NEW
YORK, Oct. 7, 2024 /PRNewswire/ -- Report on how
AI is driving market transformation - The Global Pharmaceutical
Contract Research and Manufacturing Market size is estimated to
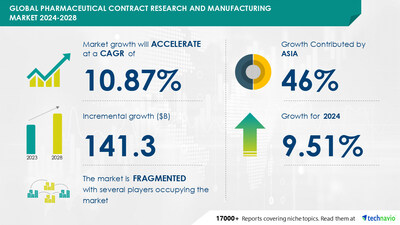
grow by USD 141.3 billion from
2024-2028, according to Technavio. The market is estimated to grow
at a CAGR of 10.87% during the forecast period.
Availability of cost-efficient resources in emerging
markets is driving market growth, with a trend
towards increasing number of US FDA approved manufacturing
facilities. However, stereotypical nature of CMOS poses
a challenge - Key market players include Almac Group Ltd.,
Boehringer Ingelheim International GmbH, Cadila Pharmaceuticals
Ltd., Catalent Inc., Charles River Laboratories International Inc.,
Cmic Holdings Co. Ltd, Dr Reddys Laboratories Ltd., ICON plc, IQVIA
Holdings Inc., Laboratory Corp. Of America Holdings, Lonza Group
Ltd., Lupin Ltd., Novotech Health Holdings, OPTIMAPHARM d.o.o.,
Parexel International Corp., PCI Pharma Services, Recipharm AB,
Samsung Electronics Co. Ltd., Syneos Health Inc., and Thermo Fisher
Scientific Inc..
Key insights into market evolution with
AI-powered analysis. Explore trends, segmentation, and growth
drivers- View the snapshot of this report
|
Pharmaceutical
Contract Research And Manufacturing Market Scope
|
|
Report
Coverage
|
Details
|
|
Base year
|
2023
|
|
Historic
period
|
2018 - 2022
|
|
Forecast
period
|
2024-2028
|
|
Growth momentum &
CAGR
|
Accelerate at a CAGR of
10.87%
|
|
Market growth
2024-2028
|
USD 141.3
billion
|
|
Market
structure
|
Fragmented
|
|
YoY growth 2022-2023
(%)
|
9.51
|
|
Regional
analysis
|
North America, Asia,
Europe, and Rest of World (ROW)
|
|
Performing market
contribution
|
Asia at 46%
|
|
Key
countries
|
US, China, India,
Germany, and UK
|
|
Key companies
profiled
|
Almac Group Ltd.,
Boehringer Ingelheim International GmbH, Cadila Pharmaceuticals
Ltd., Catalent Inc., Charles River Laboratories International Inc.,
Cmic Holdings Co. Ltd, Dr Reddys Laboratories Ltd., ICON plc, IQVIA
Holdings Inc., Laboratory Corp. Of America Holdings, Lonza Group
Ltd., Lupin Ltd., Novotech Health Holdings, OPTIMAPHARM d.o.o.,
Parexel International Corp., PCI Pharma Services, Recipharm AB,
Samsung Electronics Co. Ltd., Syneos Health Inc., and Thermo Fisher
Scientific Inc.
|
Market Driver
The Pharmaceutical Contract Research and Manufacturing (CRAM)
market is experiencing significant growth due to the increasing
number of US FDA-approved manufacturing facilities in emerging
economies, particularly China and
India. India, with around 400 such facilities, is a
top destination for pharmaceutical manufacturing services
outsourcing. This includes approximately 230 API and 150 FDF
facilities, the highest in any country. China, previously limited to CROs due to
stringent regulations on CMOS, has seen a shift with the State
Council allowing MAHs to use third-party licensed manufacturers.
This change has led to an increase in US FDA-approved facilities
and foreign pharmaceutical companies outsourcing drug manufacturing
to China. This trend is expected
to continue, driving the growth of the global pharmaceutical CRAM
market.
The Pharmaceutical Contract Research and Manufacturing (CRM)
market is witnessing significant trends in various areas. Generic
Drugs are gaining popularity due to their affordability, driving
demand for Generic APIs and Formulation Services. Artificial
Intelligence is revolutionizing Drug Discovery and Development,
enabling faster identification of Drug Candidates. Route Scouting
Services and Bioprocess Outsourcing are key areas of focus for
Biologics Manufacturing. Regulatory Compliance remains a top
priority, with Serialization and Counterfeiting mitigation measures
essential. Clinical Trials are outsourced to CROs for
cost-effective manufacturing and efficient Clinical Operations. Big
Pharmaceutical Companies and Academic Institutes collaborate for
innovative Drug Development Services. Advanced manufacturing
technologies like Continuous Manufacturing and Digitalization are
transforming the Pharmaceutical Ecosystem. Targeted Medication
Therapies, Biosimilars, and Personalized Medicines are driving the
need for Specialized Services and Cost-effective Manufacturing.
Drug Delivery Systems and Clinical Trial Support are crucial for
bringing Small Molecule Drugs and Active Pharmaceutical Ingredients
to market. Regulatory bodies and industry associations play a vital
role in ensuring Quality Control and ensuring the integrity of the
Pharmaceutical Supply Chain.
Request Sample of our comprehensive
report now to stay ahead in the AI-driven market evolution!
Market Challenges
- The pharmaceutical Contract Research and Manufacturing
Organizations (CMOs) market in the Asia
Pacific region holds the largest share but faces a
significant challenge due to limited access to advanced
technologies for manufacturing complex drugs, such as biological
drugs and vaccines. These CMOs primarily focus on producing small
molecule-based generic drugs due to high manufacturing costs and
lack of funding. This restricts growth opportunities for small and
medium-sized CMOs. Major players like Boehringer Ingelheim,
Catalent, and Lonza dominate the market for large-scale production
of biological drugs. The absence of advanced technologies and high
manufacturing costs in countries like Mexico, South
Korea, and Brazil also
hinder the growth of CMOs in these regions. Consequently, the
global pharmaceutical CRAM market growth may be impeded
substantially during the forecast period.
- The Pharmaceutical Contract Research and Manufacturing (CRM)
market faces several challenges in the production of biologics and
small molecule drugs. Big Pharma companies and academic institutes
rely on CROs for cost-effective manufacturing of targeted
medication therapies, including biologics and biosimilars.
Manufacturing services for active pharmaceutical ingredients (APIs)
and drug formulation require advanced technologies like continuous
manufacturing and specialized services for drug delivery systems.
Quality control is crucial for producing consistent, high-quality
products. CROs must support clinical trial operations and
commercial operations, ensuring compliance with quality standards
and patient needs. Digitalization and personalized medicines also
require specialized expertise. The drug development process
involves complex production workflows, from the creation of drug
candidates to the manufacturing of oral solids, liquids, emulsions,
and more. Meeting the demands of Big Pharma and academic institutes
while maintaining cost-effectiveness and quality is a significant
challenge for the CRM industry.
Discover how AI is revolutionizing market
trends- Get your access now!
Segment Overview
This pharmaceutical contract research and manufacturing market
report extensively covers market segmentation by
- Service
- End-user
- 2.1 Big pharmaceuticals
- 2.2 Small and medium-sized pharmaceuticals
- 2.3 Generic pharmaceuticals
- Geography
- 3.1 North America
- 3.2 Asia
- 3.3 Europe
- 3.4 Rest of World (ROW)
1.1 CMO- The Contract Research and
Manufacturing Organizations (CMOs) segment is the largest in the
global pharmaceutical CRAM market, accounting for a significant
market share. CMOs are entities that manufacture drugs and
healthcare products on a contract basis for pharmaceutical
companies and biotech firms. Advancements in medical sciences and
the growing popularity of specialty medicines, as well as
technological developments like nanotechnology and stem cell
research, are driving the demand for complex manufacturing
processes. These processes require extended resource deployments,
leading pharmaceutical giants like Pfizer, Johnson & Johnson,
and GlaxoSmithKline to outsource their manufacturing activities to
optimize in-house resources. The outsourcing trend is particularly
prevalent among pharmaceutical vendors worldwide, resulting in the
substantial growth of the CMO segment. Emerging markets, including
India, Brazil, China, and Mexico, are major contributors due to their
low labor and land costs, making them attractive locations for
setting up manufacturing facilities. Generic drug manufacturers in
these countries outsource approximately 80% of their production to
CMOs. The increasing focus on core competencies and cost reduction,
along with the regulatory compliance and research advancement
benefits, are fueling the expansion of the CMO segment in the
global pharmaceutical CRAM market.
Download a Sample of our
comprehensive report today to discover how AI-driven innovations
are reshaping competitive dynamics
Research Analysis
The Pharmaceutical Contract Research and Manufacturing (CRM)
market encompasses a range of services that support the development
and production of small molecule drugs, biologics, biosimilars, and
active pharmaceutical ingredients (APIs). Services include drug
discovery, route scouting, regulatory compliance, clinical trials,
outsourcing, drug formulation, quality control, and advanced
manufacturing technologies such as continuous manufacturing and
specialized services. Artificial Intelligence (AI) is increasingly
being utilized in drug discovery to identify new drug candidates
and optimize their properties. Cost-effective manufacturing is a
key driver for outsourcing, particularly for generic drugs and
APIs. Bioprocess outsourcing is essential for the production of
complex biologics and biosimilars. Continuous manufacturing offers
advantages in terms of cost savings, improved quality, and
increased efficiency. Specialized services include the production
of low potent APIs and the manufacturing of originator and generic
APIs. The market caters to both small molecule drugs and biologics,
with a focus on ensuring regulatory compliance and maintaining the
highest standards of quality control.
Market Research Overview
The Pharmaceutical Contract Research and Manufacturing (CRM)
market encompasses a range of services that support the entire drug
development process, from discovery to commercialization. This
market includes various services such as drug discovery, clinical
trials, regulatory compliance, route scouting, bioprocess
outsourcing, and manufacturing services. Generic Drugs: CRM
companies offer manufacturing services for generic drugs, producing
affordable alternatives to branded medicines. Artificial
Intelligence: AI is increasingly used in drug development to
identify new targets, optimize drug design, and analyze clinical
trial data. Drug Development: CRM companies provide comprehensive
drug development services, from discovery to commercialization,
ensuring regulatory compliance and cost-effective manufacturing.
Clinical Trials: CRM companies offer clinical trial support,
managing trial design, execution, and data analysis to ensure
efficient and effective trials. Outsourcing: Pharmaceutical
companies outsource various aspects of drug development and
manufacturing to CRM companies for cost savings and expertise.
Bioprocess Outsourcing: CRM companies specialize in bioprocessing,
producing biologics, biosimilars, and vaccines using advanced
manufacturing technologies. Serialization and Counterfeiting: CRM
companies provide serialization services to ensure product
authenticity and combat counterfeiting, enhancing patient safety.
Pharmaceutical Ecosystem: CRM companies operate within the complex
pharmaceutical ecosystem, collaborating with academic institutes,
CROs, and big pharma to bring new drugs to market. Drug Development
Services: CRM companies offer a range of drug development services,
from drug discovery and formulation to manufacturing and
commercialization. Manufacturing Services: CRM companies provide
manufacturing services for small molecule drugs, APIs, and drug
delivery systems, ensuring quality control and cost savings.
Cost-effective manufacturing: CRM companies offer cost-effective
manufacturing solutions, utilizing economies of scale and advanced
technologies to reduce production costs. Quality control: CRM
companies prioritize quality control, implementing rigorous
standards and advanced technologies to ensure product safety and
efficacy. Advanced manufacturing technologies: CRM companies employ
advanced manufacturing technologies, such as continuous
manufacturing and specialized services, to improve efficiency and
reduce costs. Personalized medicines: CRM companies support the
development and manufacturing of personalized medicines, tailoring
treatments to individual patient needs. Drug development processes:
CRM companies optimize drug development processes, from production
workflows to digitalization, to bring new drugs to market faster
and more efficiently.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Service
-
- End-user
-
- Big Pharmaceuticals
- Small And Medium-sized Pharmaceuticals
- Generic Pharmaceuticals
- Geography
-
- North America
- Asia
- Europe
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory
company. Their research and analysis focuses on emerging market
trends and provides actionable insights to help businesses identify
market opportunities and develop effective strategies to optimize
their market positions.
With over 500 specialized analysts, Technavio's report library
consists of more than 17,000 reports and counting, covering 800
technologies, spanning across 50 countries. Their client base
consists of enterprises of all sizes, including more than 100
Fortune 500 companies. This growing client base relies on
Technavio's comprehensive coverage, extensive research, and
actionable market insights to identify opportunities in existing
and potential markets and assess their competitive positions within
changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/pharmaceutical-contract-research-and-manufacturing-market-to-grow-by-usd-141-3billion-from-2024-2028--driven-by-cost-efficient-resources-in-emerging-markets-ai-powered-report---technavio-302267668.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/pharmaceutical-contract-research-and-manufacturing-market-to-grow-by-usd-141-3billion-from-2024-2028--driven-by-cost-efficient-resources-in-emerging-markets-ai-powered-report---technavio-302267668.html
SOURCE Technavio