- Independent study concluded:
- IceCure
system offers significant advantage in ability to re-treat tumors
that are initially resistant, achieving a subsequent local control
rate of 100%
- 92.4% of patients (N= 24) were
discharged the day after cyroablation
- The
technology's ability to preserve renal function post-treatment is
paramount for patients' quality of life
- Findings
serve as a guide for medical professionals in choosing efficient,
cost-effective, and patient-friendly treatment options, thereby
benefiting society at large by optimizing kidney tumor
management
- Success across multiple indications
supports ProSense®'s commercialization, particularly in
facilities that can use one device across multiple
specialties
CAESAREA, Israel, Nov. 28,
2023 /PRNewswire/ -- IceCure Medical Ltd. (Nasdaq:
ICCM), developer of the ProSense® System, a minimally-invasive
cryoablation technology that destroys tumors by freezing as an
alternative to surgical tumor removal, today announced the latest
release of a study (the "Study") in a series of independent studies
of ProSense® published in peer-reviewed journals demonstrating
safety and efficacy. The Study, titled "Single-Probe Percutaneous
Cryoablation with Liquid Nitrogen for the Treatment of T1a Renal
Tumors", published in Cancers, demonstrated the safety and
efficacy of ProSense® in treating malignant small renal masses. The
Study was authored by eight physicians in France, including interventional radiologists
and urologists from Curie Institute, Paris, Nîmes University Hospital (University
of Montpellier), Nîmes, and Carémeau University Hospital,
Nîmes.
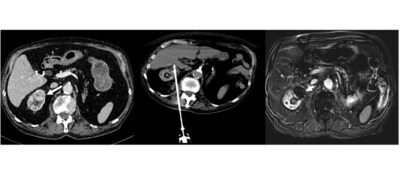
ProSense® is approved for the treatment of benign and malignant
kidney tumors in the U.S., Europe,
and numerous other countries.
The objective of this retrospective Study was to address the
challenges of managing small renal masses, including recurrence
rates, by exploring the safety and efficiency of single-probe
percutaneous cryoablation as a potential solution. The causes of
partial tumor response and persistent tumor residue after a T1a
renal cryoablation procedure were assessed. A total of 25 patients
underwent cryoablation for 26 T1a renal tumors with a median tumor
size of 25.3 mm (20 to 30.7 mm) and a median RENAL nephrometry
score, indicating tumor complexity, of 7 (5 to 9).
Main findings of the Study:
- Disease-free survival rate was 92% (23 out of 25) at a median
follow-up of 26 and a half months
- Recurrent lesions were treated again using cryoablation,
achieving a secondary local control rate of 100%
- No patients died
- No major complications arose
- 92.4% of patients (N= 24) were discharged the day after
surgery
One of the Study's authors, Professor Julien Frandon, Director of the Interventional
Radiology Department at Nîmes University Hospital commented, "In
our recent publication, we evaluated the safety and efficacy of
IceCure's cryoablation technology for the treatment of renal T1a
tumors. This innovative approach has demonstrated remarkable safety
profiles, even for challenging and unfavorably located small renal
masses. One of the study's crucial findings is the technology's
ability to preserve renal function post-treatment, which is
paramount for patients' quality of life. This technology stands out
as a forward-thinking solution in oncological treatment, providing
a combination of patient safety, procedural efficiency, and
cost-effectiveness."
"We appreciate the diligent work of Professor Frandon and his
colleagues in conducting this Study and we congratulate them on its
publication in Cancers, a prestigious European medical
journal," stated IceCure's Chief Executive Officer Eyal Shamir. "The authors' findings are similar
to the interim results from our own ICESECRET study in small renal
masses which demonstrated an 89.5% recurrence-free rate. We expect
ICESECRET's five-year patient follow-up to be completed in 2026,
with topline results available shortly afterwards."
About ProSense®
ProSense® cryoablation is a minimally invasive, non-surgical,
outpatient 40-minute, cost affective treatment option that destroys
tumors by freezing them. ProSense® has been investigated and proven
effective in various clinical applications, including breast
tumors, kidney cancer, lung cancer, and in palliative care.
Independent and company-sponsored clinical studies of ProSense®
have shown strong results, with high rates of tumor destruction,
and patient and physician satisfaction.
About IceCure Medical
IceCure Medical (Nasdaq: ICCM) develops and markets ProSense®,
an advanced liquid-nitrogen-based cryoablation therapy for the
treatment of tumors (benign and cancerous) by freezing, with
the primary focus areas being breast, kidney, bone and lung cancer.
Its minimally invasive technology is a safe and effective
alternative to hospital surgical tumor removal that is easily
performed in a relatively short procedure. The system is marketed
and sold worldwide for the indications cleared and approved to
date including in the U.S., Europe, and China.
Forward Looking Statements
This press release contains forward-looking statements within
the meaning of the "safe harbor" provisions of the Private
Securities Litigation Reform Act of 1995 and other Federal and
Israeli securities laws. Words such as "expects," "anticipates,"
"intends," "plans," "believes," "seeks," "estimates" and similar
expressions or variations of such words are intended to identify
forward-looking statements. For example, IceCure is using forward
looking statement in this press release when it discusses: the
potential of ProSense® to be an effective and viable option for
treating small renal masses; and the expectation that the five-year
patient follow-up for the ICESECRET study is to be completed in
2026, with topline results available shortly afterwards. Historic
results of scientific research and clinical and preclinical trials
do not guarantee that the conclusions of future research or trials
will suggest identical or even similar conclusions. Because such
statements deal with future events and are based on IceCure's
current expectations, they are subject to various risks and
uncertainties and actual results, performance, or achievements of
IceCure could differ materially from those described in or implied
by the statements in this press release. Important factors that
could cause actual results, developments and business decisions to
differ materially from those anticipated in these forward-looking
statements include, among others: the Company's planned level
of revenues and capital expenditures; the Company's available cash
and its ability to obtain additional funding; the Company's ability
to market and sell its products; legal and regulatory developments
in the United States and other countries; the Company's
ability to maintain its relationships with suppliers, distributors
and other partners; the Company's ability to maintain or protect
the validity of its patents and other intellectual property; the
Company's ability to expose and educate medical professionals about
its products; political, economic and military instability in
the Middle East, specifically in Israel; as well as those
factors set forth in the Risk Factors section of the Company's
Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on
March 29, 2023, and other documents
filed with or furnished to the SEC which are available on the
SEC's website, www.sec.gov. The Company undertakes no obligation to
update these statements for revisions or changes after the date of
this release, except as required by law.
IR Contact:
Email: investors@icecure-medical.com
Michael Polyviou
Phone: 732-232-6914
Todd Kehrli
Phone: 310-625-4462
Photo -
https://mma.prnewswire.com/media/2286054/IceCure_cryoprobe.jpg
Logo -
https://mma.prnewswire.com/media/1941429/IceCure_Logo.jpg
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/icecure-system-successfully-treated-kidney-cancer-tumor-with-92-disease-free-survival-rate-and-100-secondary-local-control-rate-301998960.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/icecure-system-successfully-treated-kidney-cancer-tumor-with-92-disease-free-survival-rate-and-100-secondary-local-control-rate-301998960.html
SOURCE IceCure Medical