Vertex Pharmaceuticals' Kalydeco Gets Expanded Use Authorization from Health Canada
2023年11月30日 - 4:55AM
Dow Jones News
By Denny Jacob
Vertex Pharmaceuticals' has gained market authorization from
Health Canada for the expanded use of Kalydeco to treat cystic
fibrosis in certain children aged two months and up.
The biotechnology company said the latest authorization of
Kalydeco is for children with certain mutations in the cystic
fibrosis transmembrane conductance regulator gene.
Vertex said it will work closely with government and private
payers to facilitate access for eligible patients as soon as
possible.
Kalydeco was previously approved by Health Canada for use in
people with cystic fibrosis aged four months and older with certain
gene mutations.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
November 29, 2023 14:40 ET (19:40 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
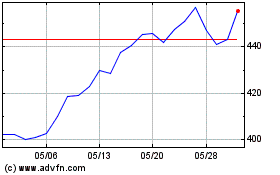
Vertex Pharmaceuticals (NASDAQ:VRTX)
過去 株価チャート
から 6 2024 まで 7 2024
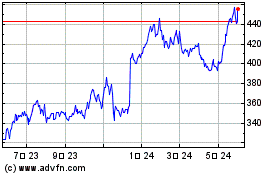
Vertex Pharmaceuticals (NASDAQ:VRTX)
過去 株価チャート
から 7 2023 まで 7 2024