New Data Supporting a New Precision Medicine Approach for ALS Patients With GeNeuro’s GNK-301 Presented at the 35th International Symposium on ALS/MND
2024年12月7日 - 2:30AM
ビジネスワイヤ(英語)
Regulatory News:
GeNeuro (Euronext Paris: CH0308403085 - GNRO), a
biopharmaceutical company focused on addressing the factors driving
the progression of neurodegenerative and autoimmune diseases, such
as amyotrophic lateral sclerosis (ALS), today announced that
groundbreaking findings on a potential precision medicine strategy
for ALS with GeNeuro’s GNK-301 were presented at the 35th
International Symposium on ALS/MND, taking place in Montreal,
Canada, from December 6-8, 2024. The presentation was delivered by
Dr. Darshan Pandya, from National Institute of Neurological
Disorders and Stroke (NINDS)/National Institutes of Health
(NIH).
The research, led by GeNeuro, through its Lyon R&D unit
GeNeuro Innovation SAS, in collaboration with leading institutions,
including the NIH/NINDS, ERBC (France), and the University of
Oxford (UK), highlights the potential of GeNeuro’s GNK-301, a
humanized monoclonal antibody targeting HERV-K ENV, a neurotoxic
protein that is often found in the cerebrospinal fluid of ALS
patients. This protein has been shown to contribute to neuronal
cell death and blood-brain barrier dysfunction, two hallmarks of
ALS. Preclinical studies have shown that GNK-301 can be used to
detect the presence of the HERV-K ENV protein in a laboratory test
and that it can then be used as a medicine to neutralize the
harmful effects of HERV-K ENV, protecting neurons and preventing
damage to the blood-brain barrier. By being able to detect this
toxic protein in ALS patients and starting a neutralising treatment
early, GeNeuro is proposing a new precision medicine approach that
offers hope for transforming the treatment of ALS.
“We are excited to share these promising findings and
congratulate the NIH/NINDS, ERBC and the University of Oxford for
this exciting new data that opens a path towards a novel precision
medicine approach in the treatment of sporadic ALS”, said Hervé
Perron, CSO of GeNeuro.
About GNK-301 and HERV-K ENV
Studies have shown that the HERV-K ENV protein acts as a
neurotoxin, contributing to neuronal cell death and blood-brain
barrier (BBB) dysfunction—two hallmarks of ALS pathology. In the
laboratory, a test with GNK-301 is able to confirm the presence of
HERV-K ENV in samples of cerebrospinal fluid (CSF) of ALS patients
and, as a consequence, identify those who would benefit from its
administration as a medicine. As a neutralizing monoclonal
antibody-based medicine, GNK-301 abolishes the ALS CSF
neurotoxicity in iPSC-derived neuron cultures and the neuronal
death in mice that had been stereotaxically injected with HERV-K
ENV. Surprisingly, when present in the brain, the HERV-K ENV
protein reproduced the previously reported BBB dysfunction observed
in ALS brains, which was also prevented by GNK-301. In animal
facilities of ERBC-Voxcan (France), when labelled GNK-301 was
injected intravenously in mice, it was found only to accumulate in
parts of the brain where HERV-K ENV protein was present. It is of
note that there have been previous studies supporting the use of
GNK-301 as a medicine: individuals with ALS who make their own
endogenous autoantibodies against HERV-K ENV live for longer.
However, GNK-301, compared to autoantibodies, has the huge
advantage of displaying a much greater affinity and neutralizing
effect. In addition, the observed effect of this endogenous
neurotoxin on the BBB helps to facilitate the transport of the
antibody into brain tissue after its intravenous administration.
Further preclinical studies are being conducted, but planning for
the required medical grade production of GNK-301 is underway to
provide the antibody for clinical studies in ALS patients who will
have tested positive for HERV-K ENV with GeNeuro’s dedicated
immunoassay. This would be the first integrated strategy for
precision medicine in sporadic ALS.
About GeNeuro
GeNeuro‘s mission is to develop safe and effective treatments
against neurological disorders and autoimmune diseases, such as
multiple sclerosis, by neutralizing causal factors encoded by
HERVs, which represent 8% of human DNA. GeNeuro is based in Geneva,
Switzerland and has R&D facilities in Lyon, France.
For more information, visit: www.geneuro.com
Disclaimer
This press release contains certain forward - looking statements
and estimates concerning GeNeuro’s financial condition, operating
results, strategy, projects and future performance and the markets
in which it operates. Such forward-looking statements and estimates
may be identified by words, such as “anticipate,” “believe,” “can,”
“could,” “estimate,” “expect,” “intend,” “is designed to,” “may,”
“might,” “plan,” “potential,” “predict,” “objective,” “should,” or
the negative of these and similar expressions. They incorporate all
topics that are not historical facts. Forward looking statements,
forecasts and estimates are based on management’s current
assumptions and assessment of risks, uncertainties and other
factors, known and unknown, which were deemed to be reasonable at
the time they were made but which may turn out to be incorrect.
Events and outcomes are difficult to predict and depend on factors
beyond the company’s control. Consequently, the actual results,
financial condition, performances and/or achievements of GeNeuro or
of the industry may turn out to differ materially from the future
results, performances or achievements expressed or implied by these
statements, forecasts and estimates. Owing to these uncertainties,
no representation is made as to the correctness or fairness of
these forward-looking statements, forecasts and estimates.
Furthermore, forward-looking statements, forecasts and estimates
speak only as of the date on which they are made, and GeNeuro
undertakes no obligation to update or revise any of them, whether
as a result of new information, future events or otherwise, except
as required by law. The content of this press release is solely the
responsibility of the authors and does not necessarily represent
the official views of the National Institutes of Health.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241206710776/en/
GeNeuro Jesús Martin-Garcia Chairman and CEO +41 22 552
48 00 investors@geneuro.com
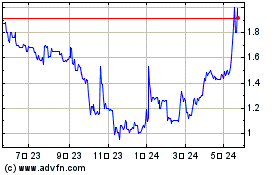
GeNeuro (EU:GNRO)
過去 株価チャート
から 12 2024 まで 1 2025
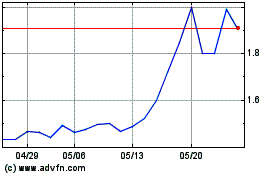
GeNeuro (EU:GNRO)
過去 株価チャート
から 1 2024 まで 1 2025