Based on an encouraging discussion in the End
of Phase 1B/2 Meeting with FDA the
Company plans to:
Proceed with a pivotal, adaptive Phase 3
clinical trial (the "MIRACLE" trial) designed for possible
accelerated approval of Annamycin in combination with cytarabine
for the treatment of relapsed or refractory AML;
Run such future studies globally and in the US
above the lifetime maximum allowable anthracycline dose;
and
Provide the FDA with additional data
supporting the selection of the optimal dosing level via the
adaptive design in the MIRACLE trial
HOUSTON, Aug. 1, 2024
/PRNewswire/ -- Moleculin Biotech, Inc., (Nasdaq: MBRX)
("Moleculin" or the "Company"), a clinical stage pharmaceutical
company with a broad portfolio of drug candidates targeting
hard-to-treat tumors and viruses, today announced the positive
discussion in and outcome of its End of Phase 1B/2 (EOP1B/2) meeting with the US Food and Drug
Administration (FDA) supporting the advancement of Annamycin in
combination with Cytarabine (also known as "Ara-C" and for which
the combination of Annamycin and Ara-C is referred to as "AnnAraC")
to a Phase 3 pivotal trial for the treatment of AML patients who
are refractory to or relapsed after induction therapy (R/R AML).
This Phase 3 "MIRACLE" trial (derived from Moleculin
R/R AML AnnAraC Clinical Evaluation)
will be a global trial, including sites in the US.
"We thank the FDA's Divisions of Hematologic Malignancies I and
Cardiology and Nephrology, as well as related divisions, for a very
constructive EOP1B/2 meeting and for their valuable feedback. Armed
with this, we are now able to finalize plans for a pivotal approval
pathway in AML," commented Walter
Klemp, Chairman and Chief Executive Officer of Moleculin.
"Importantly, consistent with the FDA's recommendations, the
adaptive Phase 3 trial will rely solely on CR (complete remission)
at day 30 as the primary endpoint versus placebo, a standard we are
confident Annamycin will meet and that provides an opportunity for
accelerated approval."
Mr. Klemp continued: "We now also have additional confidence
that our planned pivotal trial should be able to generate data
supportive of a true value inflection point for shareholders in a
timely manner. We plan to utilize a double-blind,
placebo-controlled design, where the control arm is high dose
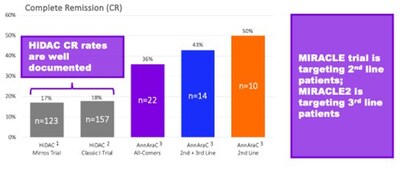
cytarabine (HiDAC) plus placebo. There is considerable historical
data on the use of HiDAC. You can see in this graphic that,
compared to this historical data, AnnAraC has already demonstrated
more than double the CR rate. The MIRACLE trial will initially
focus on 2nd line treatment for R/R AML subjects and
then follow-up with treatment for 3rd line R/R AML."
"This approach should also allow us to use this trial for
approval in Europe. Based on our
discussions with the FDA, we intend to amend our current
investigational new drug application or IND to allow dosing above
the lifetime maximum allowable dose (LTMAD) for currently
prescribed anthracyclines in this trial in the US."
The Company obtained valuable input from the FDA and having
resolved a number of key issues, believes that it has significantly
de-risked the pathway to approval. The MIRACLE study, subject to
appropriate future filings with and potential additional feedback
from the FDA and their foreign equivalents, is expected to
initially utilize an adaptive design whereby the first 75 patients
will be randomized to receive HiDAC combined with either placebo,
190 mg/m2 of Annamycin, or 230 mg/m2 of
Annamycin. At that point, the trial will be unblinded to select the
Optimum Dose for Annamycin. For the second half of the trial,
approximately 120 additional patients will be randomized to receive
either HiDAC plus placebo or HiDAC plus the Optimum Dose of
Annamycin. The selection of the Optimum Dose will be based not only
on the absence of dose limiting toxicities but also on the overall
balance of safety, pharmacokinetics and efficacy, consistent with
the FDA's new Project Optimus initiative.
Mr. Klemp concluded: "The FDA also wants to see the durability
of response (DoR) and overall survival (OS) as secondary endpoints,
as well as data for patients beyond 2nd line, which is
why our plan includes a follow-on MIRACLE2 trial in 3rd
line patients starting once the optimum dose is established in the
MIRACLE trial. From a Company perspective, we believe this approach
is the best of all worlds. We are not only making the leap into
being a Phase 3 company, but our planned approval is also based on
a primary endpoint comparing to a control that we are optimistic we
can beat with the ability to report unblinded progress after just
75 patients. We are truly excited to launch the MIRACLE trial."
Moleculin Planned Significant Milestones
The Company has established plans for the following
milestones:
- 2H 2024 – Begin contracting with MIRACLE trial sites
- Q1 2025 – First subject treated in MIRACLE trial
- Mid 2026 – Interim data (n=75) unblinded and Optimum Dose
set for MIRACLE trial
- 2026 – Begin enrollment of 3rd line subjects in
MIRACLE2
- 2027 – Enrollment ends in 2nd line subjects
- 2028 – Final Data for 2nd line subjects in
MIRACLE
- 2H 2028 – Begin submission of a new drug application (NDA) the
treatment of R/R AML for accelerated approval on primary endpoint
of CR from MIRACLE
Annamycin currently has Fast Track Status and Orphan Drug
Designation from the US Food and Drug Administration for the
treatment of relapsed or refractory acute myeloid leukemia, in
addition to Orphan Drug Designation for the treatment of soft
tissue sarcoma. Furthermore, Annamycin has Orphan Drug Designation
for the treatment of relapsed or refractory acute myeloid leukemia
from the European Medicines Agency (EMA). For more information
about the ongoing MB-106 Phase 1B/2
trial, visit clinicaltrialsregister.eu and reference EudraCT
2020-005493-10 or clinicaltrials.gov and reference NCT05319587.
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical
company with a growing pipeline, including Phase 2 clinical
programs, for hard-to-treat tumors and viruses. The Company's lead
program, Annamycin is a next-generation anthracycline designed to
avoid multidrug resistance mechanisms and to eliminate the
cardiotoxicity common with currently prescribed anthracyclines.
Annamycin is currently in development for the treatment of acute
myeloid leukemia (AML) and soft tissue sarcoma (STS) lung
metastases. All interim and preliminary data related to its active
clinical trials are subject to change until a clinical study report
is published.
Additionally, the Company is developing WP1066, an
Immune/Transcription Modulator capable of inhibiting p-STAT3 and
other oncogenic transcription factors while also stimulating a
natural immune response, targeting brain tumors, pancreatic and
other cancers. Moleculin is also engaged in the development of a
portfolio of antimetabolites, including WP1122 for the potential
treatment of viruses, as well as certain cancer indications.
For more information about the Company, please
visit www.moleculin.com and connect on X, LinkedIn and
Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking
statements within the meaning of Section 27A of the Securities Act
of 1933, Section 21E of the Securities Exchange Act of 1934 and the
Private Securities Litigation Reform Act of 1995, which involve
risks and uncertainties. Although Moleculin believes that the
expectations reflected in such forward-looking statements are
reasonable as of the date made, expectations may prove to have been
materially different from the results expressed or implied by such
forward-looking statements. Moleculin has attempted to identify
forward-looking statements by terminology including 'believes,'
'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,'
'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,'
'approximately' or other words that convey uncertainty of future
events or outcomes to identify these forward-looking statements.
These statements are only predictions and involve known and unknown
risks, uncertainties, and other factors, including those discussed
under Item 1A. "Risk Factors" in our most recently filed Form 10-K
filed with the Securities and Exchange Commission (SEC) and updated
from time to time in our Form 10-Q filings and in our other public
filings with the SEC. Any forward-looking statements contained in
this release speak only as of its date. We undertake no obligation
to update any forward-looking statements contained in this release
to reflect events or circumstances occurring after its date or to
reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
MBRX@jtcir.com
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-plans-for-miracle-phase-3-pivotal-trial-302211451.html
SOURCE Moleculin Biotech, Inc.