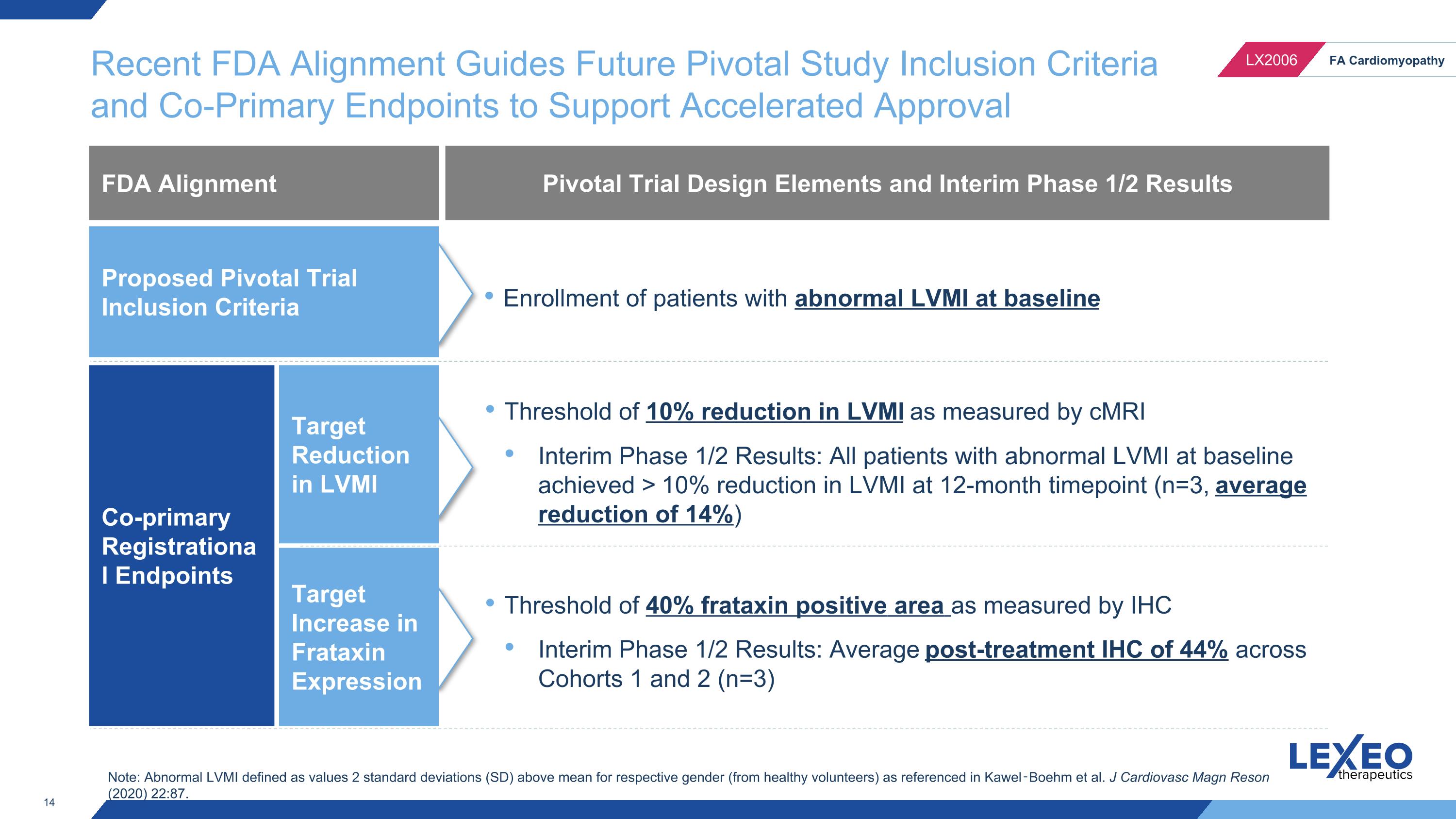
Recent FDA Alignment Guides Future Pivotal Study Inclusion Criteria and Co-Primary Endpoints to Support Accelerated Approval FDA Alignment Threshold of 10% reduction in LVMI as measured by cMRI Interim Phase 1/2 Results: All patients with abnormal LVMI at baseline achieved > 10% reduction in LVMI at 12-month timepoint (n=3, average reduction of 14%) Co-primary Registrational Endpoints Target Reduction in LVMI Target Increase in Frataxin Expression Pivotal Trial Design Elements and Interim Phase 1/2 Results Threshold of 40% frataxin positive area as measured by IHC Interim Phase 1/2 Results: Average post-treatment IHC of 44% across Cohorts 1 and 2 (n=3) Enrollment of patients with abnormal LVMI at baseline Proposed Pivotal Trial Inclusion Criteria Note: Abnormal LVMI defined as values 2 standard deviations (SD) above mean for respective gender (from healthy volunteers) as referenced in Kawel‑Boehm et al. J Cardiovasc Magn Reson (2020) 22:87.
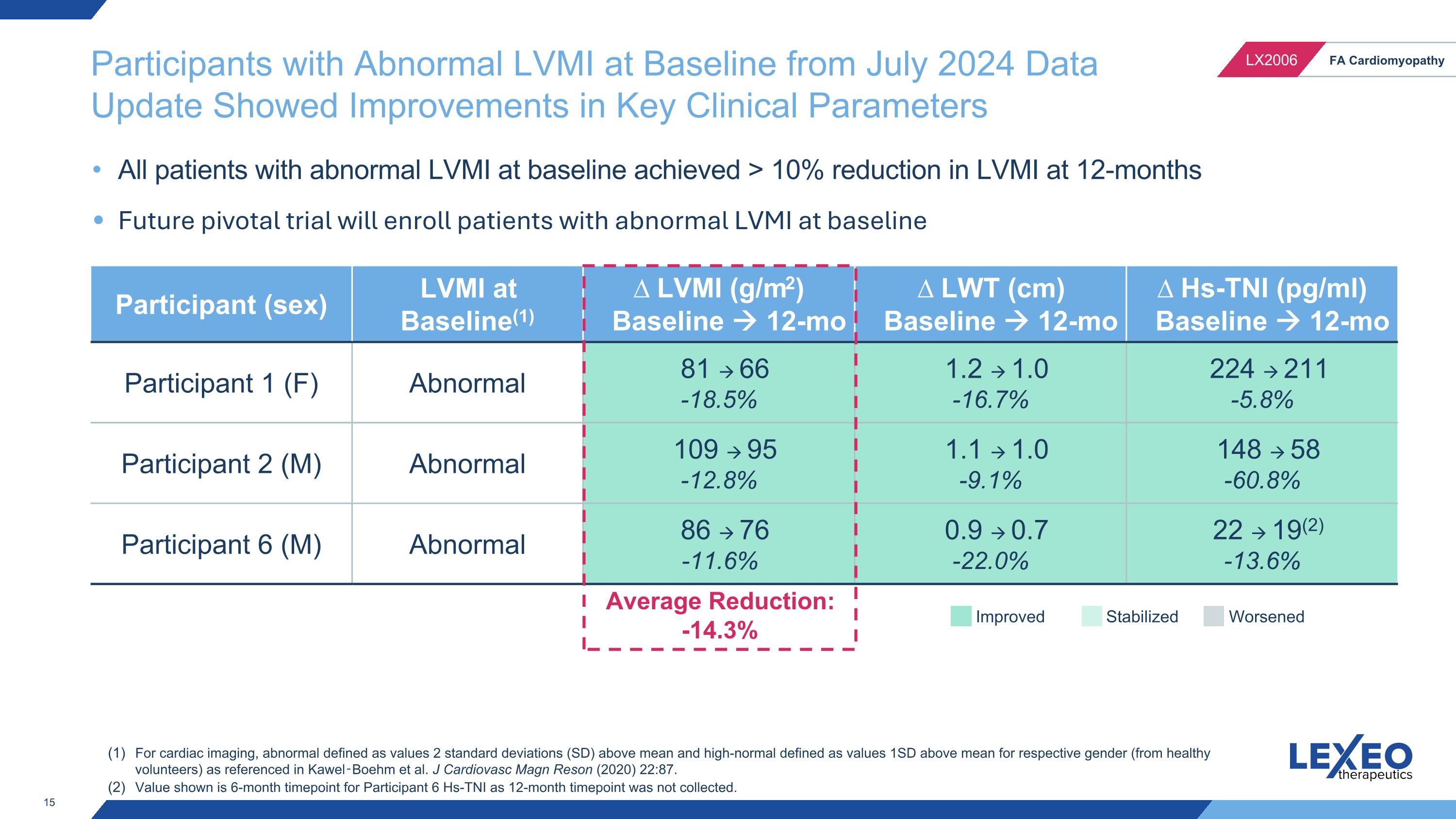
Participants with Abnormal LVMI at Baseline from July 2024 Data Update Showed Improvements in Key Clinical Parameters Participant (sex) LVMI at Baseline(1) ∆ LVMI (g/m2) Baseline 12-mo ∆ LWT (cm) Baseline 12-mo ∆ Hs-TNI (pg/ml) Baseline 12-mo Participant 1 (F) Abnormal 81 66 -18.5% 1.2 1.0 -16.7% 224 211 -5.8% Participant 2 (M) Abnormal 109 95 -12.8% 1.1 1.0 -9.1% 148 58 -60.8% Participant 6 (M) Abnormal 86 76 -11.6% 0.9 0.7 -22.0% 22 19(2) -13.6% All patients with abnormal LVMI at baseline achieved > 10% reduction in LVMI at 12-months Future pivotal trial will enroll patients with abnormal LVMI at baseline For cardiac imaging, abnormal defined as values 2 standard deviations (SD) above mean and high-normal defined as values 1SD above mean for respective gender (from healthy volunteers) as referenced in Kawel‑Boehm et al. J Cardiovasc Magn Reson (2020) 22:87. Value shown is 6-month timepoint for Participant 6 Hs-TNI as 12-month timepoint was not collected. Improved Stabilized Worsened Average Reduction: -14.3%
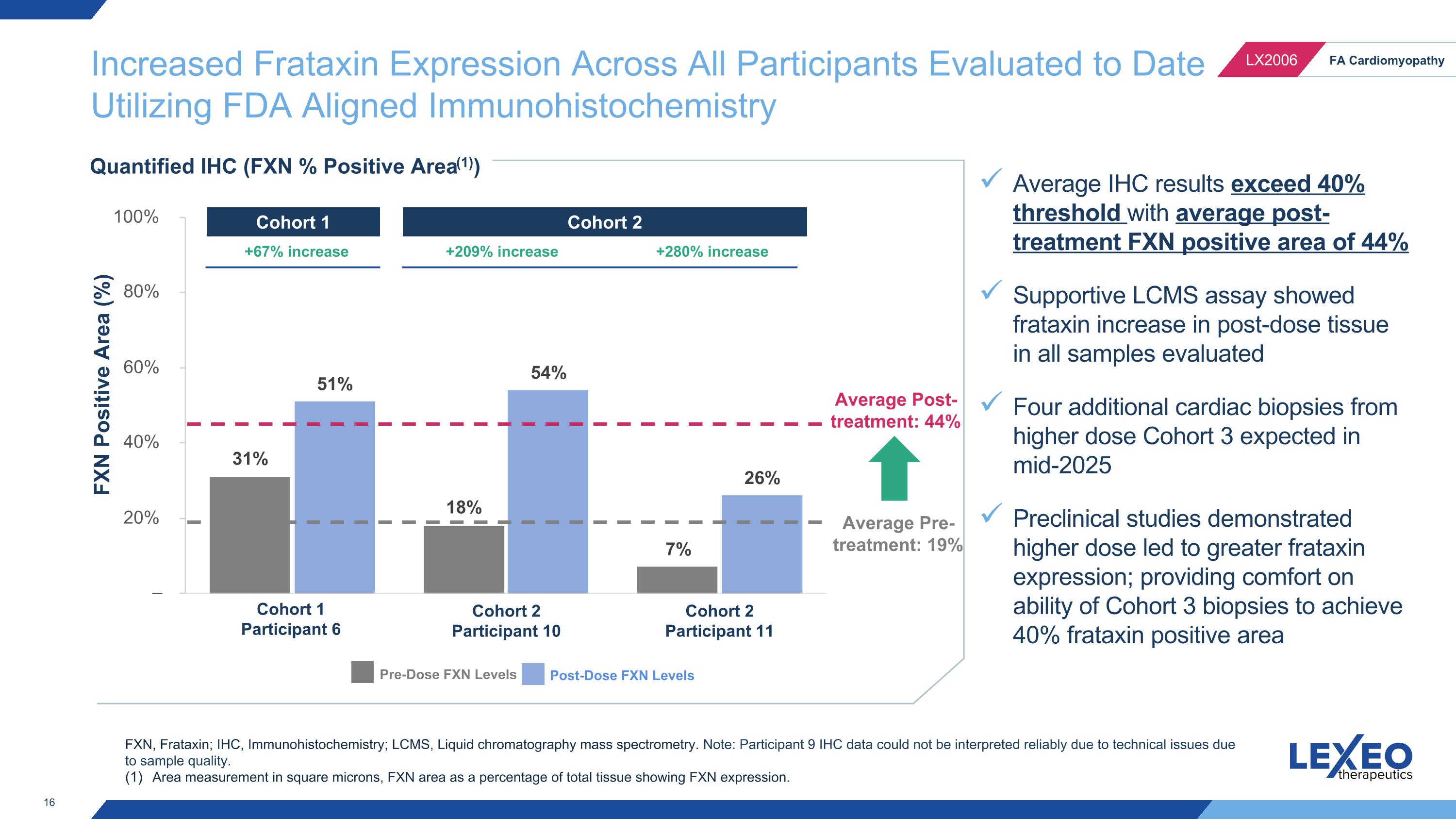
Increased Frataxin Expression Across All Participants Evaluated to Date�Utilizing FDA Aligned Immunohistochemistry FXN Positive Area (%) Quantified IHC (FXN % Positive Area(1)) Cohort 1 Participant 6 Cohort 2 Participant 10 Pre-Dose FXN Levels Post-Dose FXN Levels FXN, Frataxin; IHC, Immunohistochemistry; LCMS, Liquid chromatography mass spectrometry. Note: Participant 9 IHC data could not be interpreted reliably due to technical issues due to sample quality. Area measurement in square microns, FXN area as a percentage of total tissue showing FXN expression. +67% increase +209% increase Cohort 2 Participant 11 +280% increase Average Pre-treatment: 19% Average Post-treatment: 44% Cohort 1 Cohort 2 Average IHC results exceed 40% threshold with average post-treatment FXN positive area of 44% Supportive LCMS assay showed frataxin increase in post-dose tissue in all samples evaluated Four additional cardiac biopsies from higher dose Cohort 3 expected in mid-2025 Preclinical studies demonstrated higher dose led to greater frataxin expression; providing comfort on ability of Cohort 3 biopsies to achieve 40% frataxin positive area

LX2020 (PKP2-ACM)
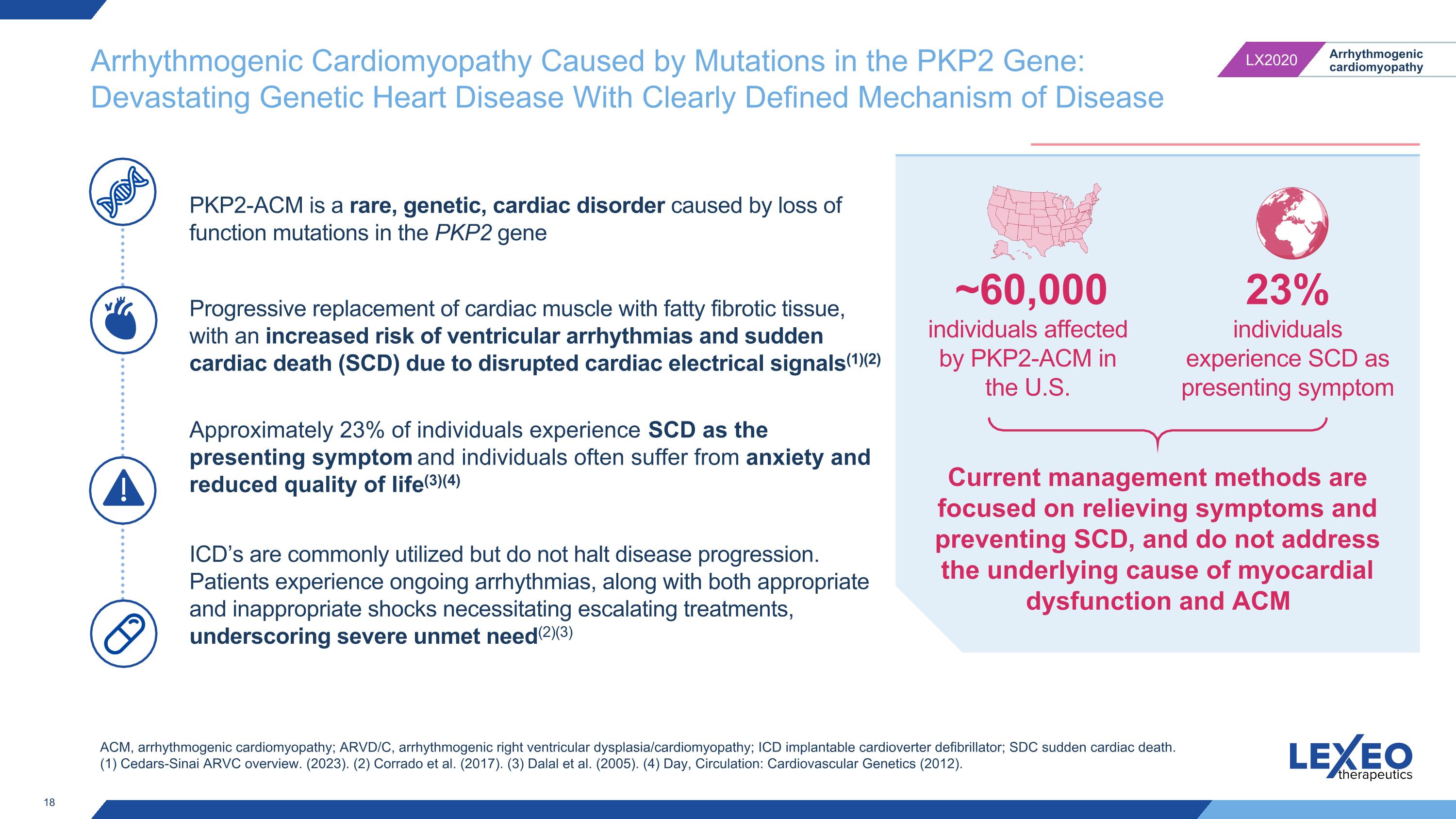
Arrhythmogenic Cardiomyopathy Caused by Mutations in the PKP2 Gene: Devastating Genetic Heart Disease With Clearly Defined Mechanism of Disease ACM, arrhythmogenic cardiomyopathy; ARVD/C, arrhythmogenic right ventricular dysplasia/cardiomyopathy; ICD implantable cardioverter defibrillator; SDC sudden cardiac death. (1) Cedars-Sinai ARVC overview. (2023). (2) Corrado et al. (2017). (3) Dalal et al. (2005). (4) Day, Circulation: Cardiovascular Genetics (2012). PKP2-ACM is a rare, genetic, cardiac disorder caused by loss of function mutations in the PKP2 gene Progressive replacement of cardiac muscle with fatty fibrotic tissue, with an increased risk of ventricular arrhythmias and sudden cardiac death (SCD) due to disrupted cardiac electrical signals(1)(2) Approximately 23% of individuals experience SCD as the presenting symptom and individuals often suffer from anxiety and reduced quality of life(3)(4) ICD’s are commonly utilized but do not halt disease progression. Patients experience ongoing arrhythmias, along with both appropriate and inappropriate shocks necessitating escalating treatments, underscoring severe unmet need(2)(3) Current management methods are focused on relieving symptoms and preventing SCD, and do not address the underlying cause of myocardial dysfunction and ACM 23% individuals experience SCD as presenting symptom ~60,000 individuals affected by PKP2-ACM in the U.S.
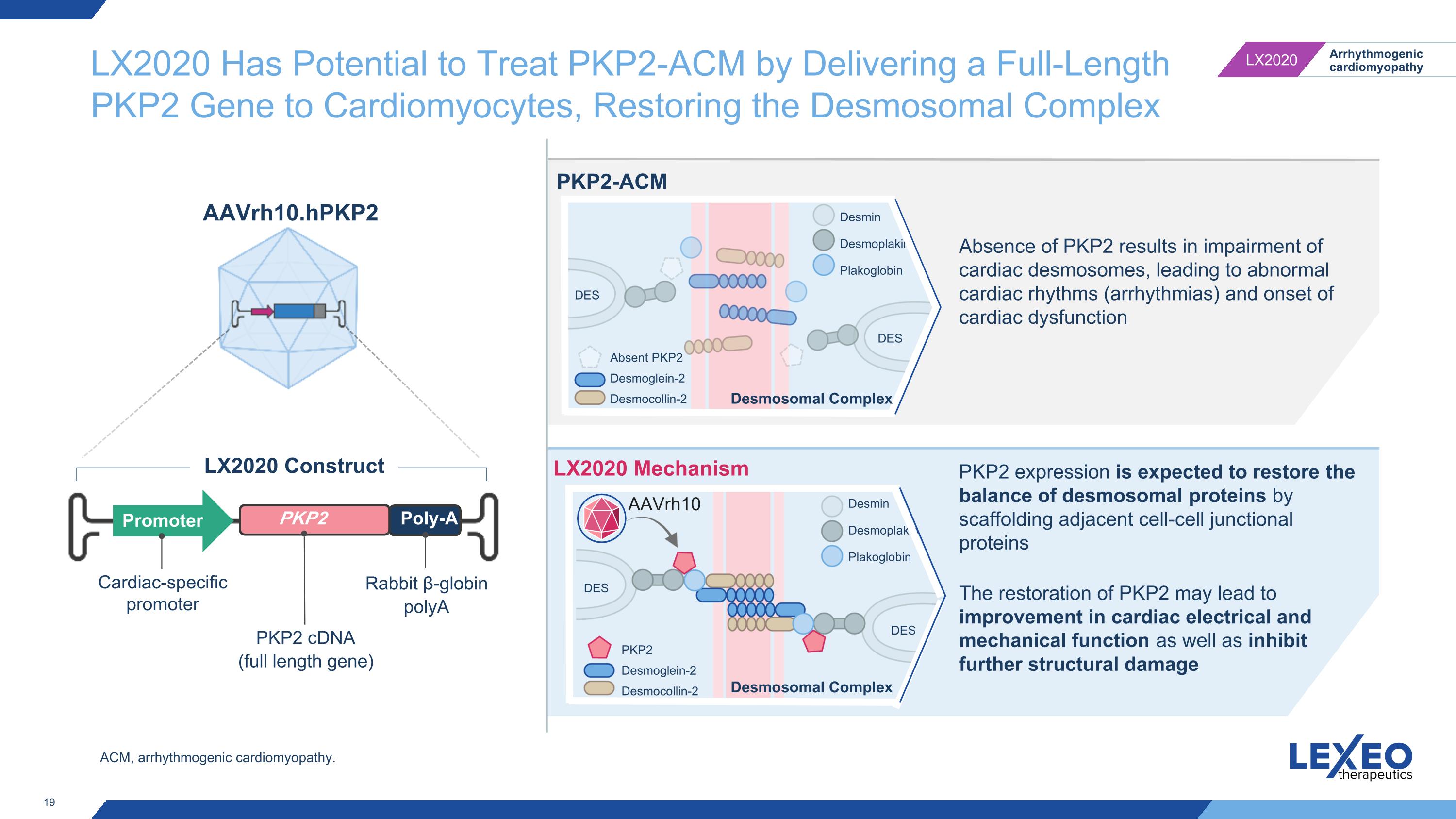
LX2020 Has Potential to Treat PKP2-ACM by Delivering a Full-Length PKP2 Gene to Cardiomyocytes, Restoring the Desmosomal Complex Cardiac-specific promoter Promoter PKP2 gene Poly-A Rabbit β-globin polyA LX2020 Construct AAVrh10.hPKP2 PKP2-ACM LX2020 Mechanism Absence of PKP2 results in impairment of cardiac desmosomes, leading to abnormal cardiac rhythms (arrhythmias) and onset of cardiac dysfunction DES DES Absent PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin DES DES PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin PKP2 expression is expected to restore the balance of desmosomal proteins by scaffolding adjacent cell-cell junctional proteins The restoration of PKP2 may lead to improvement in cardiac electrical and mechanical function as well as inhibit further structural damage AAVrh10 PKP2 cDNA (full length gene) Desmosomal Complex Desmosomal Complex ACM, arrhythmogenic cardiomyopathy.
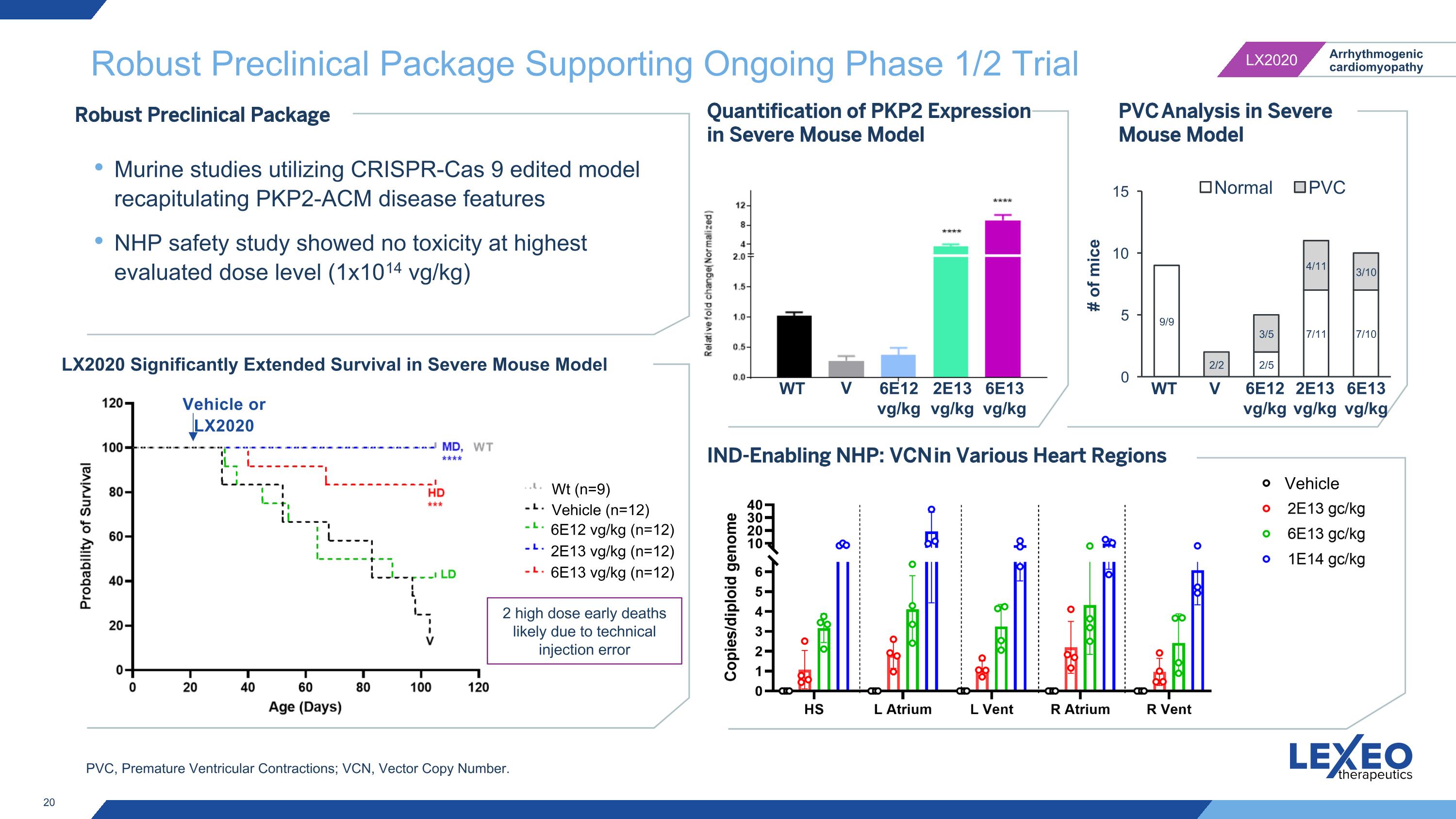
Robust Preclinical Package Supporting Ongoing Phase 1/2 Trial IND-Enabling NHP: VCN in Various Heart Regions Robust Preclinical Package Murine studies utilizing CRISPR-Cas 9 edited model recapitulating PKP2-ACM disease features NHP safety study showed no toxicity at highest evaluated dose level (1x1014 vg/kg) LX2020 Significantly Extended Survival in Severe Mouse Model Vehicle or LX2020 Quantification of PKP2 Expression in Severe Mouse Model 2 high dose early deaths likely due to technical injection error 6E12 vg/kg V WT PVC Analysis in Severe Mouse Model V WT 6E12 vg/kg (n=12) 2E13 vg/kg (n=12) 6E13 vg/kg (n=12) Vehicle (n=12) Wt (n=9) Vehicle 2E13 vg/kg 6E13 vg/kg 6E12 vg/kg 2E13 vg/kg 6E13 vg/kg PVC, Premature Ventricular Contractions; VCN, Vector Copy Number.
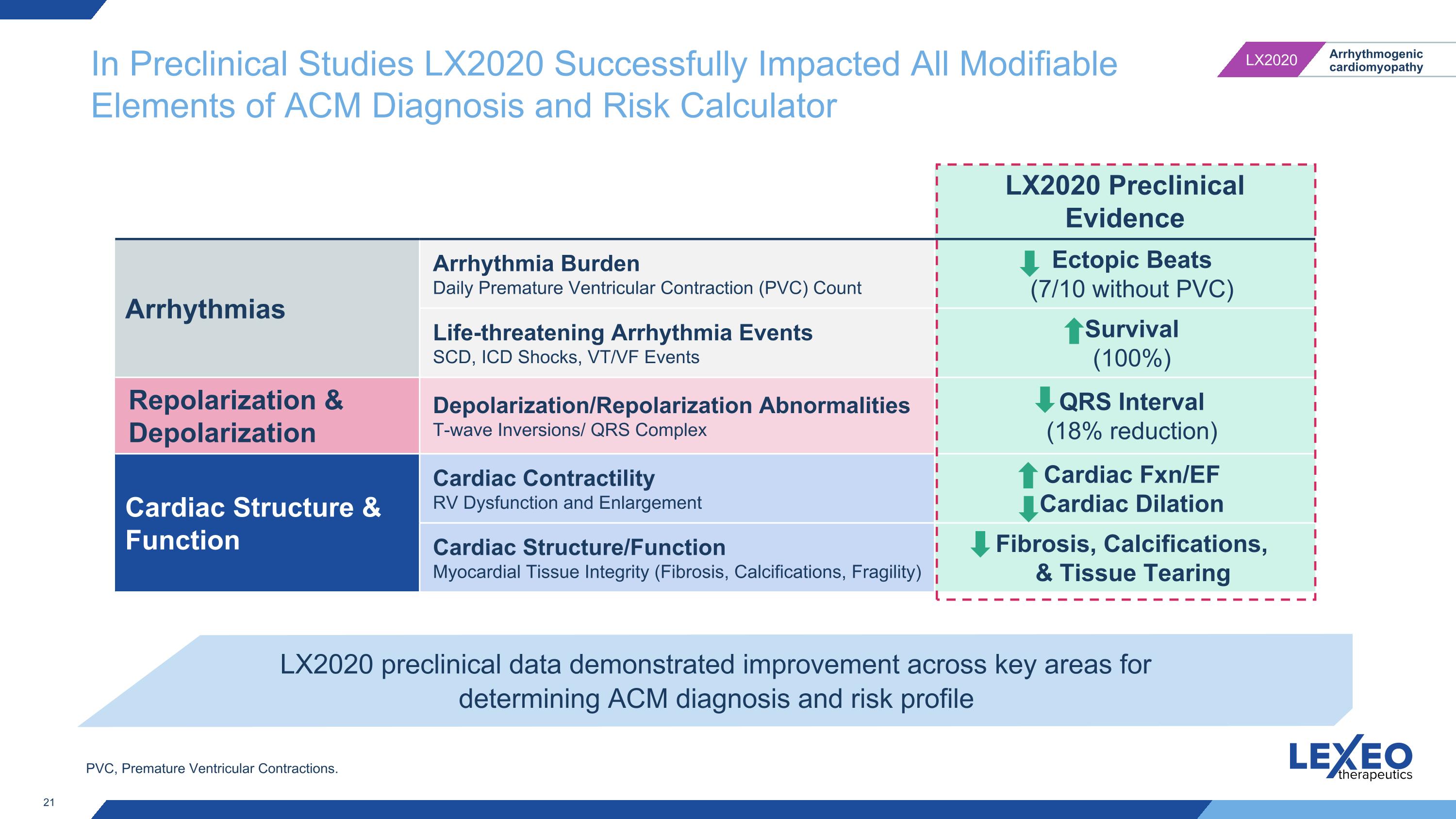
In Preclinical Studies LX2020 Successfully Impacted All Modifiable Elements of ACM Diagnosis and Risk Calculator LX2020 Preclinical Evidence Arrhythmias Arrhythmia Burden Daily Premature Ventricular Contraction (PVC) Count Ectopic Beats�(7/10 without PVC) Life-threatening Arrhythmia Events SCD, ICD Shocks, VT/VF Events Survival (100%) Repolarization & Depolarization Depolarization/Repolarization Abnormalities T-wave Inversions/ QRS Complex QRS Interval (18% reduction) Cardiac Structure & Function Cardiac Contractility RV Dysfunction and Enlargement Cardiac Fxn/EF Cardiac Dilation Cardiac Structure/Function Myocardial Tissue Integrity (Fibrosis, Calcifications, Fragility) Fibrosis, Calcifications, & Tissue Tearing LX2020 preclinical data demonstrated improvement across key areas for determining ACM diagnosis and risk profile PVC, Premature Ventricular Contractions.
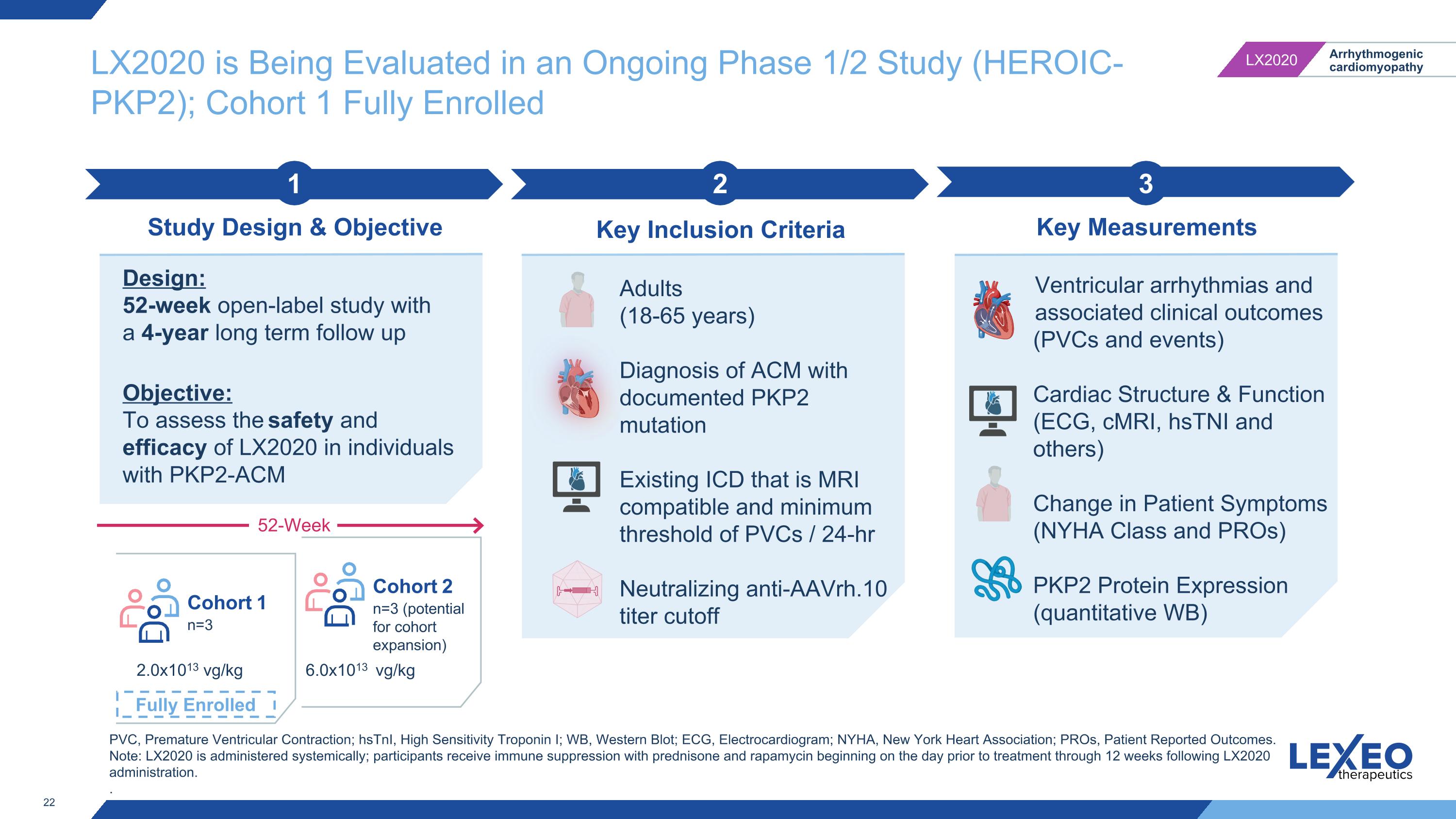
LX2020 is Being Evaluated in an Ongoing Phase 1/2 Study (HEROIC-PKP2); Cohort 1 Fully Enrolled 2 Key Inclusion Criteria 3 Key Measurements 1 Study Design & Objective Design: 52-week open-label study with a 4-year long term follow up Objective: To assess the safety and efficacy of LX2020 in individuals with PKP2-ACM Adults (18-65 years) Diagnosis of ACM with documented PKP2 mutation Existing ICD that is MRI compatible and minimum threshold of PVCs / 24-hr Neutralizing anti-AAVrh.10 titer cutoff Ventricular arrhythmias and associated clinical outcomes (PVCs and events) Cardiac Structure & Function (ECG, cMRI, hsTNI and others) Change in Patient Symptoms (NYHA Class and PROs) PKP2 Protein Expression (quantitative WB) PVC, Premature Ventricular Contraction; hsTnI, High Sensitivity Troponin I; WB, Western Blot; ECG, Electrocardiogram; NYHA, New York Heart Association; PROs, Patient Reported Outcomes. Note: LX2020 is administered systemically; participants receive immune suppression with prednisone and rapamycin beginning on the day prior to treatment through 12 weeks following LX2020 administration. . Cohort 1 n=3 2.0x1013 vg/kg 6.0x1013 vg/kg Cohort 2 n=3 (potential for cohort expansion) 52-Week Fully Enrolled
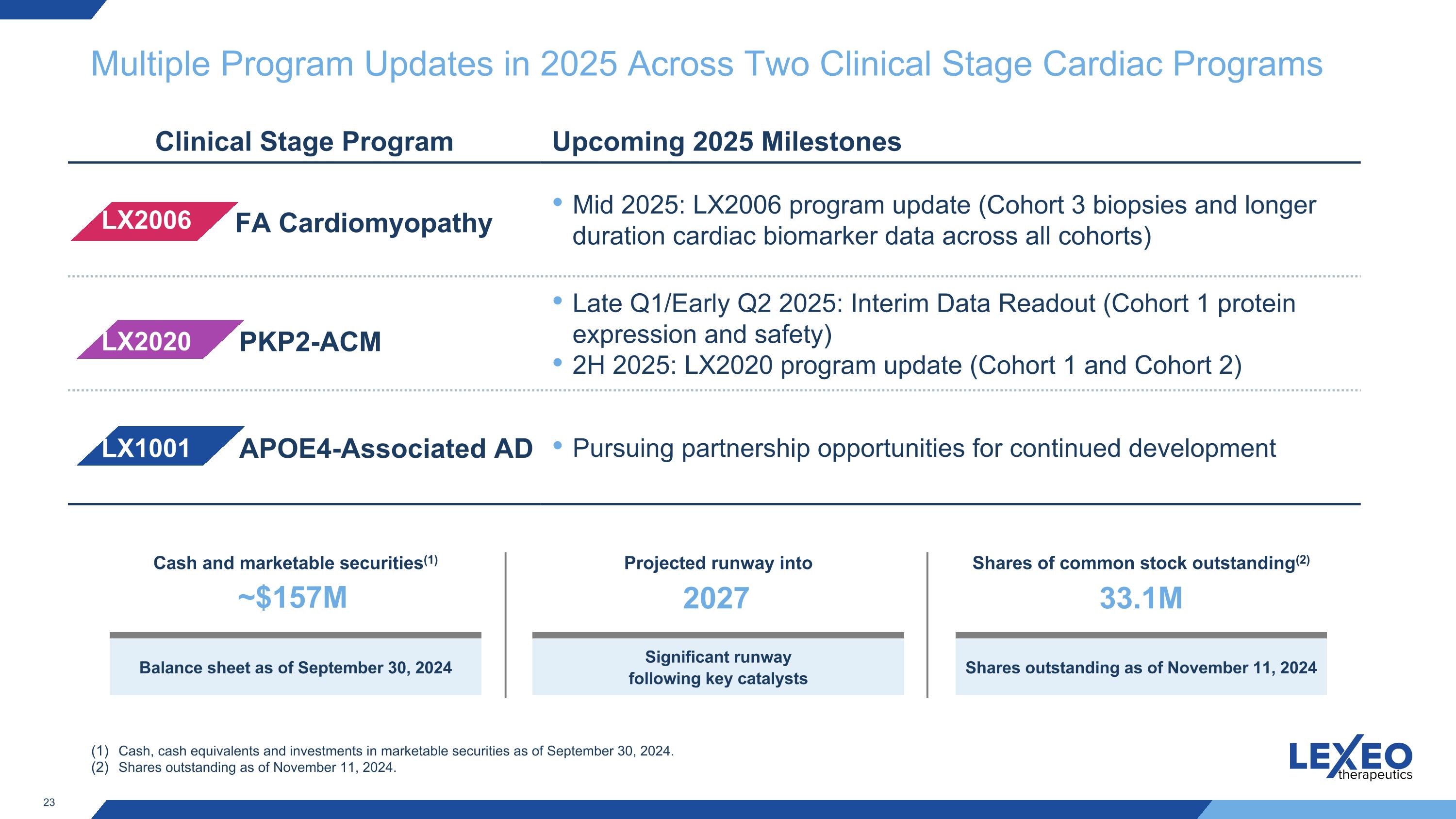
Multiple Program Updates in 2025 Across Two Clinical Stage Cardiac Programs Clinical Stage Program Upcoming 2025 Milestones Mid 2025: LX2006 program update (Cohort 3 biopsies and longer duration cardiac biomarker data across all cohorts) Late Q1/Early Q2 2025: Interim Data Readout (Cohort 1 protein expression and safety) 2H 2025: LX2020 program update (Cohort 1 and Cohort 2) Pursuing partnership opportunities for continued development LX2006 LX2020 FA Cardiomyopathy PKP2-ACM Cash, cash equivalents and investments in marketable securities as of September 30, 2024. Shares outstanding as of November 11, 2024. Cash and marketable securities(1) ~$157M Balance sheet as of September 30, 2024 Projected runway into 2027 Significant runway following key catalysts Shares of common stock outstanding(2) 33.1M Shares outstanding as of November 11, 2024 LX1001 APOE4-Associated AD

Thank you
v3.24.4
Document And Entity Information
|
Jan. 13, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 13, 2025
|
| Entity Registrant Name |
Lexeo Therapeutics, Inc.
|
| Entity Central Index Key |
0001907108
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-41855
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
85-4012572
|
| Entity Address, Address Line One |
345 Park Avenue South, Floor 6
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10010
|
| City Area Code |
212
|
| Local Phone Number |
547-9879
|
| Entity Information, Former Legal or Registered Name |
N/A
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
LXEO
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Lexeo Therapeutics (NASDAQ:LXEO)
過去 株価チャート
から 12 2024 まで 1 2025

Lexeo Therapeutics (NASDAQ:LXEO)
過去 株価チャート
から 1 2024 まで 1 2025
