false000181056000018105602023-10-122023-10-120001810560revb:CommonStockParValue0001PerShareMember2023-10-122023-10-120001810560revb:RedeemableWarrantsEachExercisableForOneByThirtyFifthShareOfCommonStockAtExercisePriceOf40250PerShareMember2023-10-122023-10-12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): October 12, 2023 |
REVELATION BIOSCIENCES, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39603 |
84-3898466 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
4660 La Jolla Village Drive Suite 100 |
|
San Diego, California |
|
92122 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (650) 800-3717 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common stock, par value $0.001 per share |
|
REVB |
|
The Nasdaq Stock Market LLC |
Redeemable warrants, each exercisable for a 1/35th share of common stock at an exercise price of $402.50 per share |
|
REVBW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On October 12, 2023, Revelation Biosciences, Inc. (the “Company”) presented at the ROTH MKM Healthcare Opportunities Conference in New York, New York. A copy of the presentation is attached to this Current Report on Form 8-K as Exhibit 99.1 and is incorporated herein by reference.
The information in Item 7.01 and in Exhibit 99.1 will not be treated as “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. This information will not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or into another filing under the Exchange Act, unless that filing expressly incorporates this information by reference.
Item 8.01 Other Events.
On October 12, 2023, the Company issued a press release titled “Gemini Induces Pharmacologic Activity and Related Physiologic Changes in Multiple Preclinical Studies - Key Biomarker Activity Confirmed for Evaluation in Upcoming Phase 1 Study”. A copy of the press release is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
REVELATION BIOSCIENCES, INC. |
|
|
|
|
Date: |
October 12, 2023 |
By: |
/s/ Chester S. Zygmont, III |
|
|
|
Chester S. Zygmont, III
Chief Financial Officer
(principal financial and accounting officer)
|

Developing innovative therapeutics to address unmet needs Corporate Presentation / October 2023 www.revbiosciences.com

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions investors not to place undue reliance on any such forward-looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the clinical utility of an increase in intranasal cytokine levels as a biomarker of viral infections; the risk that our preclinical studies will not demonstrate sufficient positive data to support commencement of clinical trials; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for REVTx-100, REVTx-300 or any other product candidates; potential indications for which product candidates may be developed; the potential impact that COVID 19 may have on Revelation’s suppliers, vendors, regulatory agencies, employees and the global economy as a whole; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; the ability of Revelation to obtain further financing and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
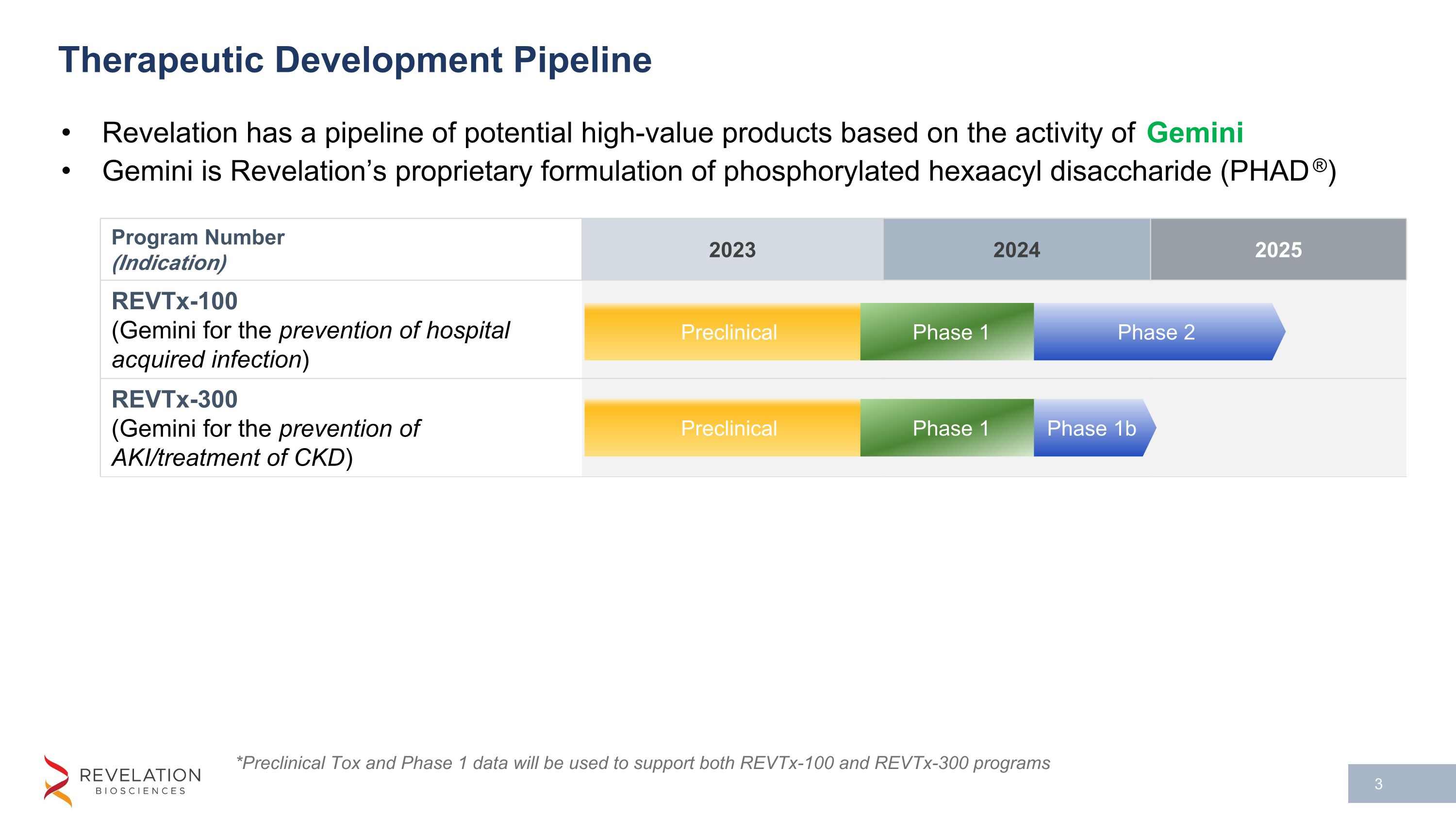
Program Number (Indication) 2023 2024 2025 REVTx-100 (Gemini for the prevention of hospital acquired infection) REVTx-300 (Gemini for the prevention of AKI/treatment of CKD) Therapeutic Development Pipeline Revelation has a pipeline of potential high-value products based on the activity of Gemini Gemini is Revelation’s proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®) Preclinical Phase 1 *Preclinical Tox and Phase 1 data will be used to support both REVTx-100 and REVTx-300 programs Phase 2 Preclinical Phase 1 Phase 1b
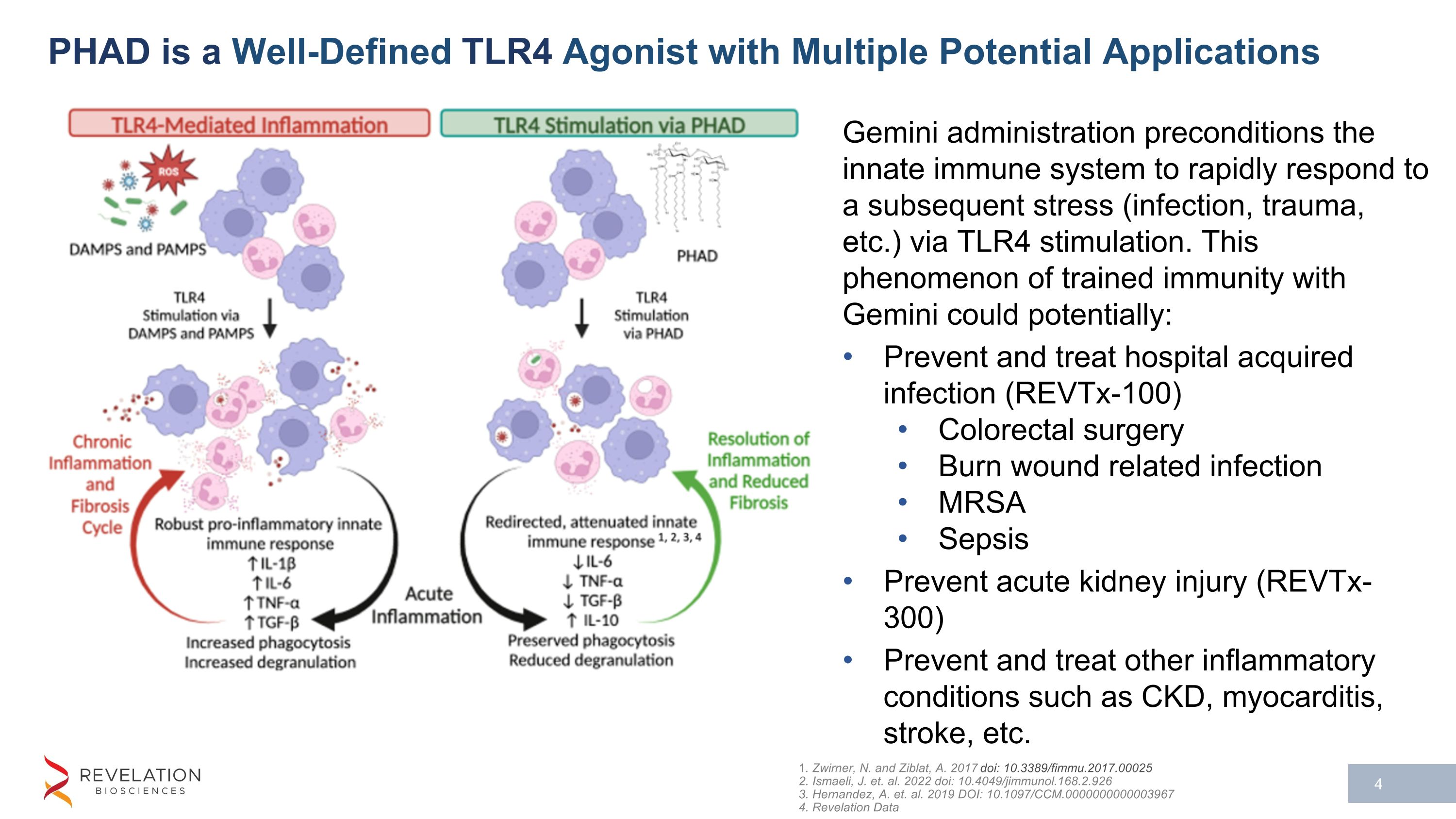
PHAD is a Well-Defined TLR4 Agonist with Multiple Potential Applications 1. Zwirner, N. and Ziblat, A. 2017 doi: 10.3389/fimmu.2017.00025 2. Ismaeli, J. et. al. 2022 doi: 10.4049/jimmunol.168.2.926 3. Hernandez, A. et. al. 2019 DOI: 10.1097/CCM.0000000000003967 4. Revelation Data Gemini administration preconditions the innate immune system to rapidly respond to a subsequent stress (infection, trauma, etc.) via TLR4 stimulation. This phenomenon of trained immunity with Gemini could potentially: Prevent and treat hospital acquired infection (REVTx-100) Colorectal surgery Burn wound related infection MRSA Sepsis Prevent acute kidney injury (REVTx-300) Prevent and treat other inflammatory conditions such as CKD, myocarditis, stroke, etc.

REVTx-100 Program Gemini for the Prevention of Hospital Acquired Infection
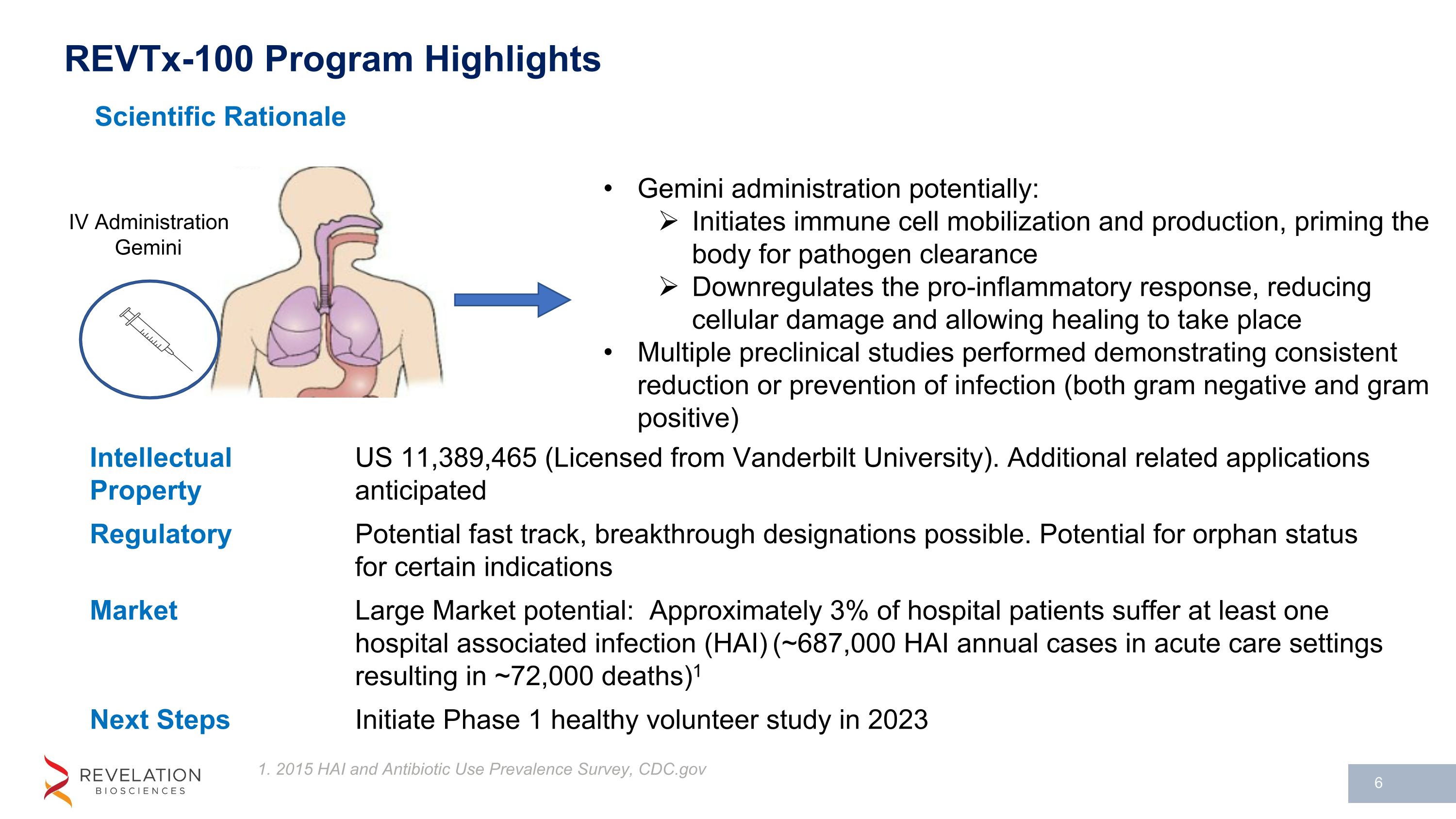
REVTx-100 Program Highlights Scientific Rationale 1. 2015 HAI and Antibiotic Use Prevalence Survey, CDC.gov Gemini administration potentially: Initiates immune cell mobilization and production, priming the body for pathogen clearance Downregulates the pro-inflammatory response, reducing cellular damage and allowing healing to take place Multiple preclinical studies performed demonstrating consistent reduction or prevention of infection (both gram negative and gram positive) IV Administration Gemini Intellectual Property US 11,389,465 (Licensed from Vanderbilt University). Additional related applications anticipated Regulatory Potential fast track, breakthrough designations possible. Potential for orphan status for certain indications Market Large Market potential: Approximately 3% of hospital patients suffer at least one hospital associated infection (HAI) (~687,000 HAI annual cases in acute care settings resulting in ~72,000 deaths)1 Next Steps Initiate Phase 1 healthy volunteer study in 2023
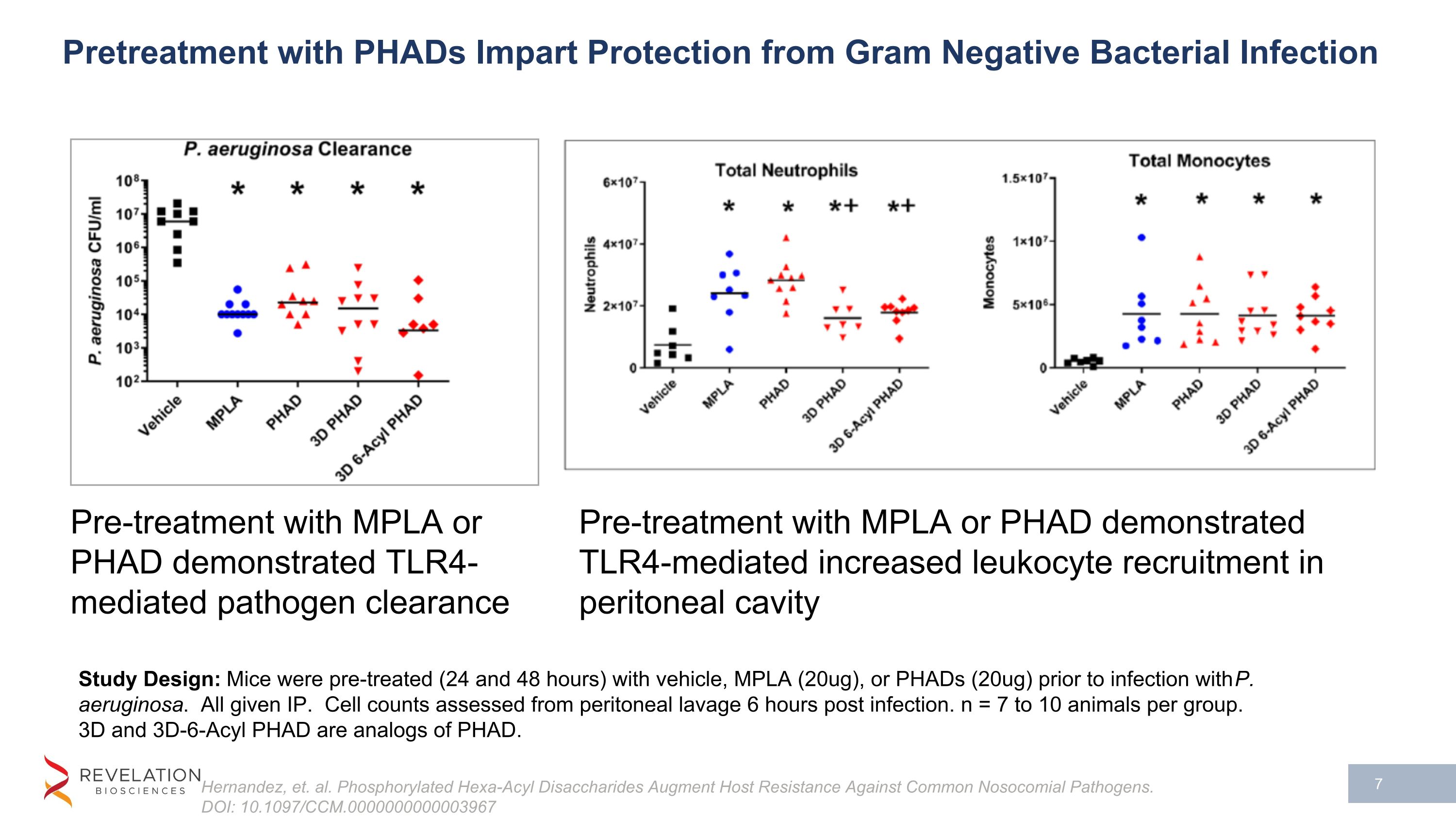
Pretreatment with PHADs Impart Protection from Gram Negative Bacterial Infection Hernandez, et. al. Phosphorylated Hexa-Acyl Disaccharides Augment Host Resistance Against Common Nosocomial Pathogens. DOI: 10.1097/CCM.0000000000003967 Study Design: Mice were pre-treated (24 and 48 hours) with vehicle, MPLA (20ug), or PHADs (20ug) prior to infection with P. aeruginosa. All given IP. Cell counts assessed from peritoneal lavage 6 hours post infection. n = 7 to 10 animals per group. 3D and 3D-6-Acyl PHAD are analogs of PHAD. Pre-treatment with MPLA or PHAD demonstrated TLR4-mediated pathogen clearance Pre-treatment with MPLA or PHAD demonstrated TLR4-mediated increased leukocyte recruitment in peritoneal cavity
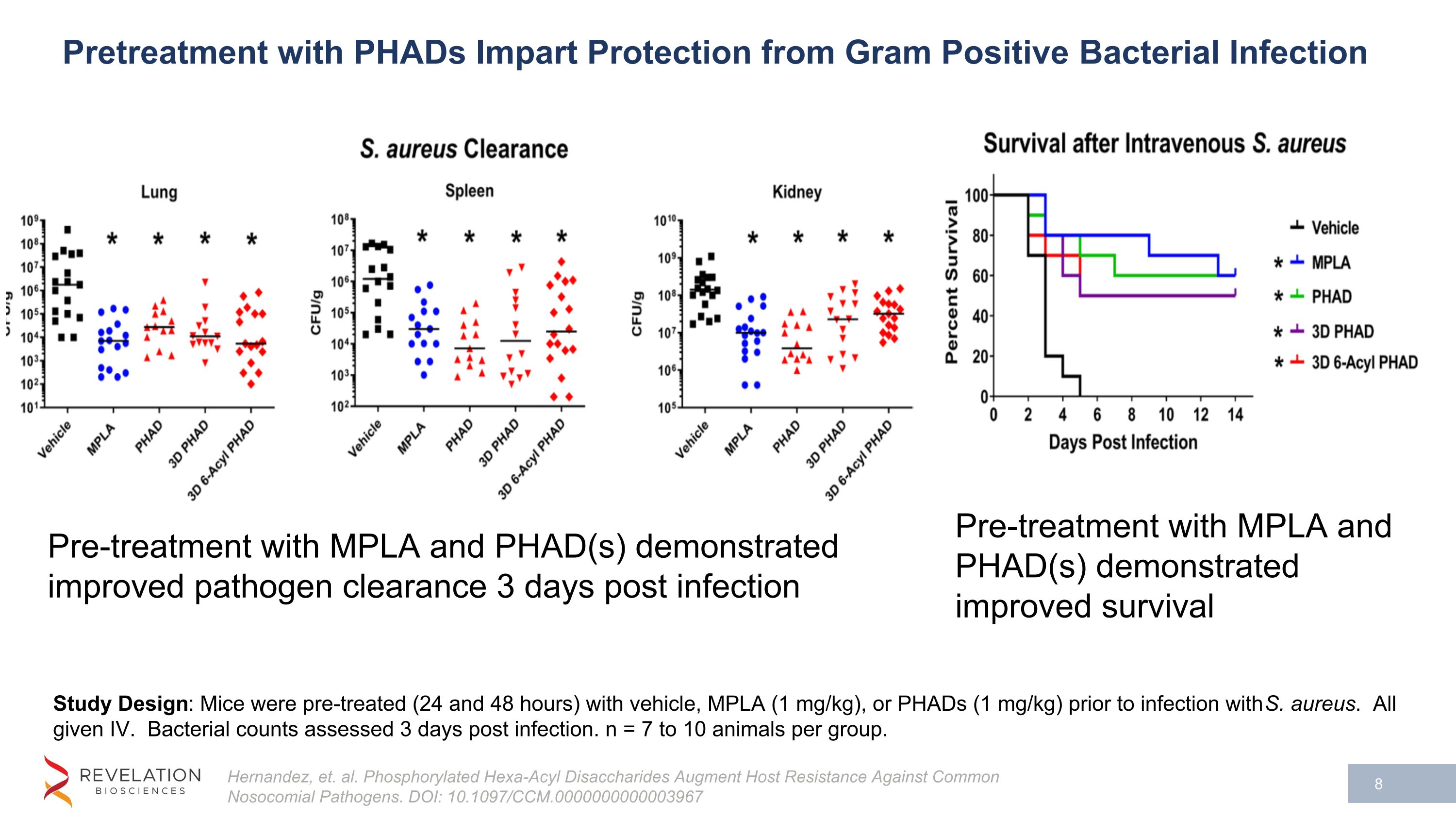
Study Design: Mice were pre-treated (24 and 48 hours) with vehicle, MPLA (1 mg/kg), or PHADs (1 mg/kg) prior to infection with S. aureus. All given IV. Bacterial counts assessed 3 days post infection. n = 7 to 10 animals per group. Pre-treatment with MPLA and PHAD(s) demonstrated improved pathogen clearance 3 days post infection Pre-treatment with MPLA and PHAD(s) demonstrated improved survival Hernandez, et. al. Phosphorylated Hexa-Acyl Disaccharides Augment Host Resistance Against Common Nosocomial Pathogens. DOI: 10.1097/CCM.0000000000003967 Pretreatment with PHADs Impart Protection from Gram Positive Bacterial Infection
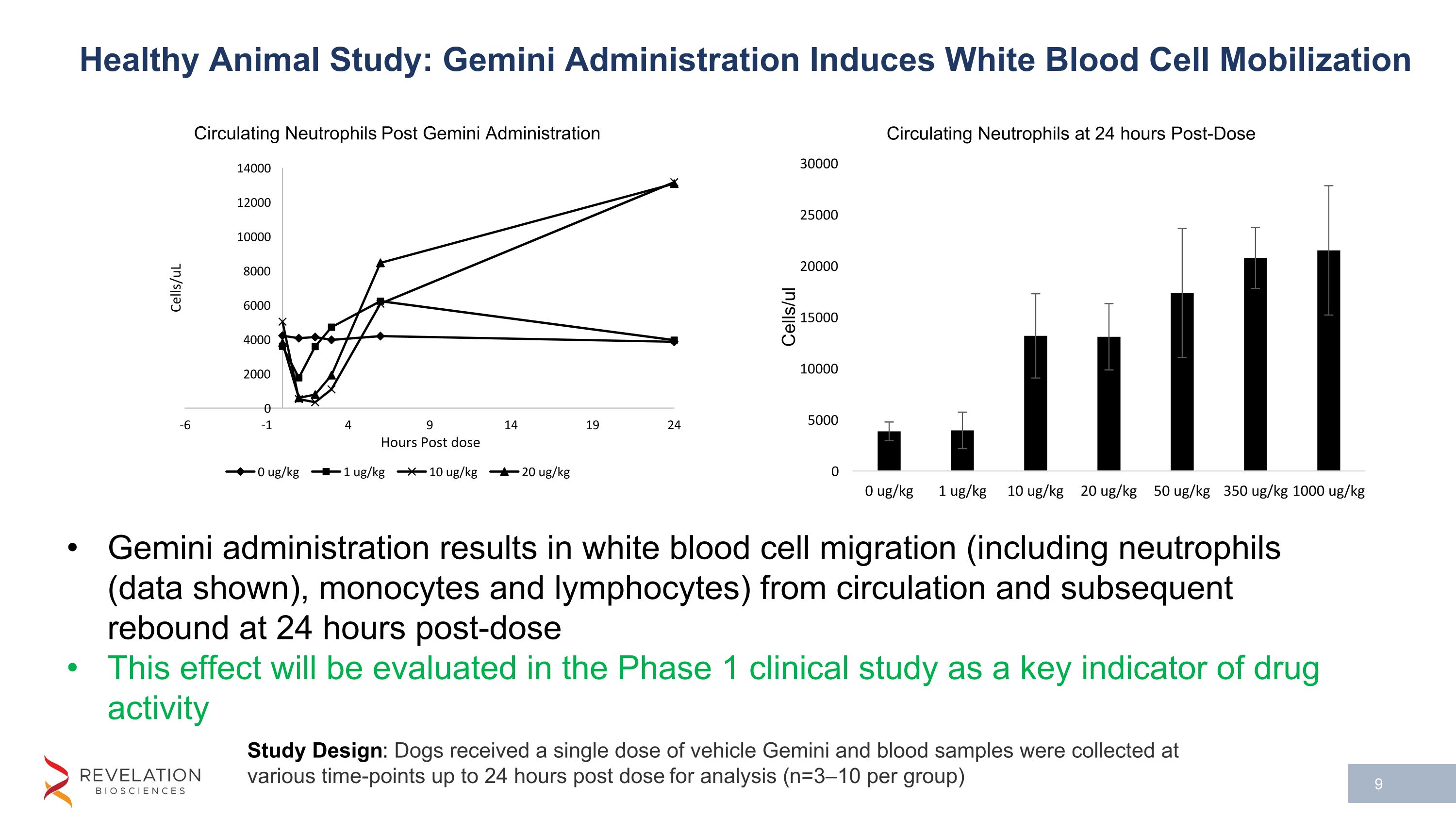
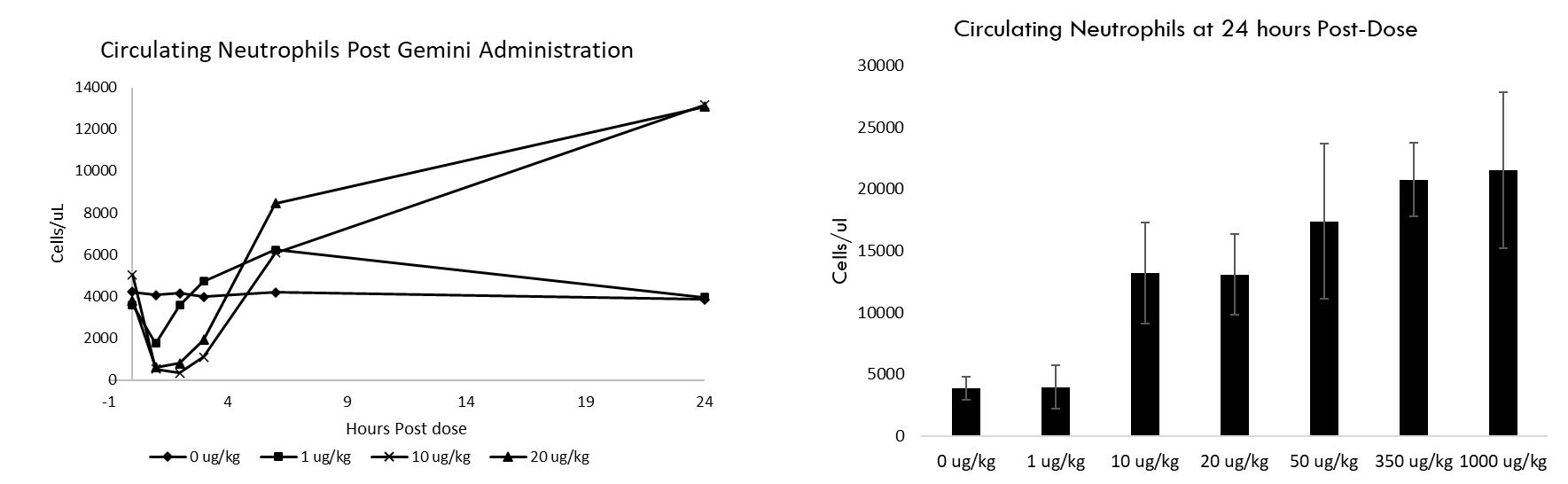
Study Design: Dogs received a single dose of vehicle Gemini and blood samples were collected at various time-points up to 24 hours post dose for analysis (n=3–10 per group) Healthy Animal Study: Gemini Administration Induces White Blood Cell Mobilization Gemini administration results in white blood cell migration (including neutrophils (data shown), monocytes and lymphocytes) from circulation and subsequent rebound at 24 hours post-dose This effect will be evaluated in the Phase 1 clinical study as a key indicator of drug activity
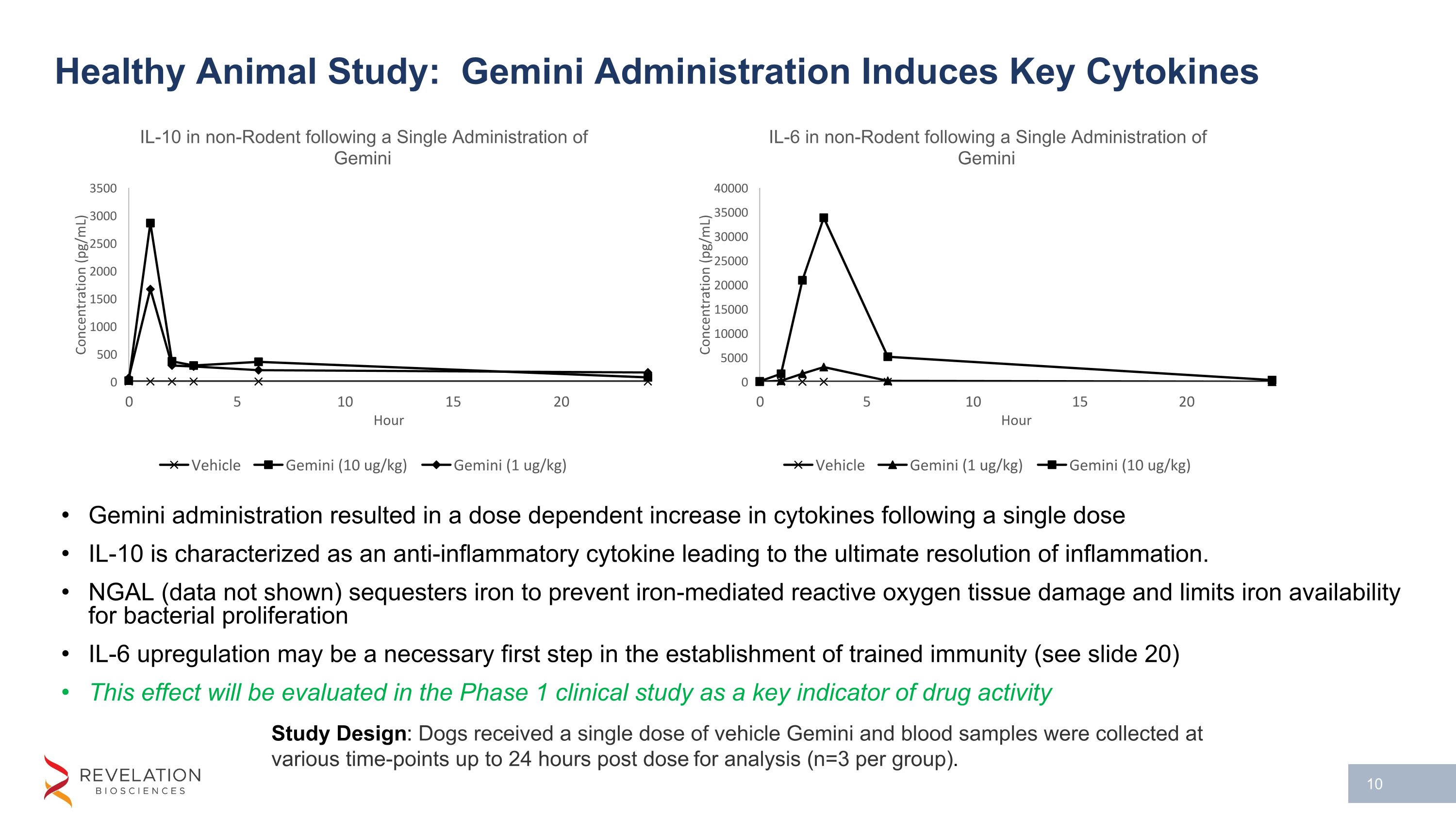
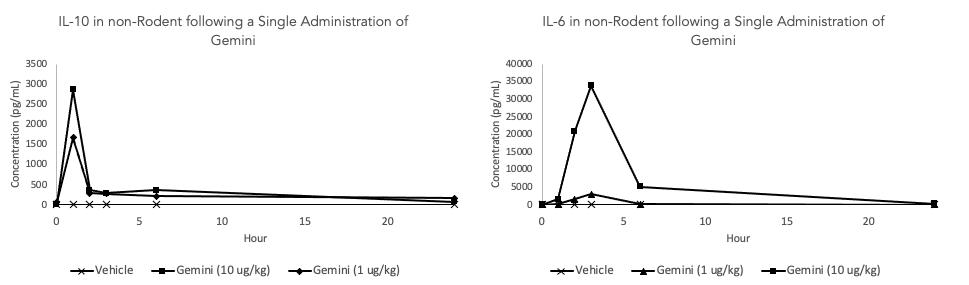
Healthy Animal Study: Gemini Administration Induces Key Cytokines Gemini administration resulted in a dose dependent increase in cytokines following a single dose IL-10 is characterized as an anti-inflammatory cytokine leading to the ultimate resolution of inflammation. NGAL (data not shown) sequesters iron to prevent iron-mediated reactive oxygen tissue damage and limits iron availability for bacterial proliferation IL-6 upregulation may be a necessary first step in the establishment of trained immunity (see slide 20) This effect will be evaluated in the Phase 1 clinical study as a key indicator of drug activity Study Design: Dogs received a single dose of vehicle Gemini and blood samples were collected at various time-points up to 24 hours post dose for analysis (n=3 per group).
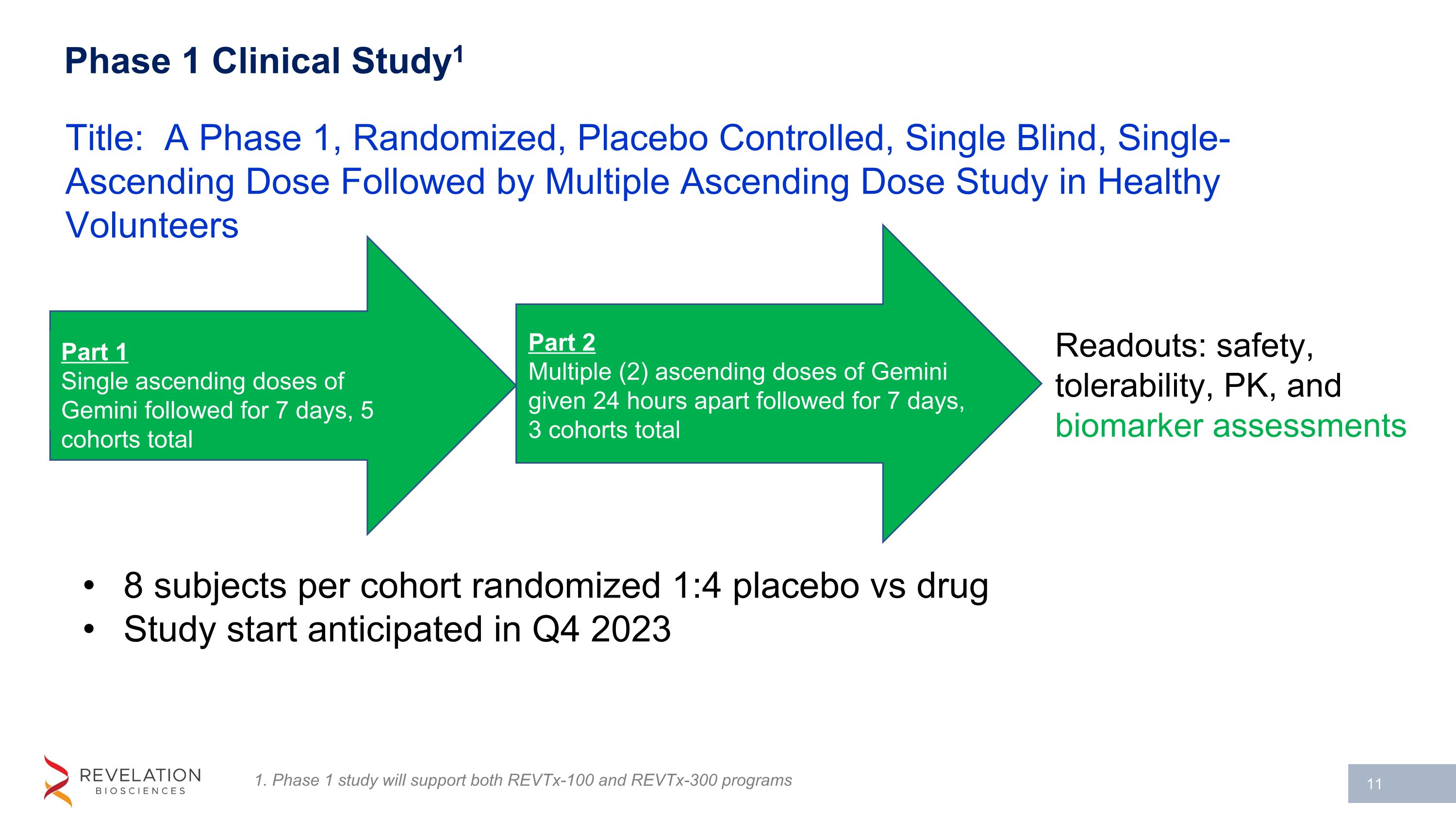
Phase 1 Clinical Study1 8 subjects per cohort randomized 1:4 placebo vs drug Study start anticipated in Q4 2023 Title: A Phase 1, Randomized, Placebo Controlled, Single Blind, Single-Ascending Dose Followed by Multiple Ascending Dose Study in Healthy Volunteers Part 1 Single ascending doses of Gemini followed for 7 days, 5 cohorts total Readouts: safety, tolerability, PK, and biomarker assessments 1. Phase 1 study will support both REVTx-100 and REVTx-300 programs Part 2 Multiple (2) ascending doses of Gemini given 24 hours apart followed for 7 days, 3 cohorts total
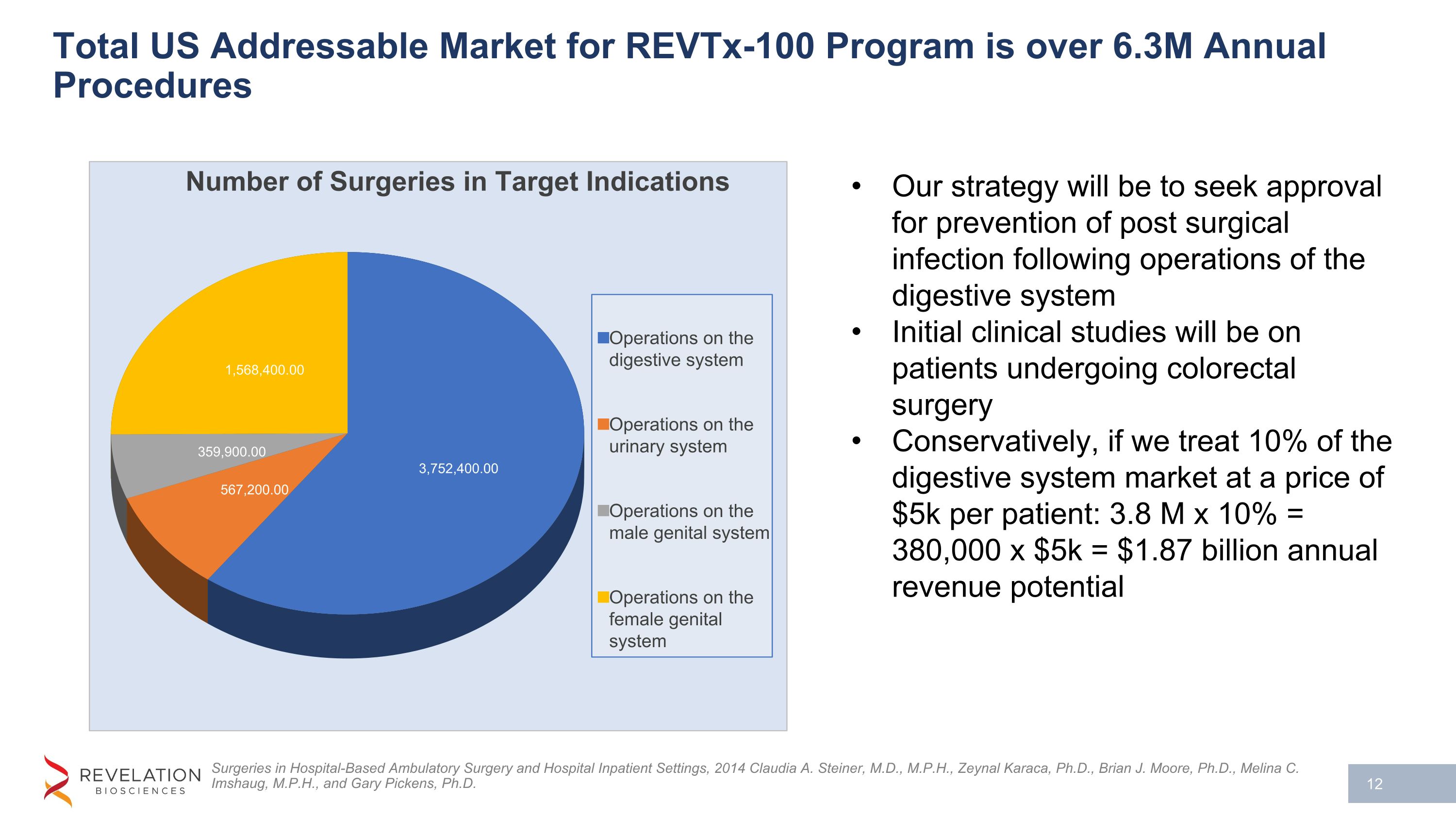
Total US Addressable Market for REVTx-100 Program is over 6.3M Annual Procedures Surgeries in Hospital-Based Ambulatory Surgery and Hospital Inpatient Settings, 2014 Claudia A. Steiner, M.D., M.P.H., Zeynal Karaca, Ph.D., Brian J. Moore, Ph.D., Melina C. Imshaug, M.P.H., and Gary Pickens, Ph.D. Our strategy will be to seek approval for prevention of post surgical infection following operations of the digestive system Initial clinical studies will be on patients undergoing colorectal surgery Conservatively, if we treat 10% of the digestive system market at a price of $5k per patient: 3.8 M x 10% = 380,000 x $5k = $1.87 billion annual revenue potential
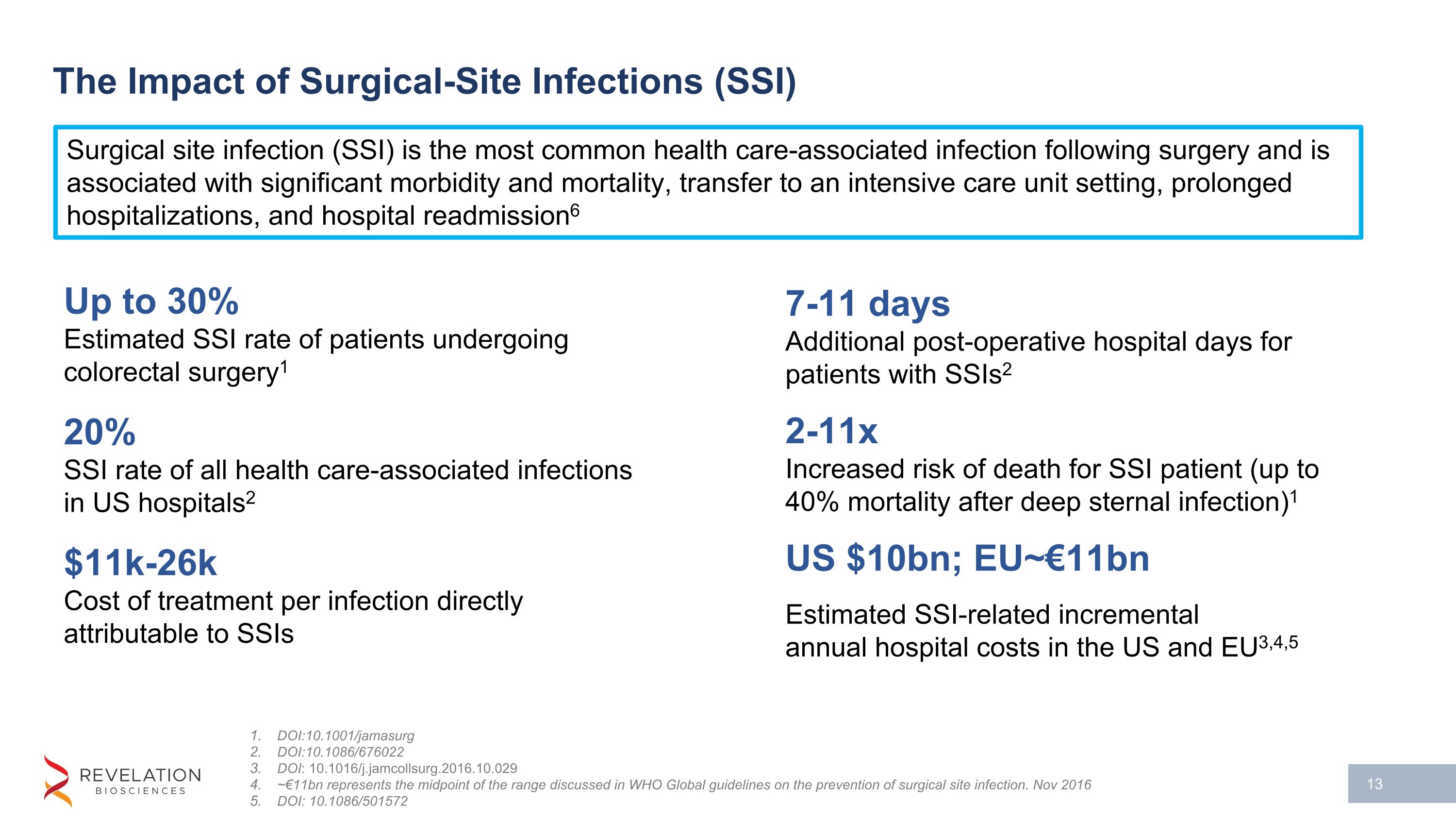
The Impact of Surgical-Site Infections (SSI) DOI:10.1001/jamasurg DOI:10.1086/676022 DOI: 10.1016/j.jamcollsurg.2016.10.029 ~€11bn represents the midpoint of the range discussed in WHO Global guidelines on the prevention of surgical site infection. Nov 2016 DOI: 10.1086/501572 Up to 30% Estimated SSI rate of patients undergoing colorectal surgery1 20% SSI rate of all health care-associated infections in US hospitals2 $11k-26k Cost of treatment per infection directly attributable to SSIs 7-11 days Additional post-operative hospital days for patients with SSIs2 2-11x Increased risk of death for SSI patient (up to 40% mortality after deep sternal infection)1 US $10bn; EU~€11bn Estimated SSI-related incremental�annual hospital costs in the US and EU3,4,5 Surgical site infection (SSI) is the most common health care-associated infection following surgery and is associated with significant morbidity and mortality, transfer to an intensive care unit setting, prolonged hospitalizations, and hospital readmission6

REVTx-300 Program Gemini For the Prevention of Acute Kidney Injury and Chronic Kidney Disease
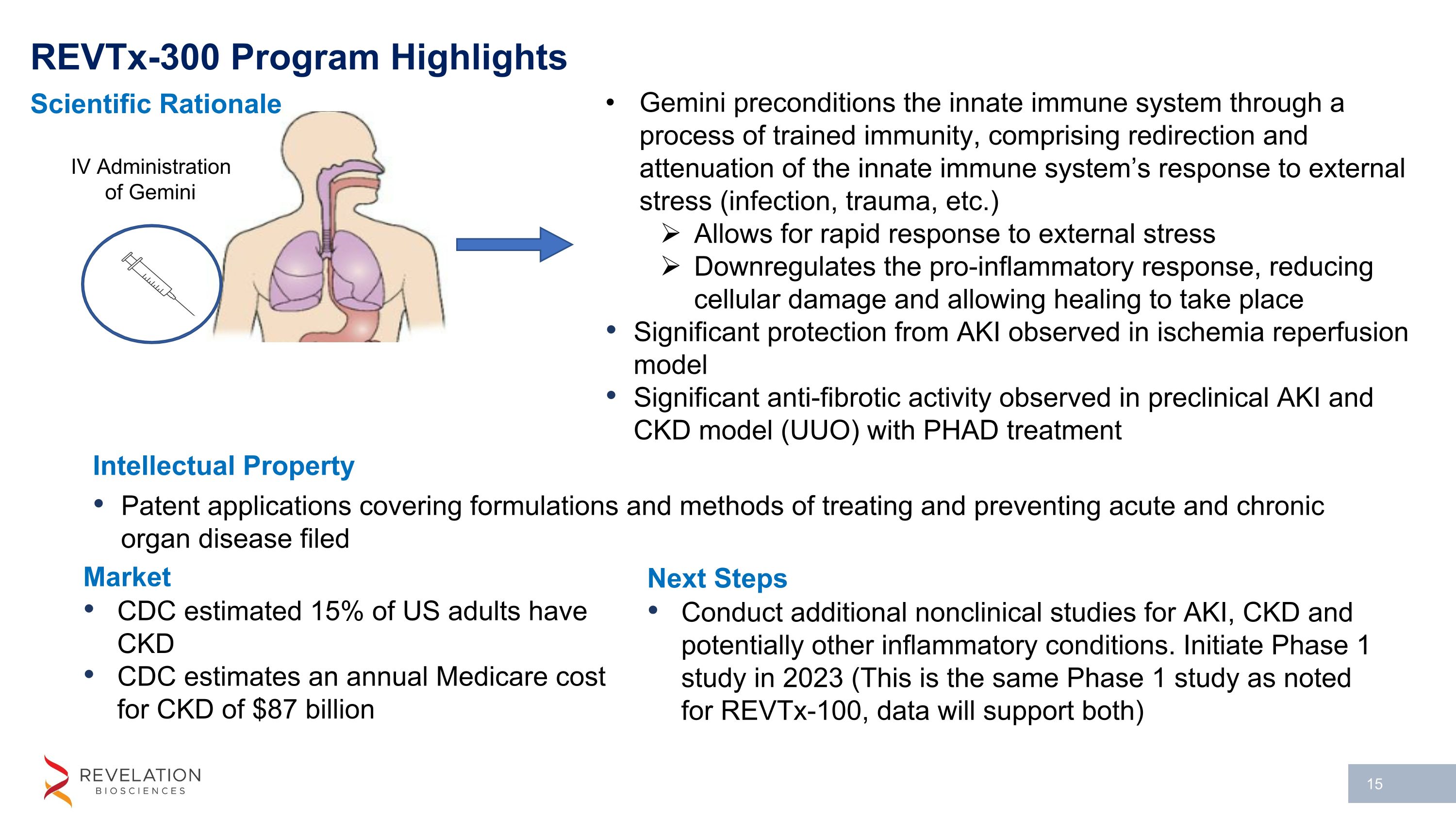
Gemini preconditions the innate immune system through a process of trained immunity, comprising redirection and attenuation of the innate immune system’s response to external stress (infection, trauma, etc.) Allows for rapid response to external stress Downregulates the pro-inflammatory response, reducing cellular damage and allowing healing to take place Significant protection from AKI observed in ischemia reperfusion model Significant anti-fibrotic activity observed in preclinical AKI and CKD model (UUO) with PHAD treatment IV Administration of Gemini REVTx-300 Program Highlights Intellectual Property Patent applications covering formulations and methods of treating and preventing acute and chronic organ disease filed Scientific Rationale Market CDC estimated 15% of US adults have CKD CDC estimates an annual Medicare cost for CKD of $87 billion Next Steps Conduct additional nonclinical studies for AKI, CKD and potentially other inflammatory conditions. Initiate Phase 1 study in 2023 (This is the same Phase 1 study as noted for REVTx-100, data will support both)
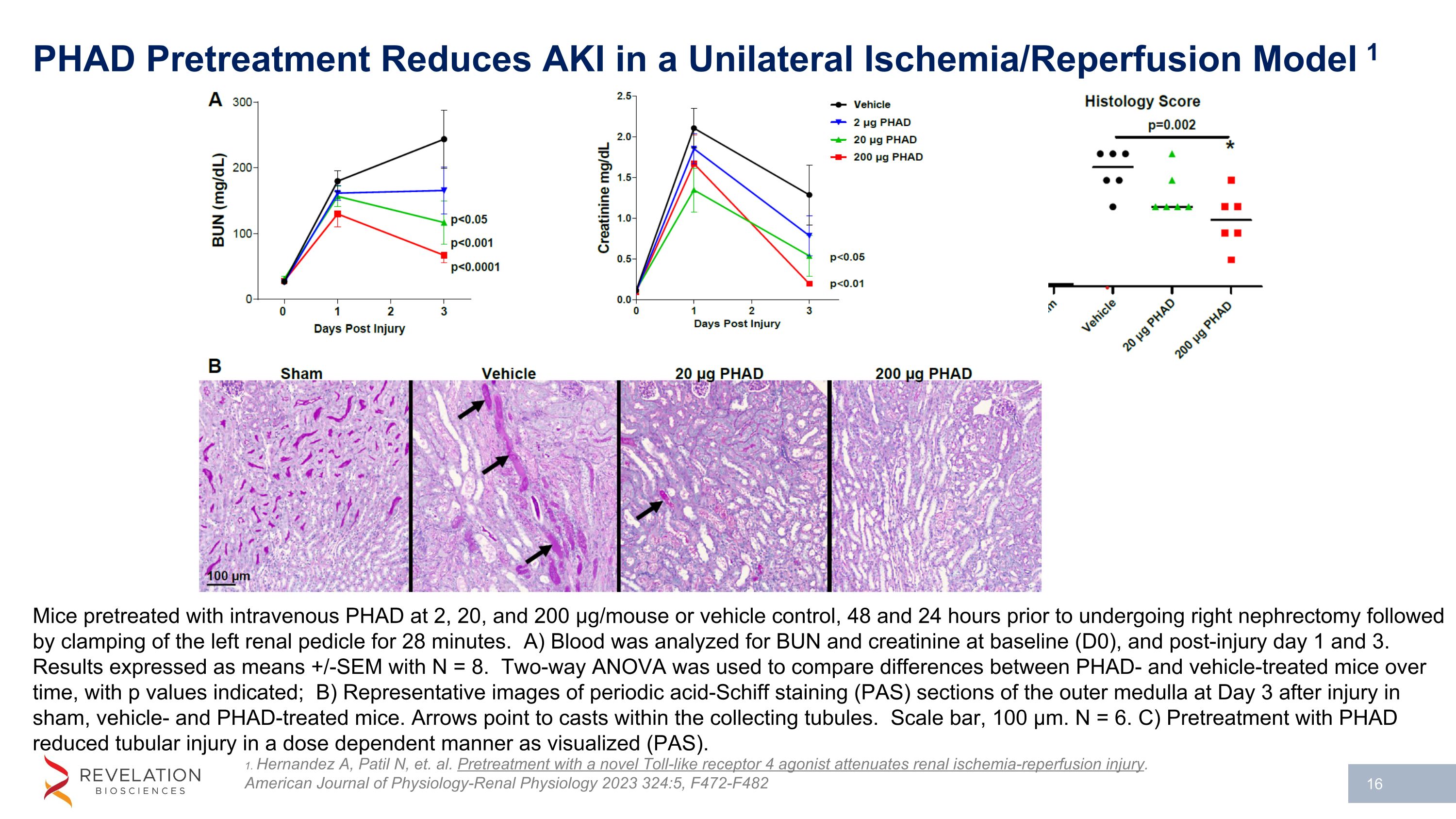
PHAD Pretreatment Reduces AKI in a Unilateral Ischemia/Reperfusion Model 1 1. Hernandez A, Patil N, et. al. Pretreatment with a novel Toll-like receptor 4 agonist attenuates renal ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology 2023 324:5, F472-F482 Mice pretreated with intravenous PHAD at 2, 20, and 200 µg/mouse or vehicle control, 48 and 24 hours prior to undergoing right nephrectomy followed by clamping of the left renal pedicle for 28 minutes. A) Blood was analyzed for BUN and creatinine at baseline (D0), and post-injury day 1 and 3. Results expressed as means +/-SEM with N = 8. Two-way ANOVA was used to compare differences between PHAD- and vehicle-treated mice over time, with p values indicated; B) Representative images of periodic acid-Schiff staining (PAS) sections of the outer medulla at Day 3 after injury in sham, vehicle- and PHAD-treated mice. Arrows point to casts within the collecting tubules. Scale bar, 100 µm. N = 6. C) Pretreatment with PHAD reduced tubular injury in a dose dependent manner as visualized (PAS).
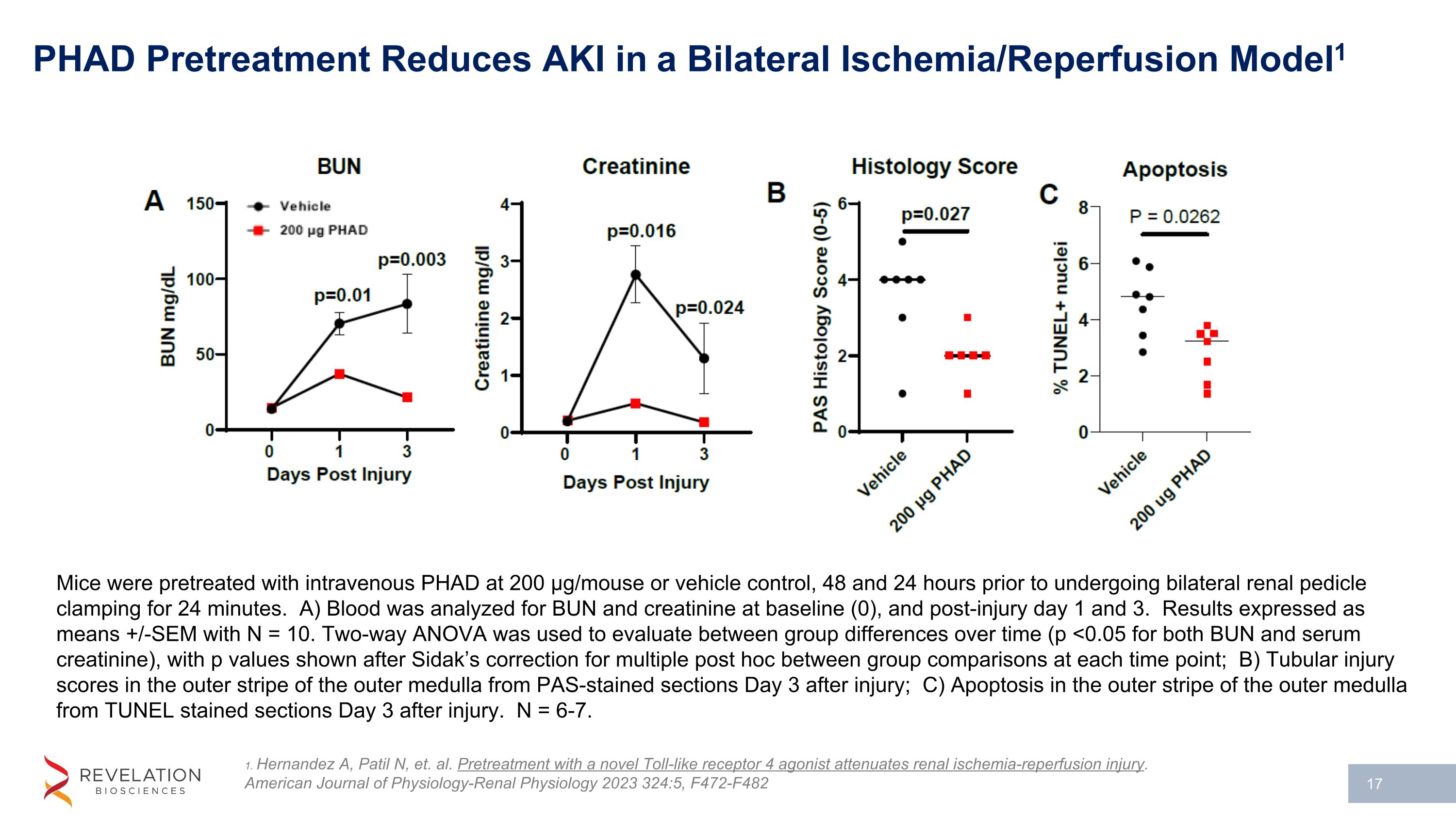
PHAD Pretreatment Reduces AKI in a Bilateral Ischemia/Reperfusion Model1 1. Hernandez A, Patil N, et. al. Pretreatment with a novel Toll-like receptor 4 agonist attenuates renal ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology 2023 324:5, F472-F482 Mice were pretreated with intravenous PHAD at 200 µg/mouse or vehicle control, 48 and 24 hours prior to undergoing bilateral renal pedicle clamping for 24 minutes. A) Blood was analyzed for BUN and creatinine at baseline (0), and post-injury day 1 and 3. Results expressed as means +/-SEM with N = 10. Two-way ANOVA was used to evaluate between group differences over time (p <0.05 for both BUN and serum creatinine), with p values shown after Sidak’s correction for multiple post hoc between group comparisons at each time point; B) Tubular injury scores in the outer stripe of the outer medulla from PAS-stained sections Day 3 after injury; C) Apoptosis in the outer stripe of the outer medulla from TUNEL stained sections Day 3 after injury. N = 6-7.
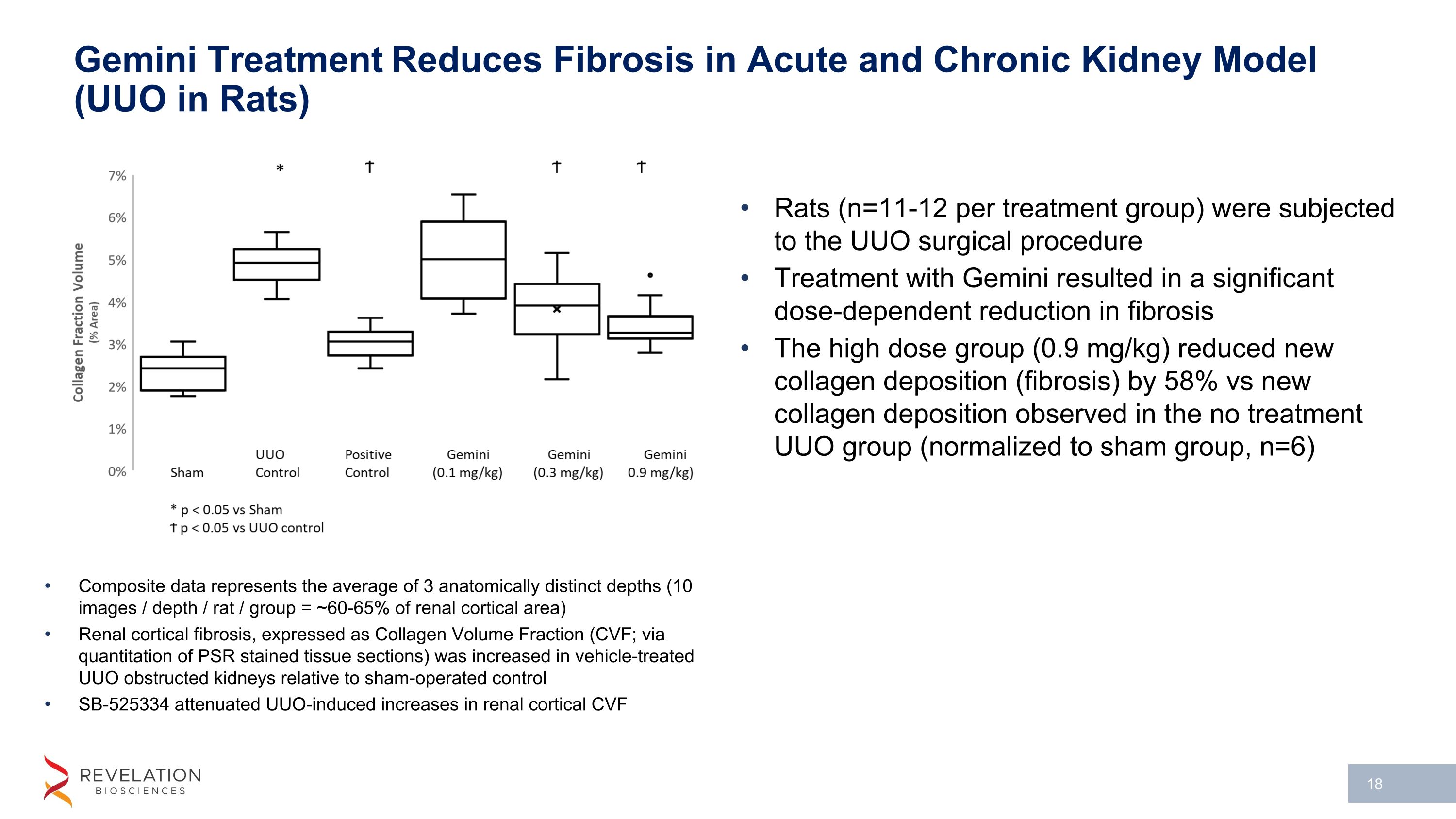
Gemini Treatment Reduces Fibrosis in Acute and Chronic Kidney Model (UUO in Rats) Composite data represents the average of 3 anatomically distinct depths (10 images / depth / rat / group = ~60-65% of renal cortical area) Renal cortical fibrosis, expressed as Collagen Volume Fraction (CVF; via quantitation of PSR stained tissue sections) was increased in vehicle-treated UUO obstructed kidneys relative to sham-operated control SB-525334 attenuated UUO-induced increases in renal cortical CVF Rats (n=11-12 per treatment group) were subjected to the UUO surgical procedure Treatment with Gemini resulted in a significant dose-dependent reduction in fibrosis The high dose group (0.9 mg/kg) reduced new collagen deposition (fibrosis) by 58% vs new collagen deposition observed in the no treatment UUO group (normalized to sham group, n=6)
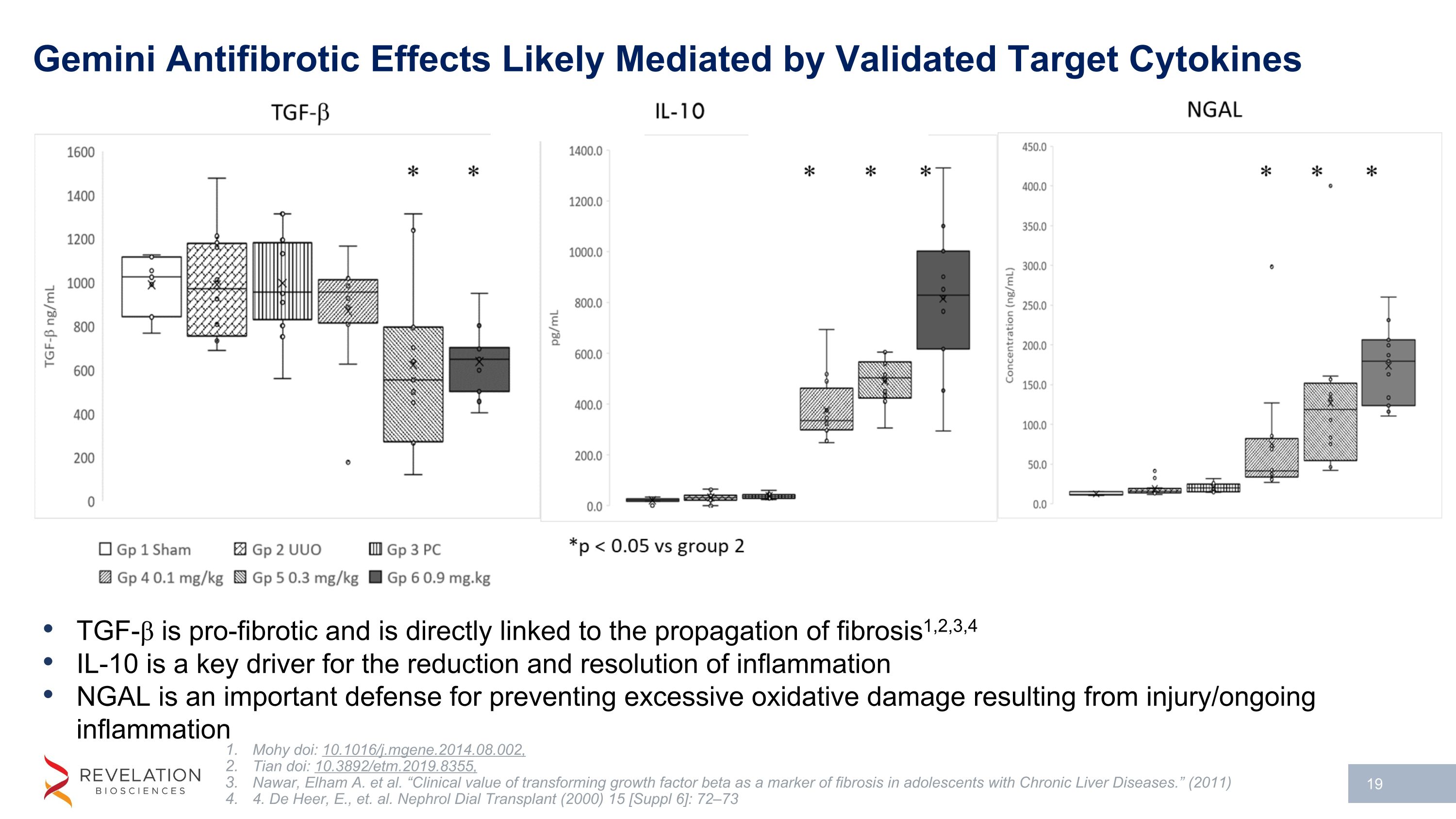
Gemini Antifibrotic Effects Likely Mediated by Validated Target Cytokines TGF-β is pro-fibrotic and is directly linked to the propagation of fibrosis1,2,3,4 IL-10 is a key driver for the reduction and resolution of inflammation NGAL is an important defense for preventing excessive oxidative damage resulting from injury/ongoing inflammation Mohy doi: 10.1016/j.mgene.2014.08.002, Tian doi: 10.3892/etm.2019.8355, Nawar, Elham A. et al. “Clinical value of transforming growth factor beta as a marker of fibrosis in adolescents with Chronic Liver Diseases.” (2011) 4. De Heer, E., et. al. Nephrol Dial Transplant (2000) 15 [Suppl 6]: 72–73
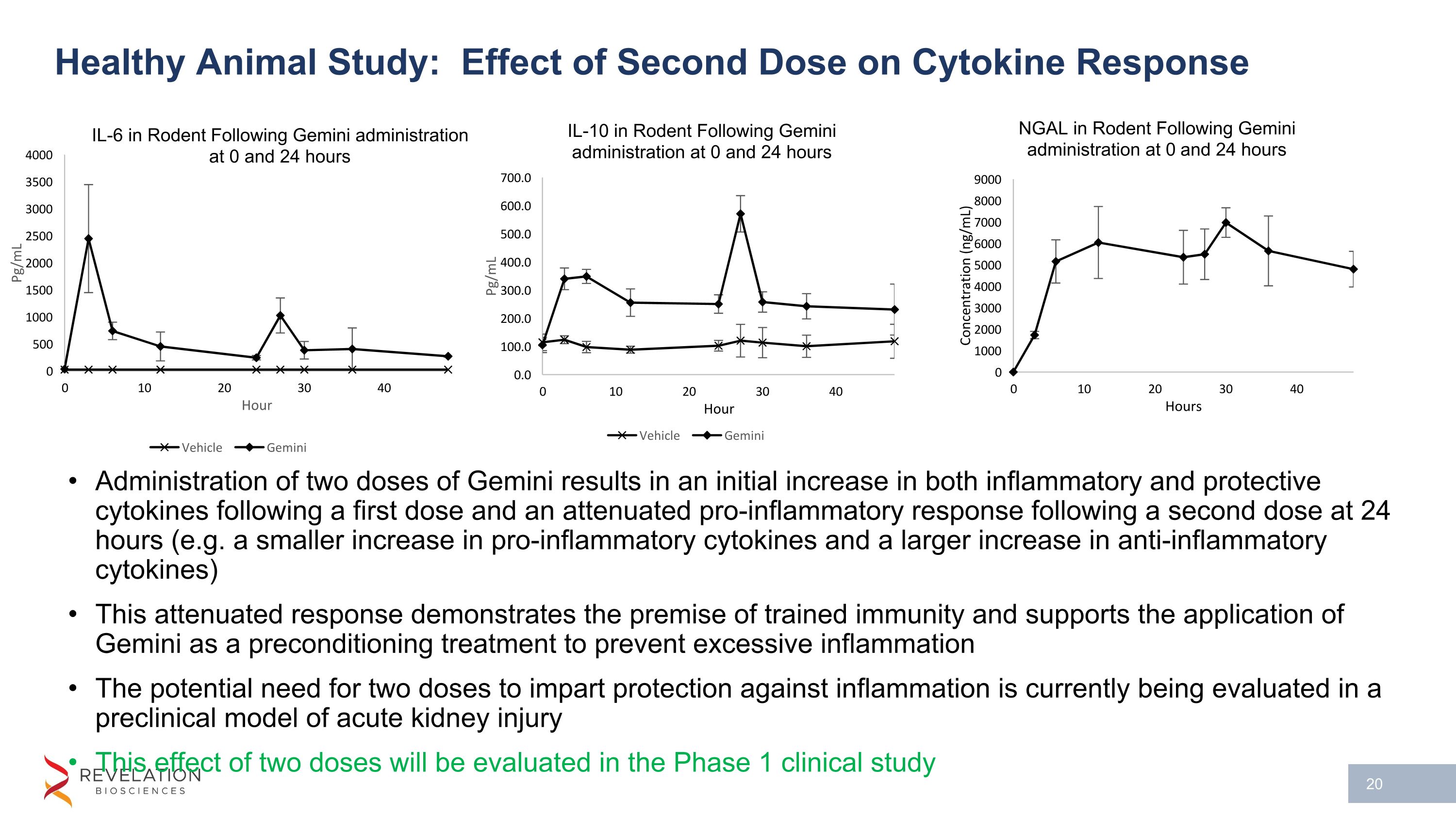
Healthy Animal Study: Effect of Second Dose on Cytokine Response Administration of two doses of Gemini results in an initial increase in both inflammatory and protective cytokines following a first dose and an attenuated pro-inflammatory response following a second dose at 24 hours (e.g. a smaller increase in pro-inflammatory cytokines and a larger increase in anti-inflammatory cytokines) This attenuated response demonstrates the premise of trained immunity and supports the application of Gemini as a preconditioning treatment to prevent excessive inflammation The potential need for two doses to impart protection against inflammation is currently being evaluated in a preclinical model of acute kidney injury This effect of two doses will be evaluated in the Phase 1 clinical study
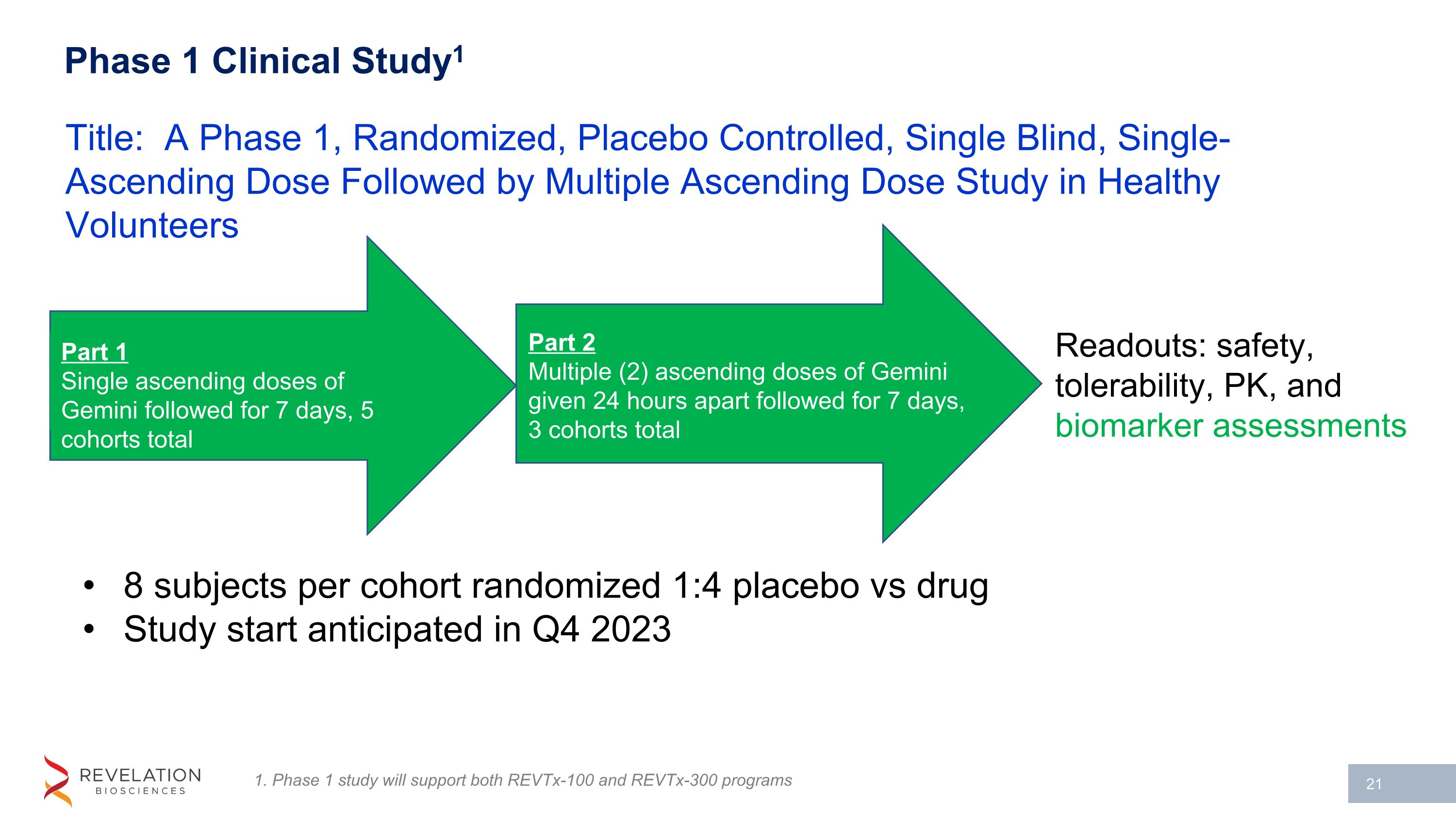
Phase 1 Clinical Study1 8 subjects per cohort randomized 1:4 placebo vs drug Study start anticipated in Q4 2023 Title: A Phase 1, Randomized, Placebo Controlled, Single Blind, Single-Ascending Dose Followed by Multiple Ascending Dose Study in Healthy Volunteers Part 1 Single ascending doses of Gemini followed for 7 days, 5 cohorts total Readouts: safety, tolerability, PK, and biomarker assessments 1. Phase 1 study will support both REVTx-100 and REVTx-300 programs Part 2 Multiple (2) ascending doses of Gemini given 24 hours apart followed for 7 days, 3 cohorts total
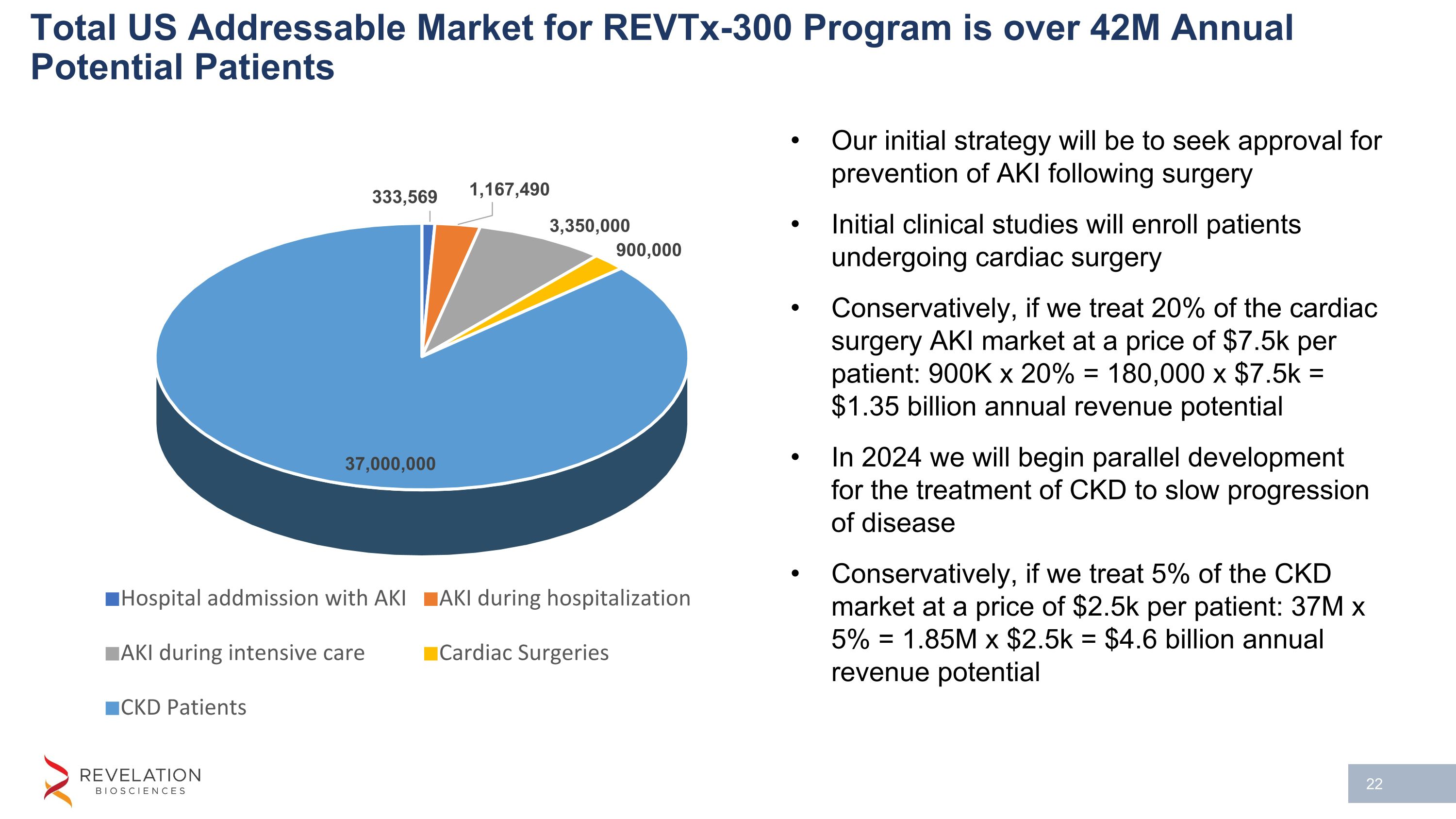
Total US Addressable Market for REVTx-300 Program is over 42M Annual Potential Patients Our initial strategy will be to seek approval for prevention of AKI following surgery Initial clinical studies will enroll patients undergoing cardiac surgery Conservatively, if we treat 20% of the cardiac surgery AKI market at a price of $7.5k per patient: 900K x 20% = 180,000 x $7.5k = $1.35 billion annual revenue potential In 2024 we will begin parallel development for the treatment of CKD to slow progression of disease Conservatively, if we treat 5% of the CKD market at a price of $2.5k per patient: 37M x 5% = 1.85M x $2.5k = $4.6 billion annual revenue potential
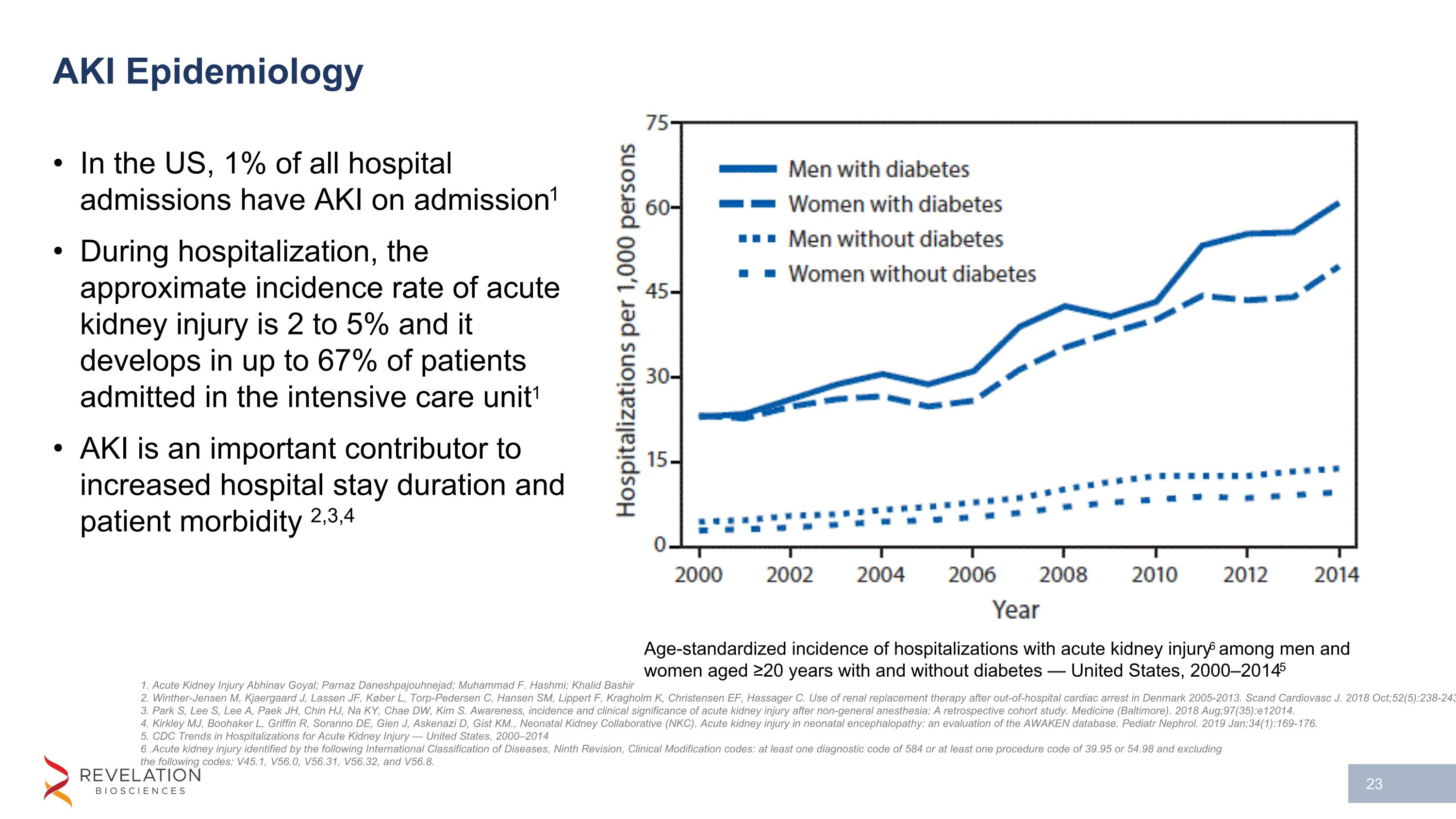
AKI Epidemiology In the US, 1% of all hospital admissions have AKI on admission1 During hospitalization, the approximate incidence rate of acute kidney injury is 2 to 5% and it develops in up to 67% of patients admitted in the intensive care unit1 AKI is an important contributor to increased hospital stay duration and patient morbidity 2,3,4 1. Acute Kidney Injury Abhinav Goyal; Parnaz Daneshpajouhnejad; Muhammad F. Hashmi; Khalid Bashir 2. Winther-Jensen M, Kjaergaard J, Lassen JF, Køber L, Torp-Pedersen C, Hansen SM, Lippert F, Kragholm K, Christensen EF, Hassager C. Use of renal replacement therapy after out-of-hospital cardiac arrest in Denmark 2005-2013. Scand Cardiovasc J. 2018 Oct;52(5):238-243 3. Park S, Lee S, Lee A, Paek JH, Chin HJ, Na KY, Chae DW, Kim S. Awareness, incidence and clinical significance of acute kidney injury after non-general anesthesia: A retrospective cohort study. Medicine (Baltimore). 2018 Aug;97(35):e12014. 4. Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, Gist KM., Neonatal Kidney Collaborative (NKC). Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. 2019 Jan;34(1):169-176. 5. CDC Trends in Hospitalizations for Acute Kidney Injury — United States, 2000–2014 6 .Acute kidney injury identified by the following International Classification of Diseases, Ninth Revision, Clinical Modification codes: at least one diagnostic code of 584 or at least one procedure code of 39.95 or 54.98 and excluding the following codes: V45.1, V56.0, V56.31, V56.32, and V56.8. Age-standardized incidence of hospitalizations with acute kidney injury6 among men and women aged ≥20 years with and without diabetes — United States, 2000–20145
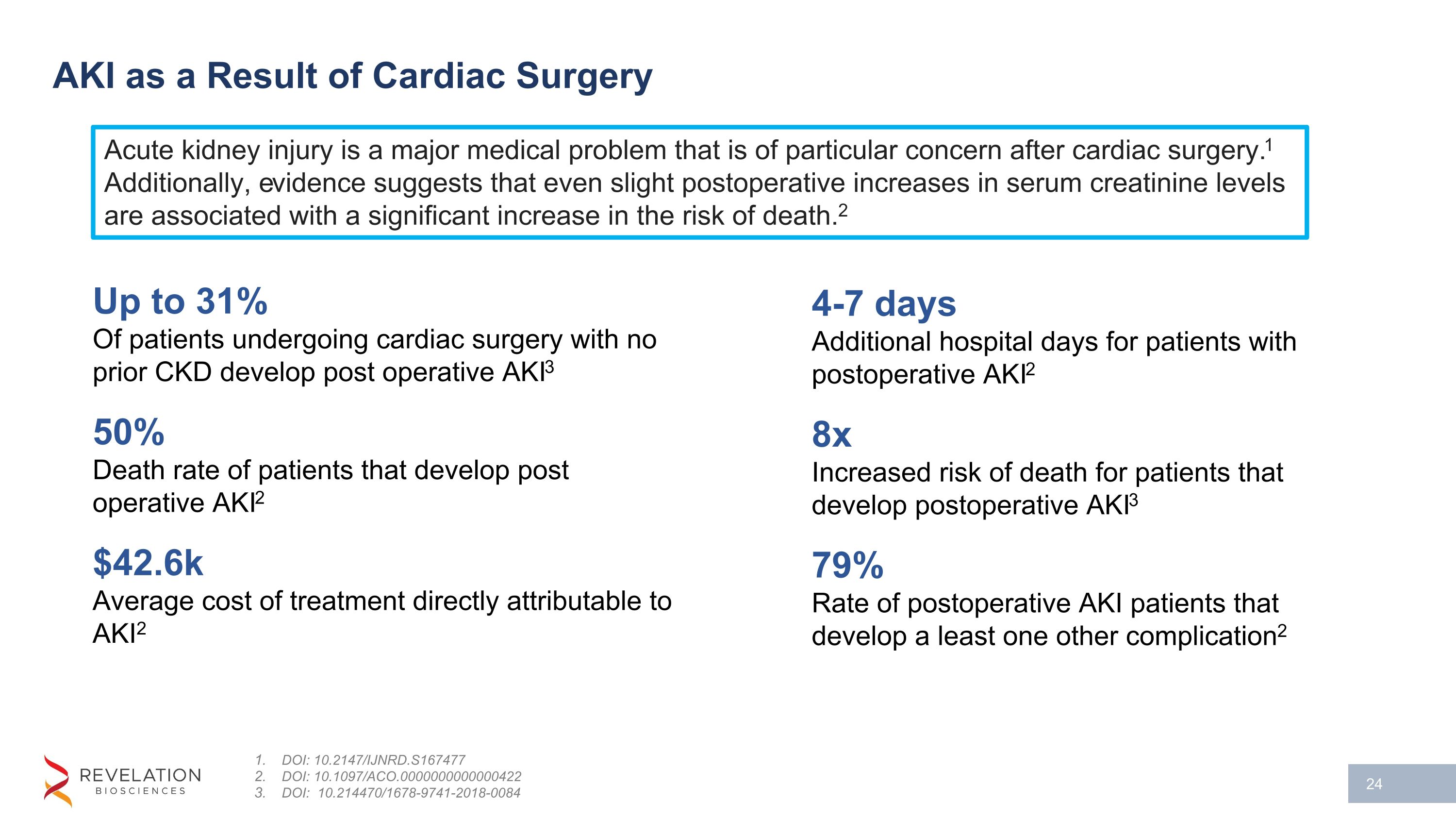
AKI as a Result of Cardiac Surgery DOI: 10.2147/IJNRD.S167477 DOI: 10.1097/ACO.0000000000000422 DOI: 10.214470/1678-9741-2018-0084 Up to 31% Of patients undergoing cardiac surgery with no prior CKD develop post operative AKI3 50% Death rate of patients that develop post operative AKI2 $42.6k Average cost of treatment directly attributable to AKI2 4-7 days Additional hospital days for patients with postoperative AKI2 8x Increased risk of death for patients that develop postoperative AKI3 79% Rate of postoperative AKI patients that develop a least one other complication2 Acute kidney injury is a major medical problem that is of particular concern after cardiac surgery.1 Additionally, evidence suggests that even slight postoperative increases in serum creatinine levels are associated with a significant increase in the risk of death.2

Financial Overview
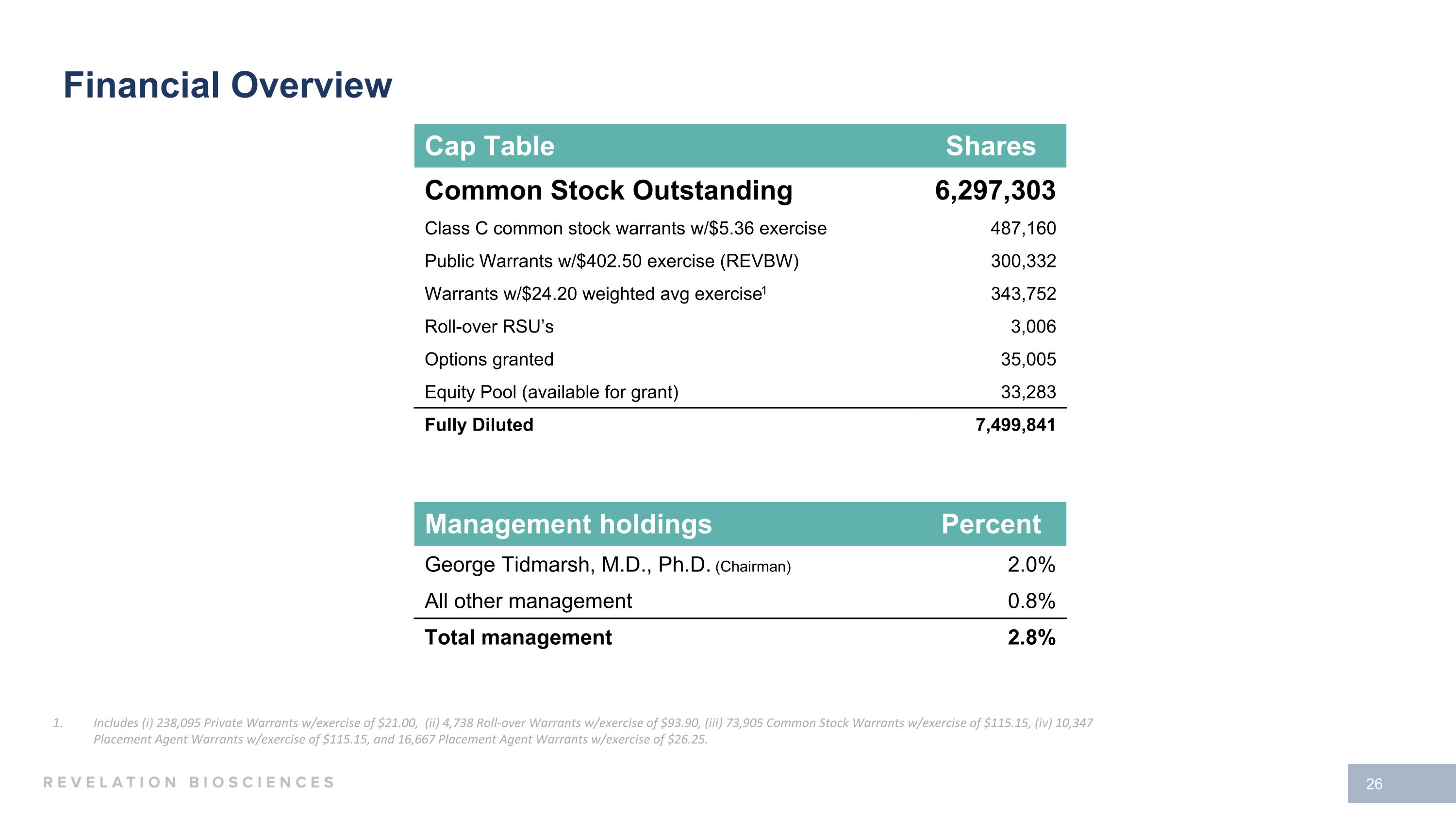
Management holdings Percent George Tidmarsh, M.D., Ph.D. (Chairman) 2.0% All other management 0.8% Total management 2.8% Cap Table Shares Common Stock Outstanding 6,297,303 Class C common stock warrants w/$5.36 exercise 487,160 Public Warrants w/$402.50 exercise (REVBW) 300,332 Warrants w/$24.20 weighted avg exercise1 343,752 Roll-over RSU’s 3,006 Options granted 35,005 Equity Pool (available for grant) 33,283 Fully Diluted 7,499,841 Financial Overview Includes (i) 238,095 Private Warrants w/exercise of $21.00, (ii) 4,738 Roll-over Warrants w/exercise of $93.90, (iii) 73,905 Common Stock Warrants w/exercise of $115.15, (iv) 10,347 Placement Agent Warrants w/exercise of $115.15, and 16,667 Placement Agent Warrants w/exercise of $26.25.

For more information please visit www.revbiosciences.com Thank you!

Gemini Induces Pharmacologic Activity and Related Physiologic Changes in Multiple Preclinical Studies
- Key Biomarker Activity Confirmed for Evaluation in Upcoming Phase 1 Study -
San Diego, CA – October 12, 2023 – Revelation Biosciences Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), announced today that data from recent preclinical studies of the Company’s Gemini formulation demonstrated its therapeutic potential as a preventative therapy across multiple indications including infection and acute kidney injury. Data, discussed below, highlights the significant pharmacologic activity following administration of Gemini in healthy animals. Additional nonclinical biomarker analyses are ongoing in preparation for a planned Phase 1 study to be initiated later this year. Further, Revelation intends to publish the full results of these nonclinical studies in conjunction with the upcoming data generated in the Phase 1 healthy volunteer clinical study.
Upregulation of key biomarkers were observed in both rodent and non-rodent healthy animal preclinical studies. This included a dose dependent migration of white blood cells (neutrophils, monocytes, and lymphocytes) from the blood stream post dose with a subsequent rebound at 24 hours (data for neutrophils in non-rodent model shown in the figure below, n=3-10 per dose level examined). White blood cell mobilization and activation plays an important role in the prevention and resolution of bacterial infection.
In addition to having an effect on white blood cells, Gemini produced a dose dependent increase in multiple cytokines of interest including interleukin-10 (IL-10), neutrophil gelatinase associated lipocalin (NGAL), and interleukin-6 (IL-6). Previously on February 7, 2023 the Company announced results from a preclinical study where administration of Gemini showed the protective effect of IL-10 and NGAL upregulation on the formation of kidney scar tissue in a validated preclinical model of acute and chronic kidney injury.
IL-10 is characterized as an anti-inflammatory cytokine leading to the ultimate resolution of inflammation. NGAL (and hepcidin, data previously shown) sequester iron to prevent iron-mediated reactive oxygen tissue damage associated with inflammation and bacterial propagation by reducing available iron stores necessary for bacterial growth. Initial upregulation of IL-6 is an important first step to establish trained immunity. This phenomenon is demonstrated after the administration of two doses of Gemini which resulted in an initial increase in both inflammatory (IL-6) and protective (IL-10) cytokines following a first dose. Then an attenuated response in IL-6 following a second dose at 24 hours (e.g. a smaller increase in inflammatory cytokines and a larger increase in protective cytokines). This attenuated response in IL-6 demonstrates the premise of trained immunity and supports the application of Gemini as a preconditioning treatment to prevent excessive inflammation. The potential need for two doses to impart protection against inflammation is currently being evaluated in a preclinical model of acute kidney injury.
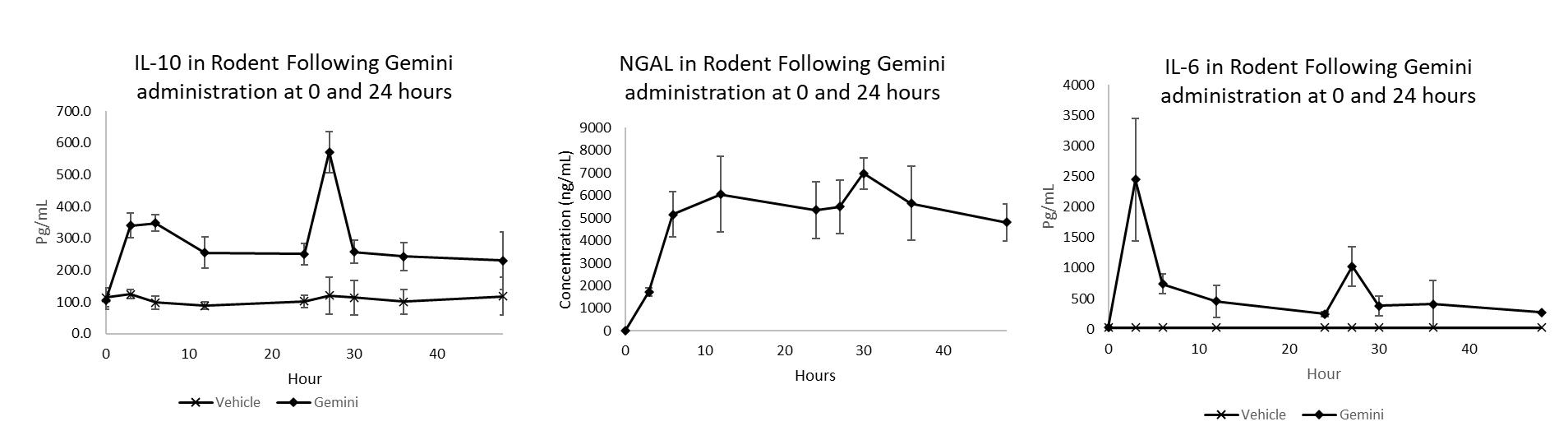
“We are delighted with the robust response observed following Gemini administration and look forward to demonstrating a comparable response in healthy human volunteers,” said James Rolke, Chief Executive Officer of Revelation. “A strong response in the Phase 1 study will be a very good indication of the potential of Gemini to prevent and treat diseases such as hospital acquired infection, acute kidney injury, chronic kidney disease and myocarditis in patients who currently have limited options.”
About Gemini
Gemini is a proprietary formulation for systemic administration of phosphorylated hexaacyl disaccharide (PHAD®) and is being developed as a potential therapy for prevention and treatment of hospital acquired infection (REVTx-100 program) and as a potential treatment for acute and chronic organ disease including prevention of acute kidney injury (REVTx-300 program), myocarditis, and chronic kidney disease (CKD). Revelation believes Gemini works through the process of trained immunity, comprising redirection and attenuation of the innate immune system’s response to external stress (infection, trauma, etc.). Revelation has conducted multiple preclinical studies demonstrating the therapeutic potential of Gemini in the target indications and plans to initiate clinical studies in 2023.
About Revelation Biosciences Inc.
Revelation Biosciences, Inc. is a life sciences company focused on harnessing the power of trained immunity for the prevention and treatment of disease using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including REVTx-100 as a prevention for hospital acquired infection and REVTx-300 as a prevention for acute kidney injury.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the clinical utility of an increase in intranasal cytokine levels as a biomarker of viral infections; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the potential impact that COVID19 may have on Revelation’s suppliers, vendors, regulatory agencies, employees and the global economy as a whole; the ability of Revelation to maintain the listing of its securities on NASDAQ; investor sentiment relating to SPAC related going public transactions; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
Company Contacts
Sandra Vedrick
Vice President, Investor Relations & Human Resources
Revelation Biosciences, Inc.
Email: svedrick@revbiosciences.com
and
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences, Inc.
Email: czygmont@revbiosciences.com
v3.23.3
Document And Entity Information
|
Oct. 12, 2023 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 12, 2023
|
| Entity Registrant Name |
REVELATION BIOSCIENCES, INC.
|
| Entity Central Index Key |
0001810560
|
| Entity Emerging Growth Company |
true
|
| Securities Act File Number |
001-39603
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
84-3898466
|
| Entity Address, Address Line One |
4660 La Jolla Village Drive
|
| Entity Address, Address Line Two |
Suite 100
|
| Entity Address, City or Town |
San Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92122
|
| City Area Code |
(650)
|
| Local Phone Number |
800-3717
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Redeemable Warrants, Each Exercisable For One By Thirty Fifth Share Of Common Stock At Exercise Price Of $402.50 Per Share [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Redeemable warrants, each exercisable for a 1/35th share of common stock at an exercise price of $402.50 per share
|
| Trading Symbol |
REVBW
|
| Security Exchange Name |
NASDAQ
|
| Common Stock, Par Value $0.001 Per Share [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common stock, par value $0.001 per share
|
| Trading Symbol |
REVB
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=revb_RedeemableWarrantsEachExercisableForOneByThirtyFifthShareOfCommonStockAtExercisePriceOf40250PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=revb_CommonStockParValue0001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Revelation Biosciences (NASDAQ:REVB)
過去 株価チャート
から 11 2024 まで 12 2024

Revelation Biosciences (NASDAQ:REVB)
過去 株価チャート
から 12 2023 まで 12 2024
