UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO
RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF OCTOBER 2023
COMMISSION FILE NUMBER 001-41084
NeuroSense Therapeutics
Ltd.
(Translation of registrant’s name into English)
NeuroSense Therapeutics Ltd.
11 HaMenofim Street, Building B
Herzliya 4672562 Israel
+972-9-7996183
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file
annual reports under cover Form 20-F or Form 40-F: Form 20-F ☒ Form
40-F ☐
INFORMATION
CONTAINED IN THIS REPORT ON FORM 6-K
On October 4, 2023, NeuroSense Therapeutics Ltd. (the “Company”) issued a press release titled “NeuroSense’s PrimeC Demonstrates
Outstanding Effect on ALS Survival in Innovative iPSC Model”, announcing the results of a non-sponsored in vitro study of PrimeC in amyotrophic
lateral sclerosis (ALS) conducted by Dr. Justin Ichida, PhD at the University of Southern California’s (USC) Stem Cell Ichida Lab. A copy
of the press release is filed herewith as Exhibit 99.1
This Report on Form 6-K (with respect to exhibit 99.1, only the
first three paragraphs) is hereby incorporated by reference into the registrant’s Registration Statements on Form
F-3 (File No. 333-269306) and Form
S-8 (File No. 333-262480), to be a part thereof from the date on which this report is submitted, to the extent not superseded by
documents or reports subsequently filed or furnished.
Exhibit Index
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
NeuroSense Therapeutics Ltd. |
| |
|
|
| |
By: |
/s/ Alon Ben-Noon |
| |
|
Name: |
Alon Ben-Noon |
| |
|
Title: |
Chief Executive Officer |
Date: October 4, 2023
2
Exhibit
99.1
NeuroSense’s PrimeC Demonstrates Outstanding
Effect on ALS Survival in Innovative iPSC Model
| ● | In
collaboration with the University of Southern California’s Ichida Stem Cell Lab, PrimeC
was shown to significantly increase survival rate of induced motor neurons in an in vitro
study utilizing induced pluripotent stem cells (iPSCs) generated from people living with
ALS |
| ● | Results
reinforce previous findings in multiple models |
| ● | In
a previous independent study carried out by Dr. Ichida, PrimeC performed among the best
in improving motor neuron survival when compared to several other ALS drugs in development
and two ALS FDA approved drugs |
CAMBRIDGE,
Mass., Oct. 4, 2023 /PRNewswire/ -- NeuroSense Therapeutics Ltd. (Nasdaq: NRSN) (“NeuroSense”), a company
developing treatments for severe neurodegenerative diseases, today announced the results of a non-sponsored in vitro study
of PrimeC in amyotrophic lateral sclerosis (ALS) conducted by Dr. Justin Ichida, PhD at the University of Southern California’s (USC) Stem
Cell Ichida Lab, part of the Eli and Edythe Broad CIRM Center for Regenerative Medicine and Stem Cell Research at USC.
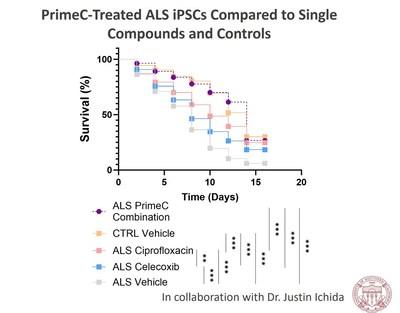
Dr.
Ichida’s in vitro study used induced pluripotent stem cells (iPSCs) that were derived from blood samples of people living
with ALS. The survival rate of induced motor neurons when administered with PrimeC in comparison to each of PrimeC’s components, ciprofloxacin
and celecoxib, alone was evaluated. PrimeC showed a significant beneficial effect relative to each of its components alone, demonstrating
the synergistic effect of PrimeC and supporting the rationale of combining the two active components into NeuroSense’s proprietary formulation.
PrimeC attenuated ALS related pathology, as depicted by the increased survival rate. The PrimeC treated iPSCs had increased neuronal
survival compared to ALS control cells, rendering their survival rate to be similar to healthy controls.
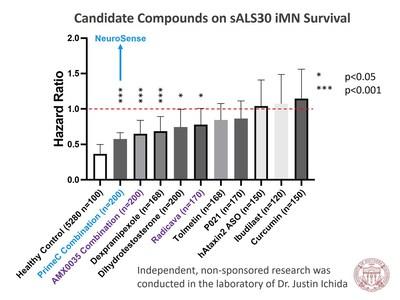
A
previous study conducted independently by Dr. Ichida in iPSCs has already demonstrated NeuroSense’s combination therapy performed among
the best in improving motor neuron survival alongside several other ALS drugs in development and two FDA approved ALS drugs, Amylyx Pharmaceuticals’
combination therapy, RELYVRIO, and Mitsubishi Tanabe’s Radicava.
Dr.
Ichida is the John Douglas French Alzheimer’s Foundation Associate Professor of Stem Cell Biology and Regenerative Medicine and a New
York Stem Cell Foundation–Robertson Investigator. He is widely recognized as being on the cutting edge of future treatments
and cures for ALS and Alzheimer’s disease, as well as the use of iPSCs, a state-of-the art in vitro cellular technique,
in drug testing and development.
“At
our lab, we screen thousands of compounds in search of one that may be effective in ALS, and we were very impressed by the data resulting
from our iPCS in vitro study of PrimeC. We chose to evaluate PrimeC based on the growing body of clinical, pre-clinical,
and biomarker data on its efficacy in ALS,” Dr. Ichida stated. “As a candidate compound, NeuroSense’s combination was among
the best in improving motor neuron survival. Furthermore, in a follow-on study, where we explored specifically the synergistic effect
of PrimeC combination relative to each one of its therapeutic agents, the results exceeded our expectations, as PrimeC increased the
survival rate to the level of the healthy control and that got us very excited.”
“Dr.
Ichida has been described as being beyond the cutting edge in his field and we are honored that he and his lab chose to evaluate PrimeC
in this non-sponsored study,” stated NeuroSense Founder and CEO, Alon Ben Noon. “These results further bolster our confidence
that PrimeC may offer a much-needed therapy for this debilitating disease which is ALS. We are proud that PrimeC is recognized as a leading
ALS drug candidate by the top medical institutions in the world.”
Dr.
Shiran Zimri, VP R&D of NeuroSense added, “These findings present a new opportunity to potentially screen super responder patients
using iPSCs, a non-invasive method which only requires a blood draw from the patient. Using this screening model may result in higher
efficacy in people living with ALS who are most likely to benefit from PrimeC.”
Dr.
Zimri will be presenting these results at the 22nd Annual Northeast Amyotrophic Lateral Sclerosis (NEALS) Meeting which takes place October4-6,
2023 in Clearwater, Florida.
NeuroSense
expects clinical topline results from PARADIGM, its Phase 2b study of PrimeC in the treatment of ALS in the fourth quarter of 2023.
About
iPSCs in Drug Development
Cellular
reprogramming, particularly the generation of induced pluripotent stem cells (iPSCs), has significant benefits for drug development,
as they can be generated from patient-specific cells, such as skin or blood cells, and differentiated into various cell types. Deriving
patient induced motor neurons (iMNs) from a patient’s blood sample recapitulates the neurodegeneration involved in ALS, allowing for
the creation of ALS in vitro models which ultimately provide a platform for drug screening and testing. Furthermore,
cellular reprogramming enables high-throughput, personalized drug screening on patient derived nerve cells. Revolutionizing drug development,
iPSCs provide accurate and more efficient models for disease research and drug discovery.
About
NeuroSense
NeuroSense
Therapeutics, Ltd. is a clinical-stage biotechnology company focused on discovering and developing treatments for patients suffering
from debilitating neurodegenerative diseases. NeuroSense believes that these diseases, which include amyotrophic lateral sclerosis (ALS),
Alzheimer’s disease and Parkinson’s disease, among others, represent one of the most significant unmet medical needs of our time, with
limited effective therapeutic options available for patients to date. Due to the complexity of neurodegenerative diseases and based on
strong scientific research on a large panel of related biomarkers, NeuroSense’s strategy is to develop combined therapies targeting multiple
pathways associated with these diseases.
For
additional information, we invite you to visit our website and follow us on LinkedIn and Twitter.
Forward-Looking
Statements
This
press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements,
other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements
contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,”
“could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,”
“plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,”
“will” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements
contain these words. Forward-looking statements are based on NeuroSense Therapeutics’ current expectations and are subject to inherent
uncertainties, risks and assumptions that are difficult to predict and include statements regarding the timing of clinical top-line results
of, and the results of, the PARADIGM clinical trial. Further, certain forward-looking statements are based on assumptions as to future
events that may not prove to be accurate. The future events and trends may not occur and actual results could differ materially and adversely
from those anticipated or implied in the forward looking statements. These risks include a delay in the reporting of clinical top-line
results from PARADIGM clinical trial; the potential for PrimeC to safely and effectively target ALS; preclinical and clinical data for
PrimeC; the timing of current and future clinical trials, timing for reporting data; the development and commercial potential of any
product candidates of the company; and other risks and uncertainties set forth in NeuroSense’s filings with the Securities and Exchange
Commission (SEC)., You should not rely on these statements as representing our views in the future. More information about the risks
and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Annual Report on Form 20-F filed
with the Securities and Exchange Commission on March 22, 2023. Forward-looking statements contained in this announcement are made as
of this date, and NeuroSense Therapeutics Ltd. undertakes no duty to update such information except as required under applicable law.
Infographic
- https://mma.prnewswire.com/media/2238433/NeuroSense_Infographic.jpg
Infographic - https://mma.prnewswire.com/media/2238434/NeuroSense_Infographic_2.jpg
Logo - https://mma.prnewswire.com/media/1707291/NeuroSense_Therapeutics_Logo.jpg

4
NeuroSense Therapeutics (NASDAQ:NRSN)
過去 株価チャート
から 12 2024 まで 1 2025
NeuroSense Therapeutics (NASDAQ:NRSN)
過去 株価チャート
から 1 2024 まで 1 2025