Form 8-K - Current report
2024年1月24日 - 7:28AM
Edgar (US Regulatory)
false
0001509745
0001509745
2024-01-23
2024-01-23
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(D)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported):
January 23, 2024
Leap Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
|
001-37990 |
|
27-4412575 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
47 Thorndike Street, Suite B1-1
Cambridge, MA |
02141 |
| (Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone
number, including area code: (617) 714-0360
N/A
(Former name or former address,
if changed since last report)
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any
of the following provisions:
| ¨ | Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425). |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange
Act (17 CFR 240.14a-12). |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under
the Exchange Act (17 CFR 240.14d-2(b)). |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c)). |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name
of each exchange on which
registered |
| Common Stock, par value $0.001 |
LPTX |
Nasdaq Capital Market |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter)
Emerging growth company ¨
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 8.01. Other Events
On January 23, 2024, Leap
Therapeutics, Inc. (the “Company”) posted an updated corporate presentation on its website, www.leaptx.com. A copy of
the presentation is filed as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference. The information
contained on, or that can be accessed from, the Company’s website is not incorporated into, and does not constitute a part of, this
Current Report on Form 8-K.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| |
LEAP THERAPEUTICS, INC. |
| |
|
| Dated: January 23, 2024 |
By: |
/s/ Douglas E. Onsi |
| |
Name: |
Douglas E. Onsi |
| |
Title: |
Chief Executive Officer and President |
Exhibit 99.1

| company presentation
LEAP THERAPEUTICS
January 23, 2024 |

| Forward looking statements
2
This presentation contains forward-looking statements
that involve substantial risks and uncertainties.
All statements, other than statements of historical facts,
contained in this presentation, including statements regarding our
strategy, future operations, clinical trials, collaborations and
partnerships, future financial position, future revenues, projected
costs, prospects, plans and objectives of management,
are forward-looking statements within the meaning of U.S.
securities laws. The words “anticipate,” “believe,” “estimate,”
“expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,”
“potential,” “will,” “would,” “could,” “should,” “continue,”
and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain
these identifying words.
Forward-looking statements are neither historical facts nor
assurances of future performance. Instead, they are based only
on our current beliefs, expectations and assumptions regarding
the future of our business, future plans and strategies,
projections, anticipated events and trends, the economy and
other future conditions.
Because forward-looking statements relate to the future,
they are subject to inherent uncertainties, risks
and changes in circumstances that are difficult to predict
and many of which are outside of our control. We may
not actually achieve the plans, intentions or expectations
disclosed in our forward-looking statements, and you
should not place undue reliance on our forward-looking
statements. Actual results or events could differ
materially from the plans, intentions and expectations
disclosed in the forward-looking statements we make.
These and other risk factors are listed from time to time
in reports filed with the Securities and Exchange
Commission, including, but not limited to, our Annual
Reports on Form 10-K and our Quarterly Reports
on Form 10-Q. We assume no obligation to update
any forward-looking statements, except as required
by applicable law.
This presentation does not constitute an offer to sell,
or the solicitation of an offer to buy, any securities. |
| Developing biomarker-targeted antibody therapies for cancer patients
3
Two clinical stage
antibody programs –
DKN-01 targeting DKK1
FL-301 targeting CLDN18.2
Upcoming multiple
milestones from two
randomized clinical trials
Biomarker strategy,
focus on GI cancers
Cash runaway to
Q2 2025 with $70M at
December 31, 2023 |
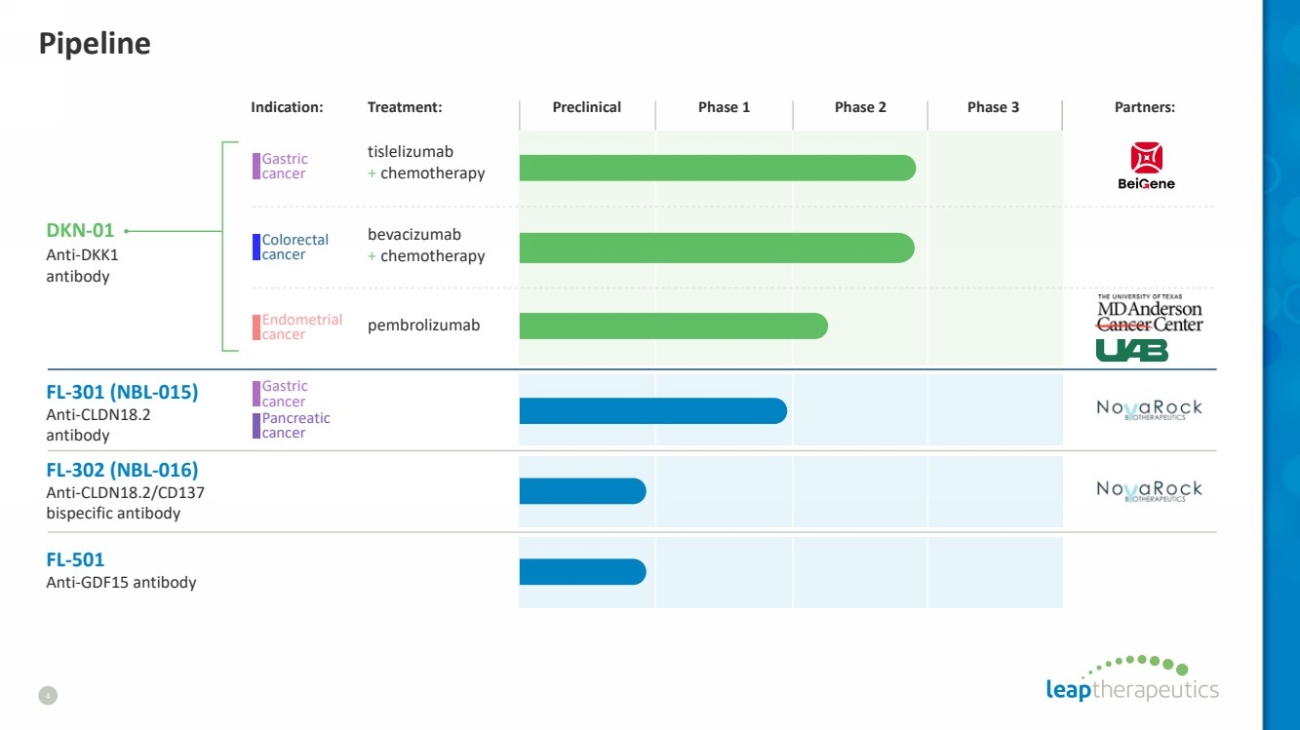
| Pipeline
4
Indication: Treatment: Preclinical Phase 1 Phase 2 Phase 3 Partners:
DKN-01
Anti-DKK1
antibody
tislelizumab
+ chemotherapy
bevacizumab
+ chemotherapy
pembrolizumab
FL-302 (NBL-016)
Anti-CLDN18.2/CD137
bispecific antibody
FL-301 (NBL-015)
Anti-CLDN18.2
antibody
Gastric
cancer
Gastric
cancer
Colorectal
cancer
Endometrial
cancer
FL-501
Anti-GDF15 antibody
Pancreatic
cancer |

| DKN-01
Anti-DKK1 monoclonal
antibody |
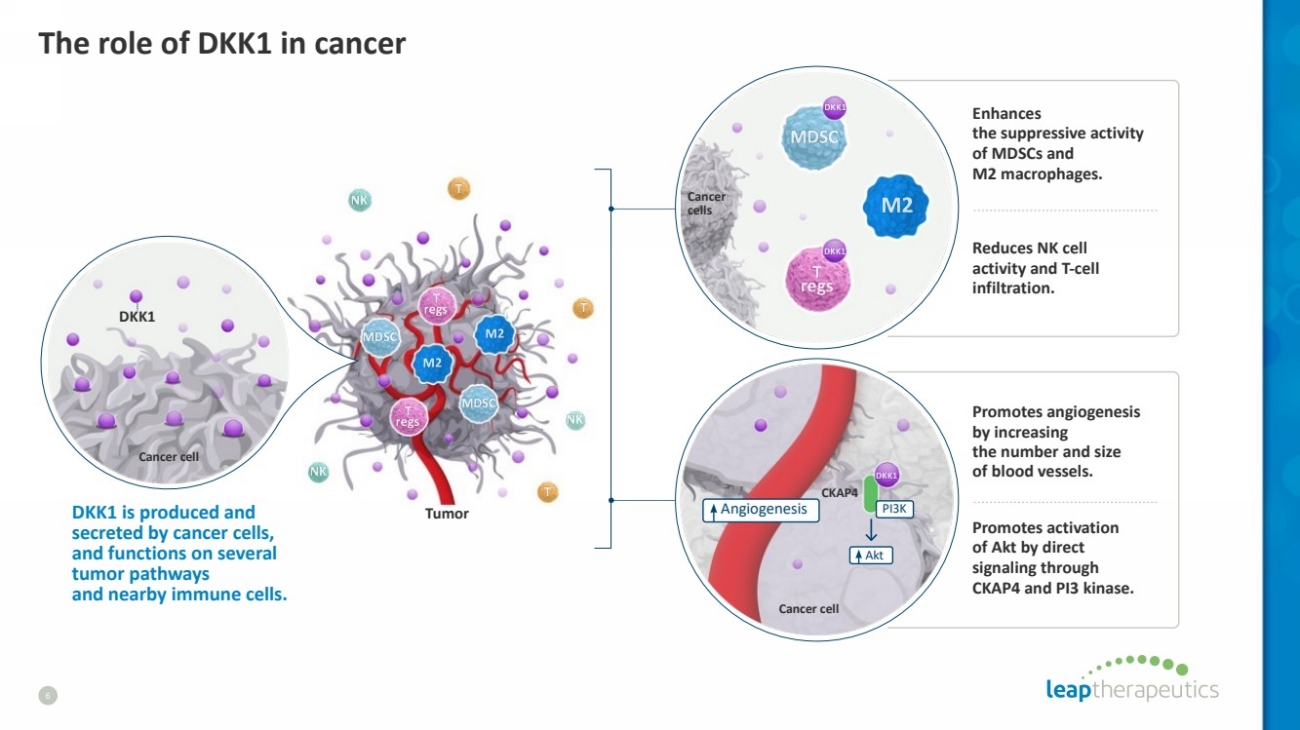
| The role of DKK1 in cancer
6
Tumor
CKAP4
Angiogenesis
Akt
PI3K
Cancer cell
Cancer
cells
DKK1 is produced and
secreted by cancer cells,
and functions on several
tumor pathways
and nearby immune cells.
Enhances
the suppressive activity
of MDSCs and
M2 macrophages.
Reduces NK cell
activity and T-cell
infiltration.
Promotes angiogenesis
by increasing
the number and size
of blood vessels.
Promotes activation
of Akt by direct
signaling through
CKAP4 and PI3 kinase.
Cancer cell
DKK1 |
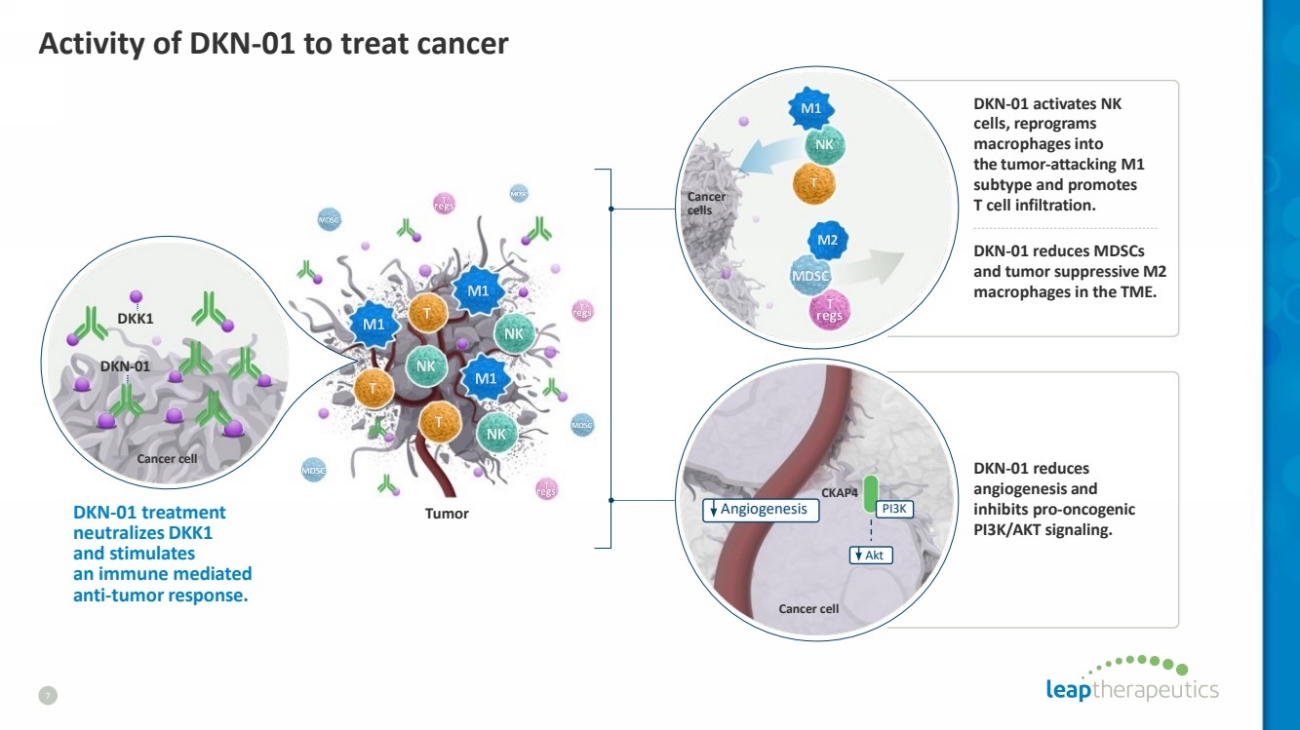
| Activity of DKN-01 to treat cancer
7
CKAP4
Angiogenesis
Akt
PI3K
Cancer cell
Cancer
cells
Tumor
Cancer cell
DKK1
DKN-01 treatment
neutralizes DKK1
and stimulates
an immune mediated
anti-tumor response.
DKN-01
DKN-01 activates NK
cells, reprograms
macrophagesinto
the tumor-attacking M1
subtype and promotes
T cell infiltration.
DKN-01 reduces MDSCs
and tumor suppressive M2
macrophages in the TME.
DKN-01 reduces
angiogenesis and
inhibits pro-oncogenic
PI3K/AKT signaling. |
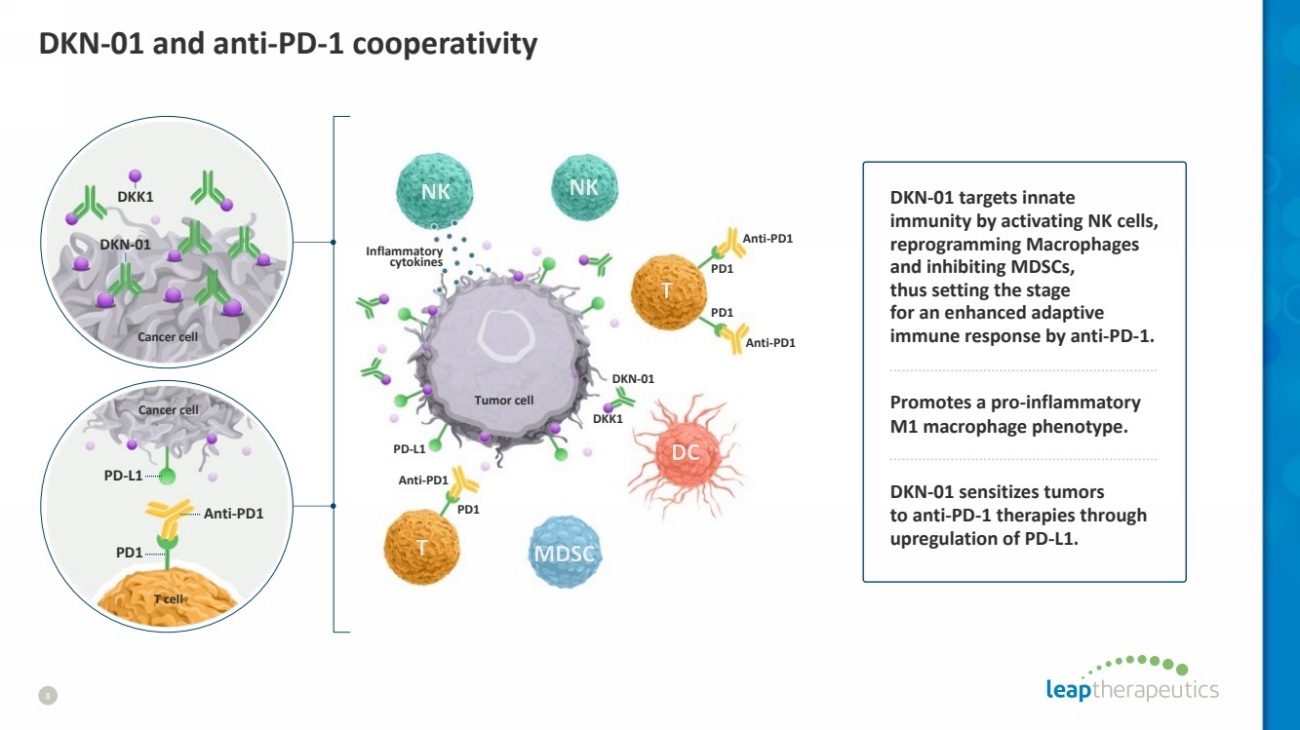
| DKN-01 and anti-PD-1 cooperativity
8
Anti-PD1
Anti-PD1
PD1
DKN-01 targets innate
immunity by activating NK cells,
reprogramming Macrophages
and inhibiting MDSCs,
thus setting the stage
for an enhanced adaptive
immune response by anti-PD-1.
Promotes a pro-inflammatory
M1 macrophage phenotype.
DKN-01 sensitizes tumors
to anti-PD-1 therapies through
upregulation of PD-L1.
PD1
Anti-PD1
PD-L1
Cancer cell
T cell
Cancer cell
DKK1
DKN-01 Inflammatory cytokines
PD1
Anti-PD1
PD1
PD-L1
Tumor cell
DKK1
DKN-01 |

| DKN-01
Gastric сancer development |
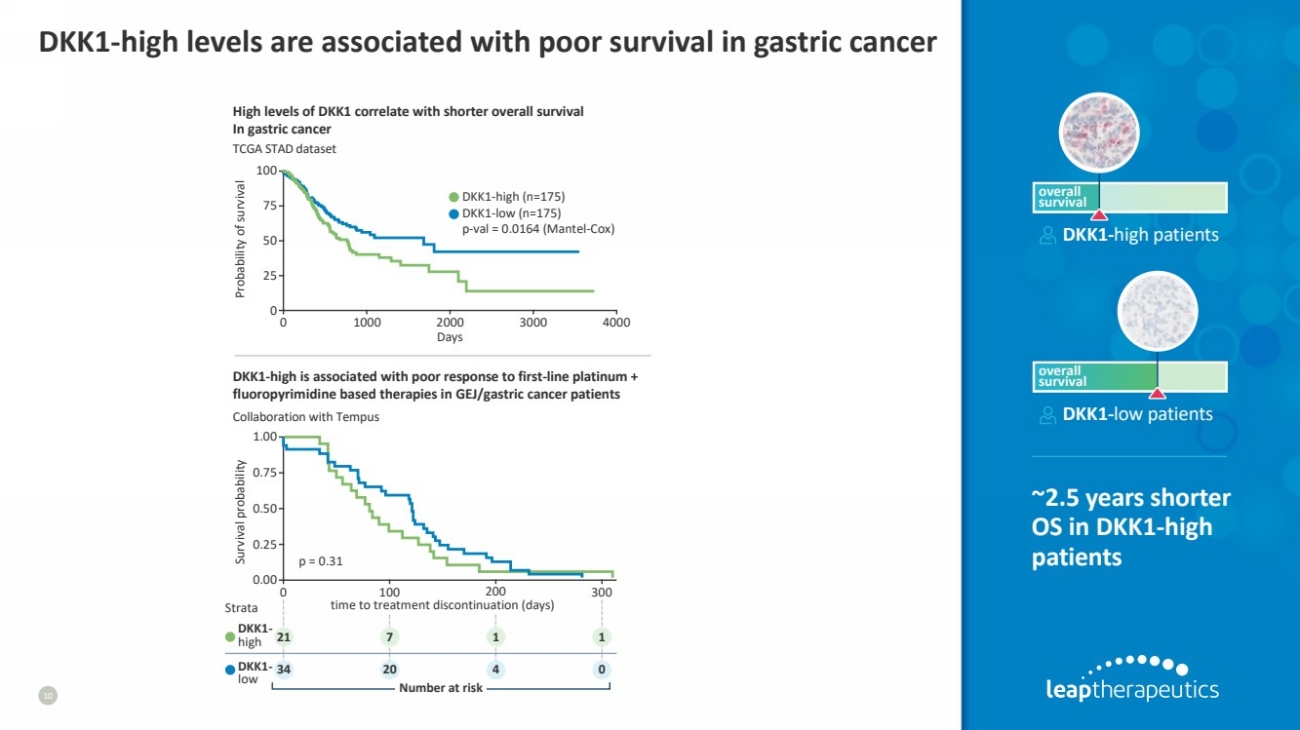
| DKK1-high levels are associated with poor survival in gastric cancer
10
Strata
21
34
7 1 1
20 4 0
0 100 200 300
time to treatment discontinuation (days)
High levels of DKK1 correlate with shorter overall survival
In gastric cancer
TCGA STAD dataset
DKK1-high is associated with poor response to first-line platinum +
fluoropyrimidine based therapies in GEJ/gastric cancer patients
Collaboration with Tempus
DKK1-high patients
~2.5 years shorter
OS in DKK1-high
patients
overall
survival
overall
survival
DKK1-low patients
Days
0 1000 2000 3000 4000
100
Probability of survival
50
0
25
75
DKK1-high (n=175)
DKK1-low (n=175)
p-val = 0.0164 (Mantel-Cox)
0.00
Survival probability
1.00
0.75
0.50
0.25
p = 0.31
overall
survival
overall
survival
DKK1-
high
DKK1- low Number at risk |
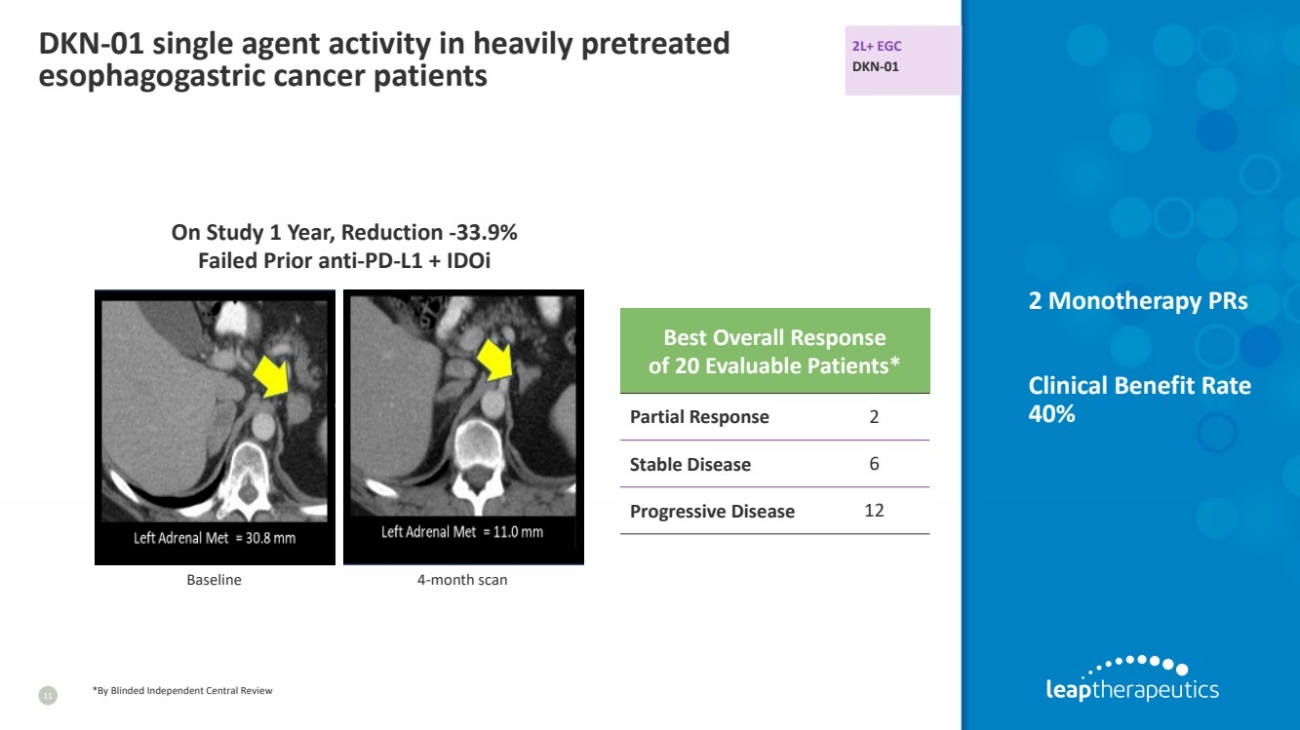
| DKN-01 single agent activity in heavily pretreated
esophagogastric cancer patients
11
Baseline 4-month scan
On Study 1 Year, Reduction -33.9%
Failed Prior anti-PD-L1 + IDOi
Best Overall Response
of 20 Evaluable Patients*
Partial Response 2
Stable Disease 6
Progressive Disease 12
*By Blinded Independent Central Review
2L+ EGC
DKN-01
2 Monotherapy PRs
Clinical Benefit Rate
40% |
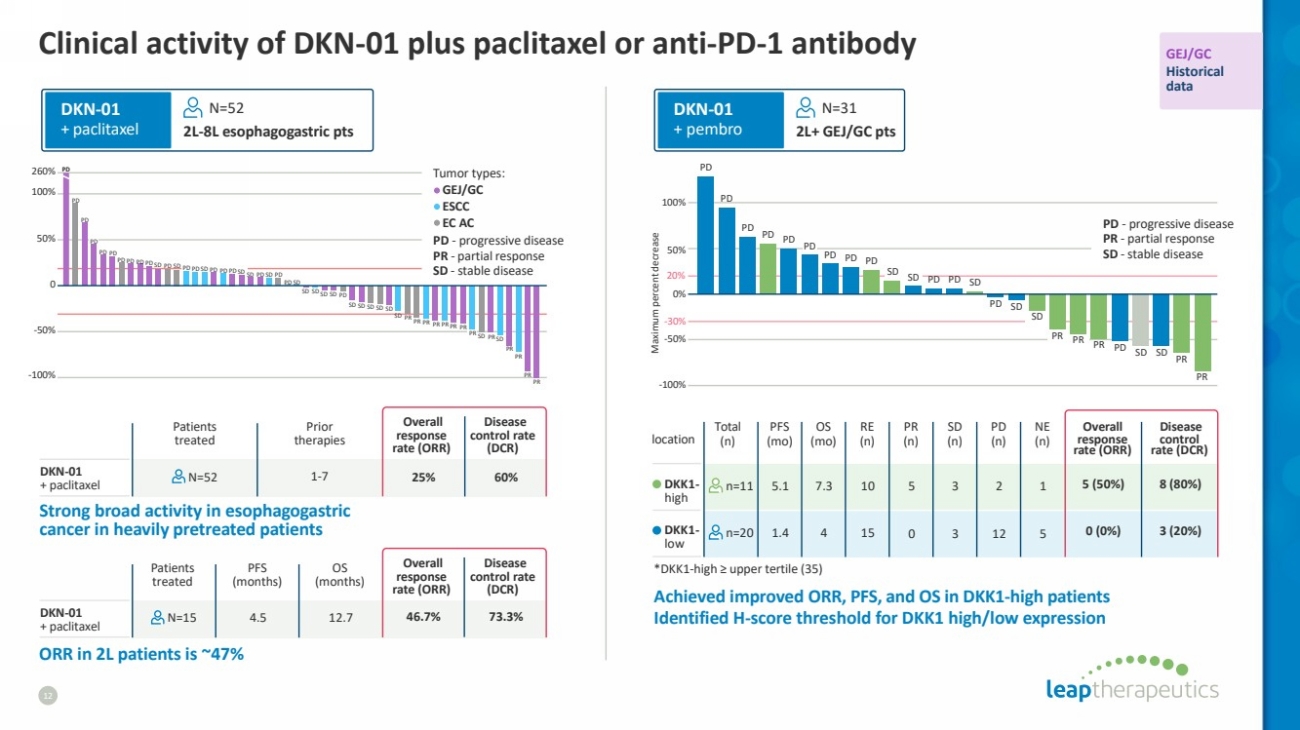
| *DKK1-high ≥ upper tertile (35)
Clinical activity of DKN-01 plus paclitaxel or anti-PD-1 antibody
12
PD
PD
PD
PD
PD PDPD PD PD PD SD SD SD SD SD SD
SD
SD SD SD SD
SD
SD SD
SD SD SD SD SD
PD PD PD PD PD PD PD PD
PD
PD
PR PR PR PR PR PR PR PR PR
PR
PR
PR
PR
PD
PD
PD PD PD PD
PD PD PD
PD PD
PD
SD
SD SD SD
SD
SD PD
SD
PR PR PR
PR
PR
50%
-50%
-100%
100%
DKN-01
+ paclitaxel
N=52
DKN-01
+ paclitaxel
Patients
treated
Prior
therapies
N=52 60%
Disease
control rate
(DCR)
25%
Overall
response
rate (ORR)
2L-8L esophagogastric pts
GEJ/GC
ESCC
EC AC
PD - progressive disease
PR - partial response
SD - stable disease
Tumor types:
0
260%
ORR in 2L patients is ~47%
Strong broad activity in esophagogastric
cancer in heavily pretreated patients
DKN-01
+ paclitaxel
Patients
treated
PFS
(months)
N=15 12.7 73.3%
OS
(months)
Disease
control rate
(DCR)
46.7%
Overall
response
rate (ORR)
4.5
1-7
Maximumpercent decrease -100%
50%
-50%
-30%
100%
20%
GEJ/GC
Historical
data
DKN-01
+ pembro
N=31
2L+ GEJ/GC pts
Total
location (n)
RE
(n)
PR
(n)
SD
(n)
PD
(n)
NE
(n)
Disease
control
rate (DCR)
Overall
response
rate (ORR)
0%
Achieved improved ORR, PFS, and OS in DKK1-high patients
Identified H-score threshold for DKK1 high/low expression
n=11
n=20
10 5 3 2 1
0 3 12 5
8 (80%)
3 (20%)
5 (50%)
0 (0%)
DKK1- high
DKK1-
low
15
PD - progressive disease
PR - partial response
SD - stable disease
OS
(mo)
7.3
4
PFS
(mo)
5.1
1.4 |
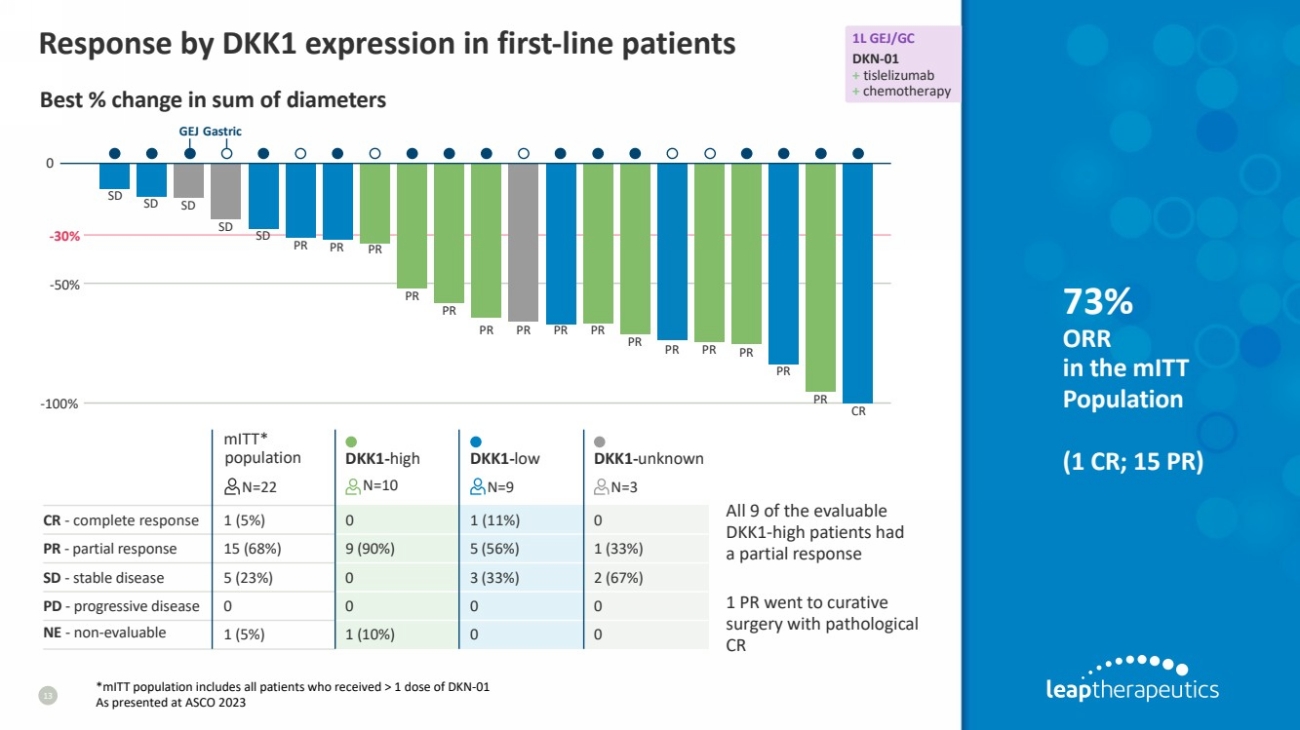
| mITT*
population
N=22
DKK1-high
N=10
DKK1-low
N=9
DKK1-unknown
N=3
CR - complete response 1 (5%) 0 1 (11%) 0
PR - partial response 15 (68%) 9 (90%) 5 (56%) 1 (33%)
SD - stable disease 5 (23%) 0 3 (33%) 2 (67%)
PD - progressive disease 0 0 0 0
NE - non-evaluable 1 (5%) 1 (10%) 0 0
Response by DKK1 expression in first-line patients
13
73%
ORR
in the mITT
Population
(1 CR; 15 PR)
*mITT population includes all patients who received > 1 dose of DKN-01
As presented at ASCO 2023
Best % change in sum of diameters
All 9 of the evaluable
DKK1-high patients had
a partial response
1 PR went to curative
surgery with pathological
CR
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy
GEJ Gastric
SD SD SD
SD SD
PR PR PR
PR
PR
PR PR PR PR
PR PR PR PR
PR
PR
CR
-50%
-30%
0
-100% |
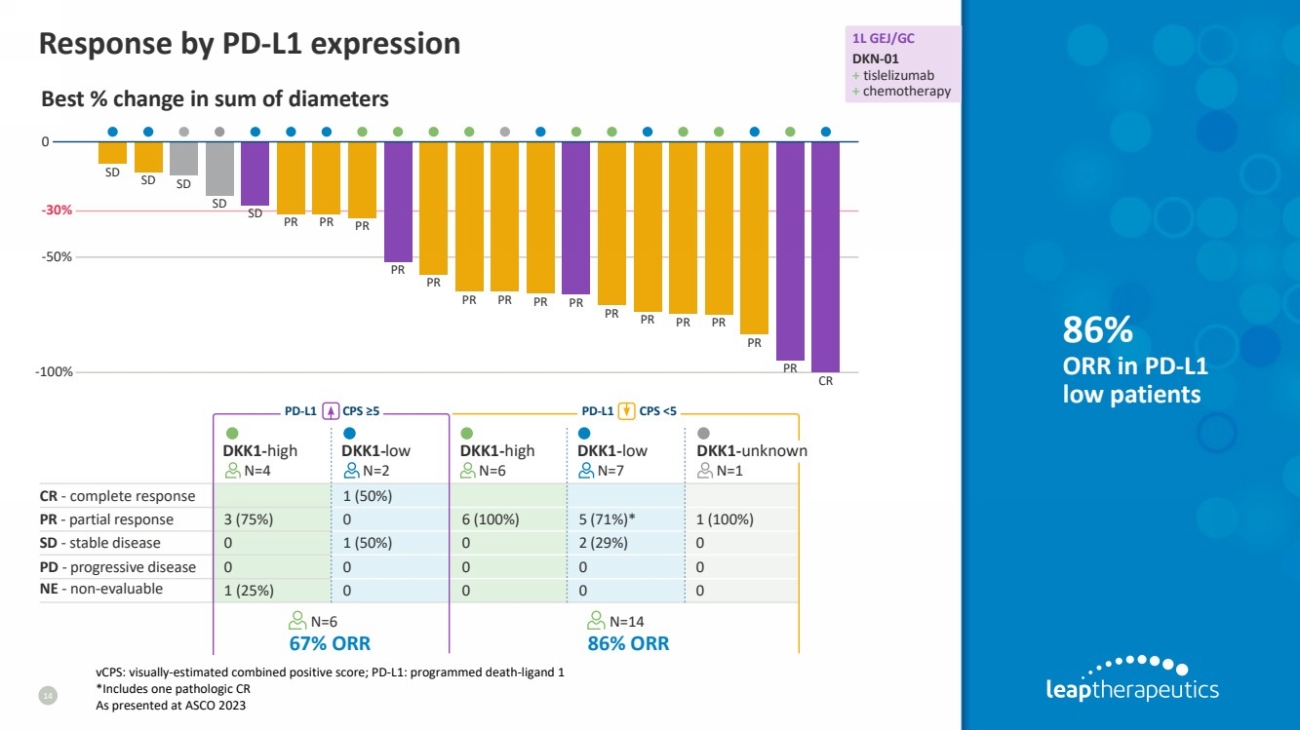
| Response by PD-L1 expression
14
86%
ORR in PD-L1
low patients
Best % change in sum of diameters
vCPS: visually-estimated combined positive score; PD-L1: programmed death-ligand 1
*Includes one pathologic CR
As presented at ASCO 2023
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy
CR - complete response 1 (50%)
PR - partial response 3 (75%) 0 6 (100%) 5 (71%)* 1 (100%)
SD - stable disease 0 1 (50%) 0 2 (29%) 0
PD - progressive disease 0 0 0 0 0
NE - non-evaluable 1 (25%) 0 0 0 0
DKK1-high DKK1-low DKK1-high DKK1-low DKK1-unknown
N=4 N=2 N=6 N=7 N=1
N=6 N=14
PD-L1 CPS ≥5
67% ORR 86% ORR
PD-L1 CPS <5
0
-50%
-30%
PR
SD SD SD
SD
SD PR PR PR
PR PR PR
PR
PR
CR
PR
PR
PR PR PR
PR
-100% |
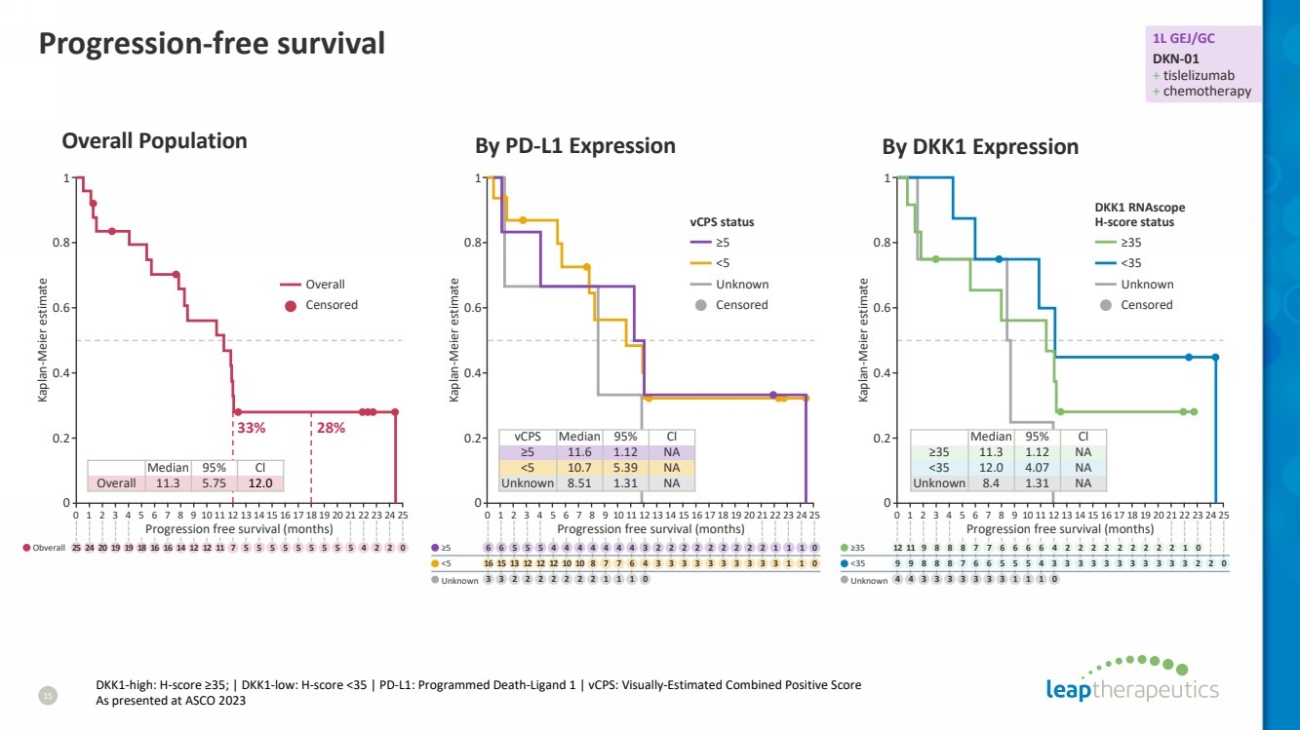
| Progression-free survival
15
Overall Population
DKK1-high: H-score ≥35; | DKK1-low: H-score <35 | PD-L1: Programmed Death-Ligand 1 | vCPS: Visually-Estimated Combined Positive Score
As presented at ASCO 2023
≥5
<5
Unknown
6 5 4 3 2 2
16 15 13 12 10 10 8 7 7 6 4 3 0
3 3 2 2 2 2 2 2 2 1 1 1 0
12 12 3 3
6 5 5 4 4 4 4 4 4 2 2 2 2 2 2 2 1 1 1 0
3 3 3 3
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Progression free survival (months)
Obverall 25 24 20 19 19 18 16 16 14 12 12 11 7 5 5 5 5 5 5 5 5 5 4 2 2 0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Progression free survival (months)
vCPS Median 95% Cl
≥5 11.6 1.12 NA
<5 10.7 5.39 NA
Unknown 8.51 1.31 NA
Median 95% Cl
≥35 11.3 1.12 NA
<35 12.0 4.07 NA
Unknown 8.4 1.31 NA
By PD-L1 Expression By DKK1 Expression
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Progression free survival (months)
33% 28%
Median 95% Cl
Overall 11.3 5.75 12.0
DKK1 RNAscope
H-score status
≥35
<35
Unknown
Censored
vCPS status
≥5
<5
Unknown
Censored
Overall
Censored
≥35
<35
Unknown
12 11 9 8 7 6 4 2 2 2 2 2 2 2 2 1 0
4 4 3 3 3 3 3 3 3 1 1 1 0
9 9 8 8 8 7 6 6 5 5 5 4 3 3 3 3 3 3 3 3 3 3 3 2 2 0
8 8 7 6 6 6 2
3 3 3 1 1
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy |
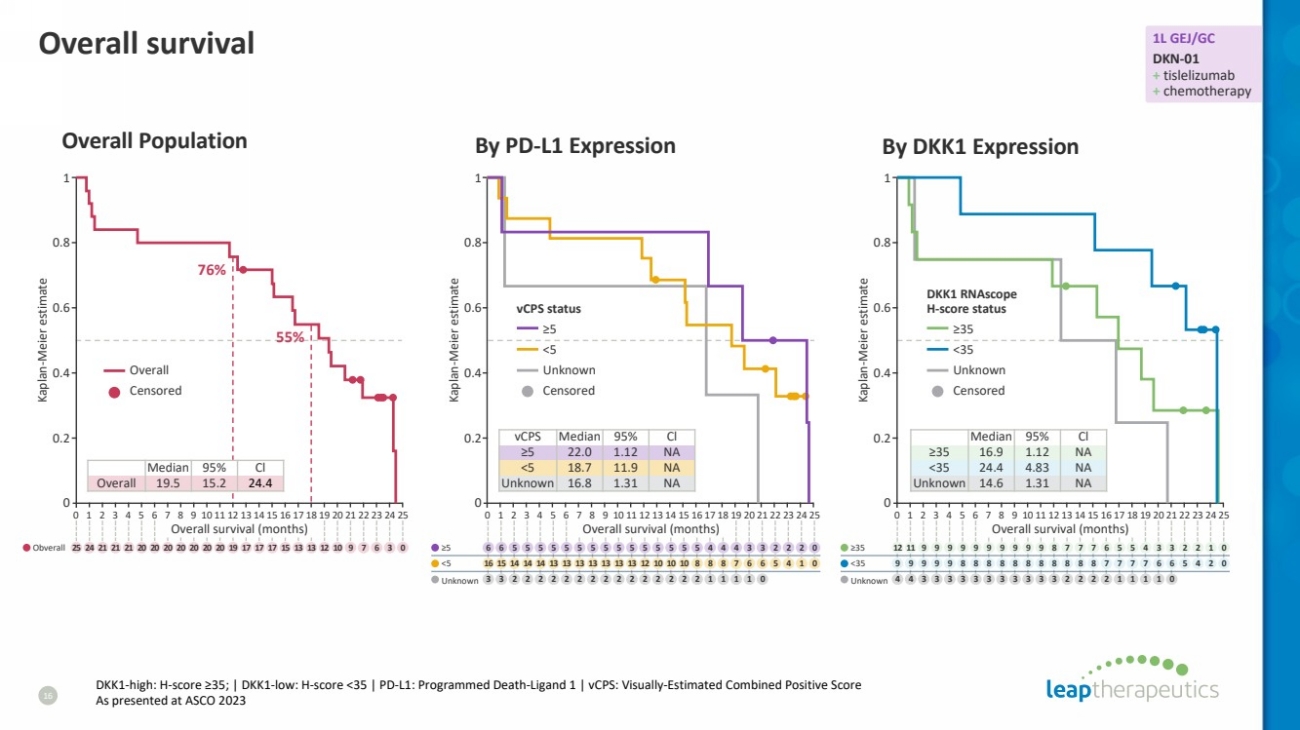
| Overall survival
16
DKK1-high: H-score ≥35; | DKK1-low: H-score <35 | PD-L1: Programmed Death-Ligand 1 | vCPS: Visually-Estimated Combined Positive Score
As presented at ASCO 2023
Overall Population
≥5
<5
Unknown
Obverall 25 24 21 20 19 9 7 6 3 0 6 6 5 5 5 4 4 4 3
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Overall survival (months)
17 15 13 12 10
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Overall survival (months)
By PD-L1 Expression By DKK1 Expression
≥35
<35
Unknown
12 11 9 8 7 6 5 2 1 0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21
Kaplan-Meier estimate
1
0.6
0
0.8
0.4
0.2
22 23 24 25
Overall survival (months)
76%
55%
Median 95% Cl
Overall 19.5 15.2 24.4
DKK1 RNAscope
H-score status
≥35
<35
Unknown
Censored
vCPS status
≥5
<5
Unknown
Censored
Overall
Censored
7 2
vCPS Median 95% Cl
≥5 22.0 1.12 NA
<5 18.7 11.9 NA
Unknown 16.8 1.31 NA
Median 95% Cl
≥35 16.9 1.12 NA
<35 24.4 4.83 NA
Unknown 14.6 1.31 NA
21 21 20 20 20 20 20 20 17 17 13
3 3 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 1 1 1 1 0
16 15 14 14 14 13 13 13 13 13 13 13 12 10 10 10 8 8 8 7 6
5 5 5 5 5 5 5 5 5 5 5 5 2 2 2 0
6 5 4 1 0
3
4 4 3 3 3 3 3 3 3 3 3 3 3 2 2 2 2 1 1 1 1 0
9 9 9 9 9 8 8 8 8 8 8 8 8 8 8 8 7 7 7 7 6 6 5 4 2 0
9 9 9 9 9 9 9 9 9 7 5 4 3 3
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy |
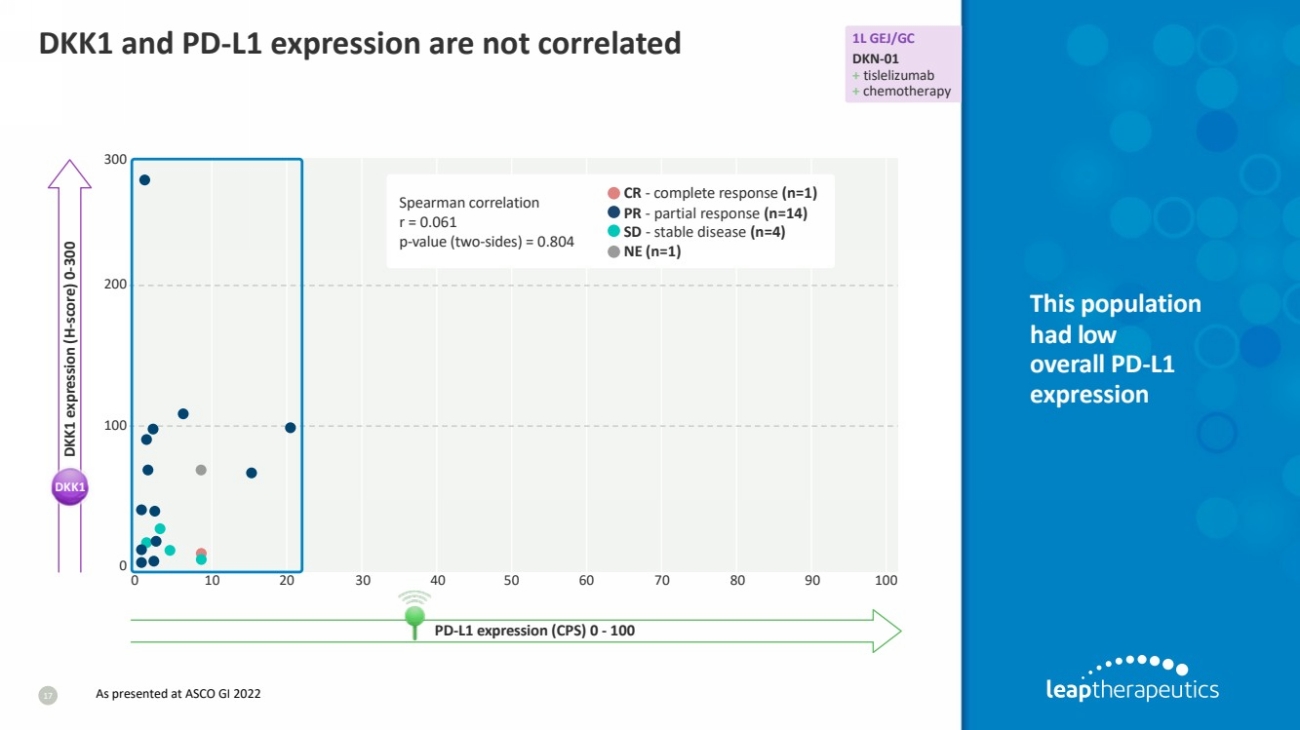
| DKK1 and PD-L1 expression are not correlated
17
This population
had low
overall PD-L1
expression
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy
200
300
100
10 20 30 40 50 60 70 80 90 100
0
DKK1 expression (H-score)
0-300
PD-L1 expression (CPS) 0 - 100
DKK1
0
Spearman correlation
r = 0.061
p-value (two-sides) = 0.804
CR - complete response (n=1)
PR - partial response (n=14)
SD - stable disease (n=4)
NE (n=1)
As presented at ASCO GI 2022 |
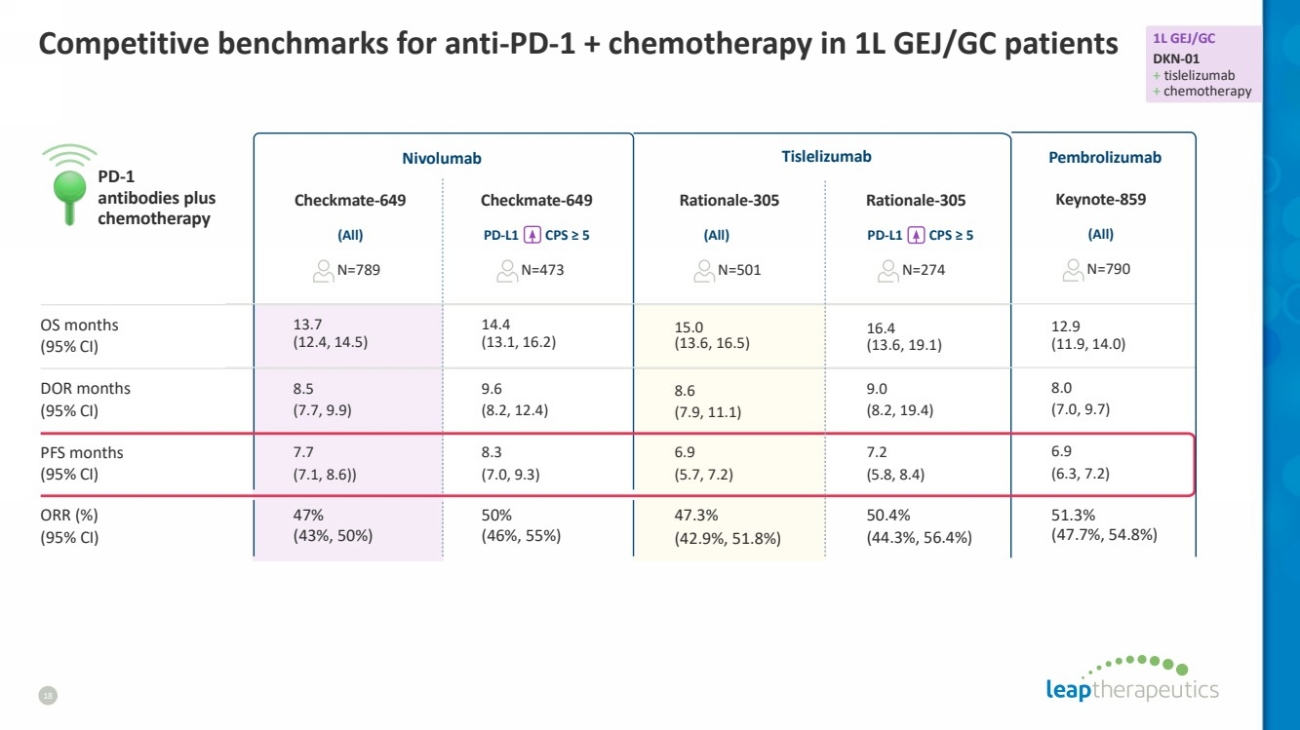
| Competitive benchmarks for anti-PD-1 + chemotherapy in 1L GEJ/GC patients
18
Nivolumab
Checkmate-649
ORR (%)
(95% CI)
50.4%
(44.3%, 56.4%)
50%
(46%, 55%)
47%
(43%, 50%)
9.0
(8.2, 19.4)
9.6
(8.2, 12.4)
8.5
(7.7, 9.9)
8.3
(7.0, 9.3)
7.7
(7.1, 8.6))
8.6
(7.9, 11.1)
DOR months
(95% CI)
PFS months
(95% CI)
Checkmate-649 Rationale-305
Tislelizumab
PD-1
antibodies plus
chemotherapy
N=789 N=473 N=501 N=274
(All) PD-L1 CPS ≥ 5
16.4
(13.6, 19.1)
14.4
(13.1, 16.2)
13.7
(12.4, 14.5)
15.0
(13.6, 16.5)
OS months
(95% CI)
7.2
(5.8, 8.4)
6.9
(5.7, 7.2)
47.3%
(42.9%, 51.8%)
1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy
Rationale-305
(All) PD-L1 CPS ≥ 5
51.3%
(47.7%, 54.8%)
8.0
(7.0, 9.7)
Keynote-859
Pembrolizumab
N=790
12.9
(11.9, 14.0)
6.9
(6.3, 7.2)
(All) |
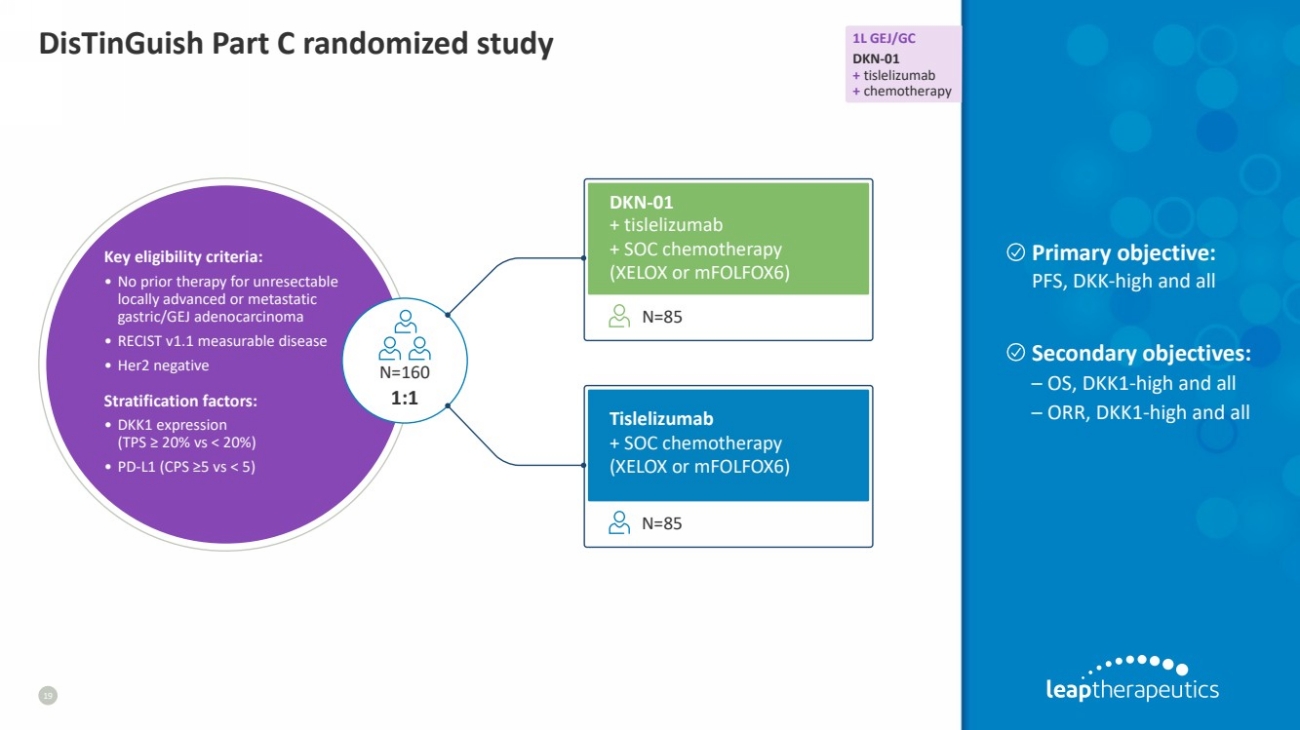
| DisTinGuish Part C randomized study 1L GEJ/GC
DKN-01
+ tislelizumab
+ chemotherapy
19
DKN-01
+ tislelizumab
+ SOC chemotherapy
(XELOX or mFOLFOX6)
Tislelizumab
+ SOC chemotherapy
(XELOX or mFOLFOX6)
N=85
N=85
Stratification factors:
• DKK1 expression
(TPS ≥ 20% vs < 20%)
• PD-L1 (CPS ≥5 vs < 5)
Key eligibility criteria:
• No prior therapy for unresectable
locally advanced or metastatic
gastric/GEJ adenocarcinoma
• RECIST v1.1 measurable disease
• Her2 negative N=160
1:1
Primary objective:
PFS, DKK-high and all
Secondary objectives:
– OS, DKK1-high and all
– ORR, DKK1-high and all |

| DKN-01
Colorectal cancer development |
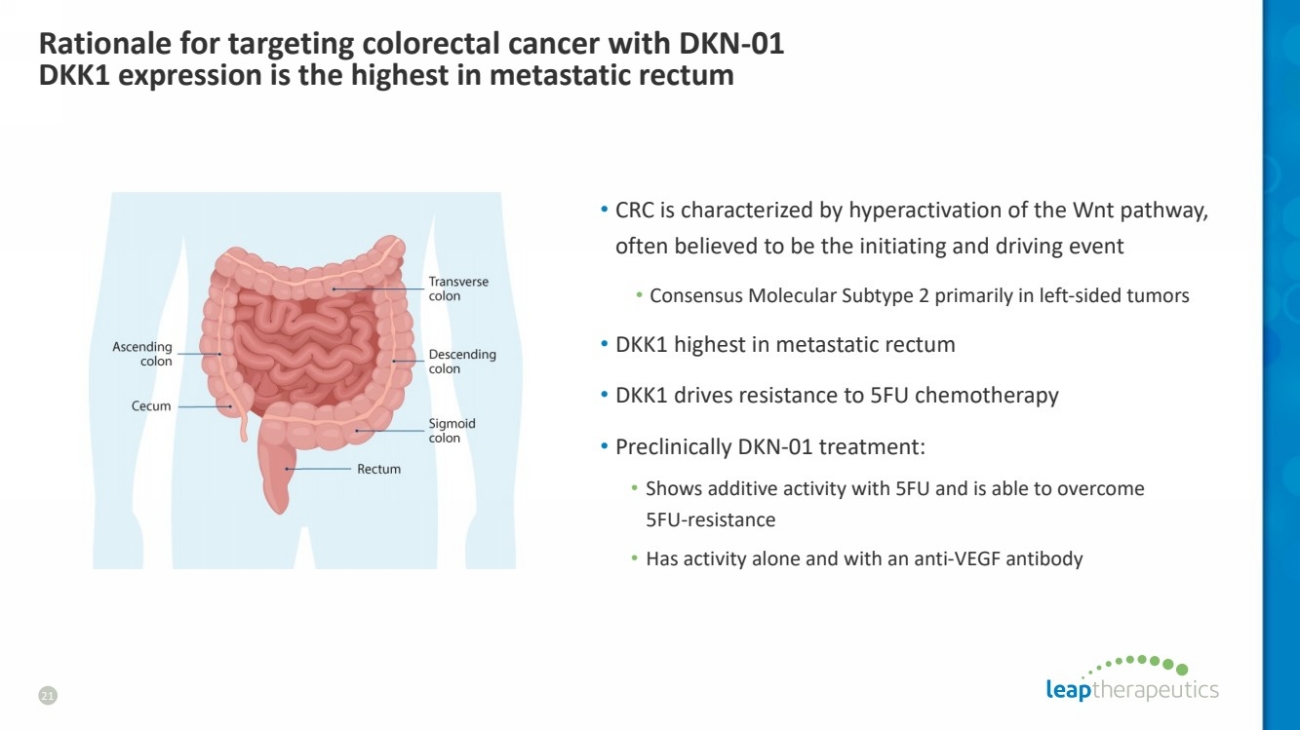
| Rationale for targeting colorectal cancer with DKN-01
DKK1 expression is the highest in metastatic rectum
21
• CRC is characterized by hyperactivation of the Wnt pathway,
often believed to be the initiating and driving event
• Consensus Molecular Subtype 2 primarily in left-sided tumors
• DKK1 highest in metastatic rectum
• DKK1 drives resistance to 5FU chemotherapy
• Preclinically DKN-01 treatment:
• Shows additive activity with 5FU and is able to overcome
5FU-resistance
• Has activity alone and with an anti-VEGF antibody |
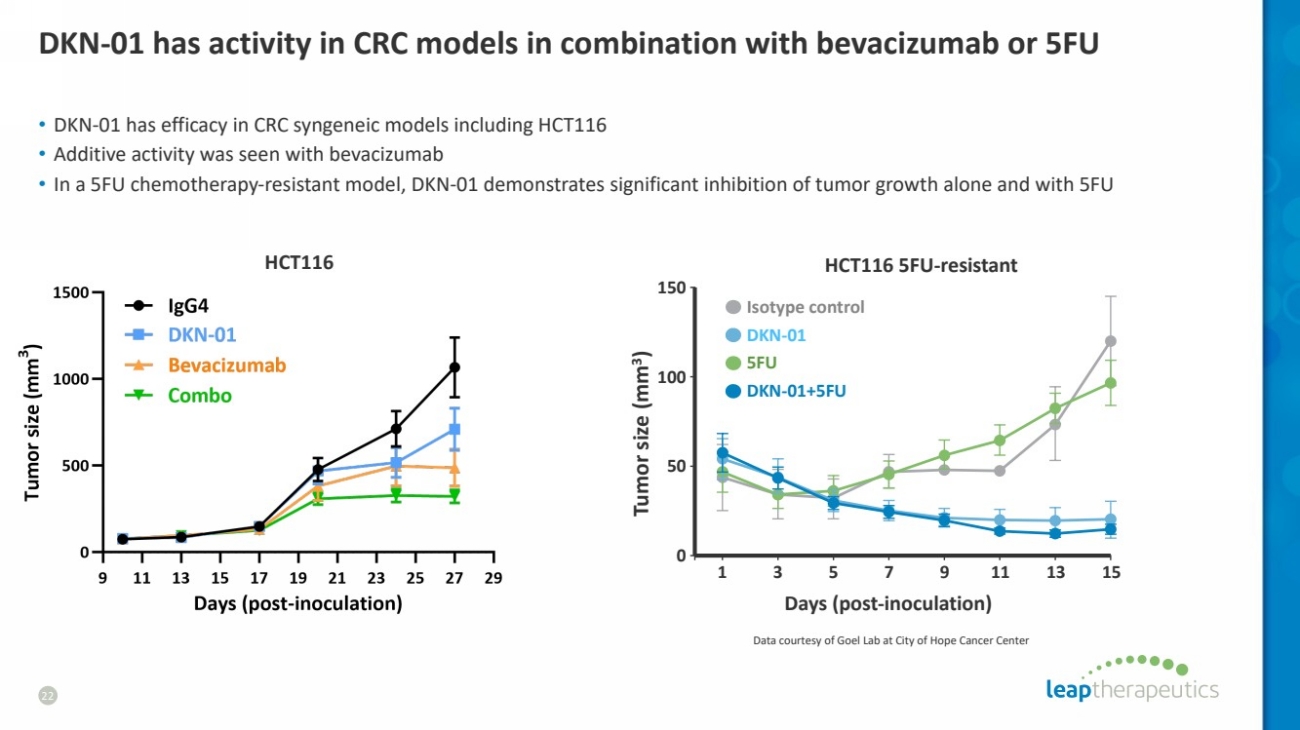
| DKN-01 has activity in CRC models in combination with bevacizumab or 5FU
HCT116
• DKN-01 has efficacy in CRC syngeneic models including HCT116
• Additive activity was seen with bevacizumab
• In a 5FU chemotherapy-resistant model, DKN-01 demonstrates significant inhibition of tumor growth alone and with 5FU
22
HCT116 5FU-resistant
150
100
50
0
Tumor size (mm
3)
Days (post-inoculation)
1 3 5 7 9 11 13 15
Isotype control
DKN-01+5FU
DKN-01
5FU
Data courtesy of Goel Lab at City of Hope Cancer Center |
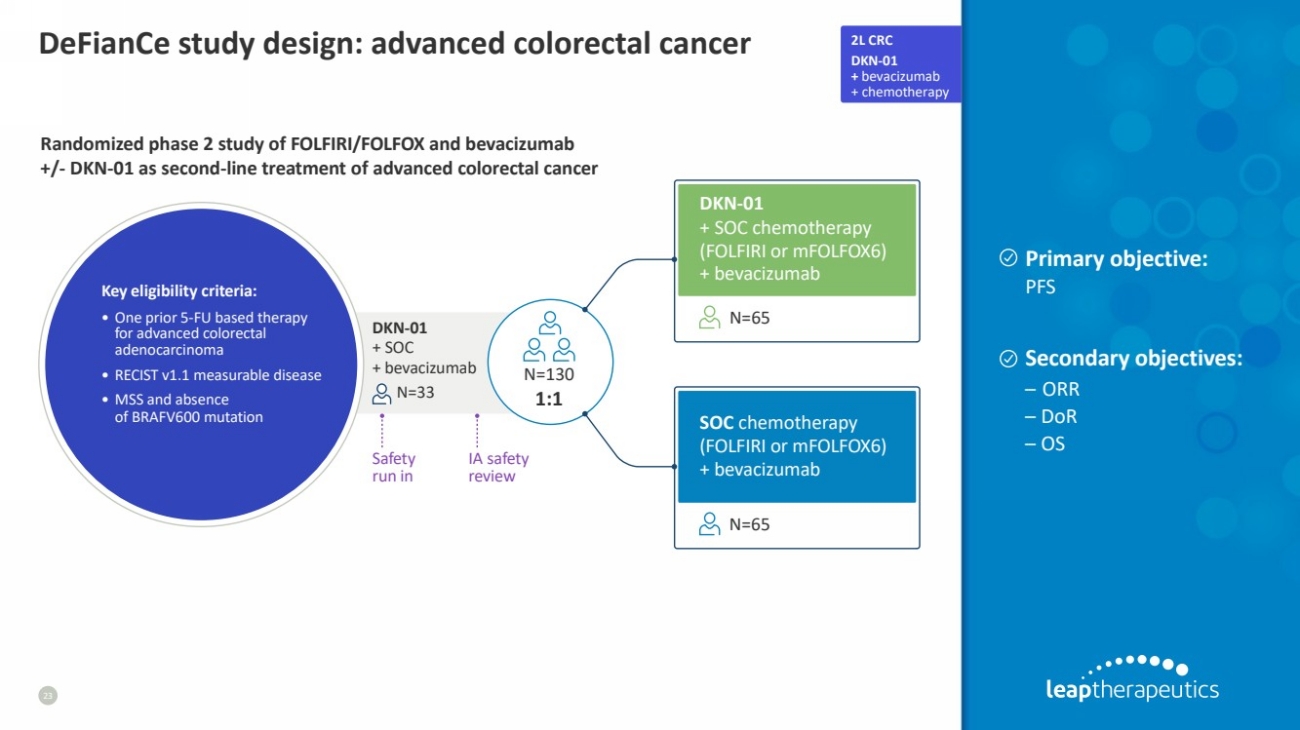
| DeFianCe study design: advanced colorectal cancer
23
Randomized phase 2 study of FOLFIRI/FOLFOX and bevacizumab
+/- DKN-01 as second-line treatment of advanced colorectal cancer
2L CRC
DKN-01
+ bevacizumab
+ chemotherapy
Primary objective:
PFS
Secondary objectives:
– ORR
– DoR
– OS
DKN-01
+ SOC chemotherapy
(FOLFIRI or mFOLFOX6)
+ bevacizumab
SOC chemotherapy
(FOLFIRI or mFOLFOX6)
+ bevacizumab
N=65
N=65
Key eligibility criteria:
• One prior 5-FU based therapy
for advanced colorectal
adenocarcinoma
• RECIST v1.1 measurable disease
• MSS and absence
of BRAFV600 mutation
N=130
1:1
Safety
run in
IA safety
review
DKN-01
+ SOC
+ bevacizumab
N=33 |
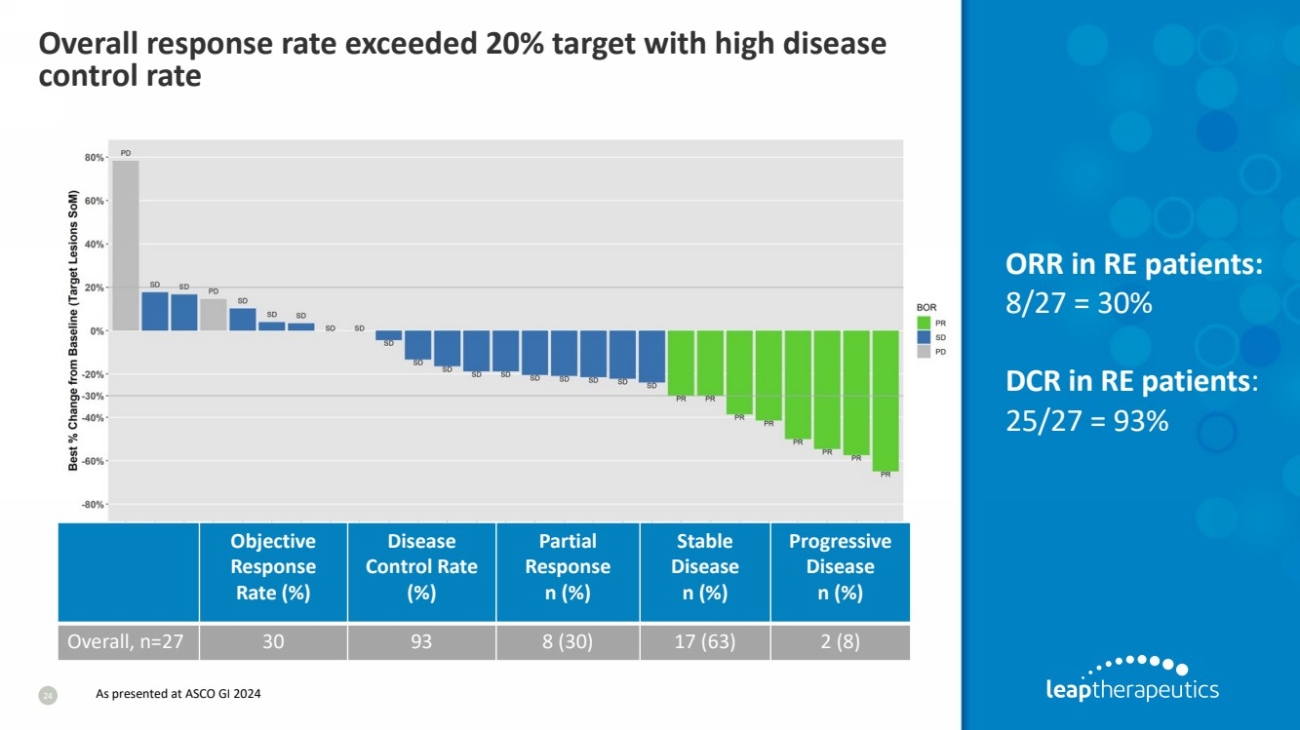
| Overall response rate exceeded 20% target with high disease
control rate
24
ORR in RE patients:
8/27 = 30%
DCR in RE patients:
25/27 = 93%
Objective
Response
Rate (%)
Disease
Control Rate
(%)
Partial
Response
n (%)
Stable
Disease
n (%)
Progressive
Disease
n (%)
Overall, n=27 30 93 8 (30) 17 (63) 2 (8)
As presented at ASCO GI 2024 |
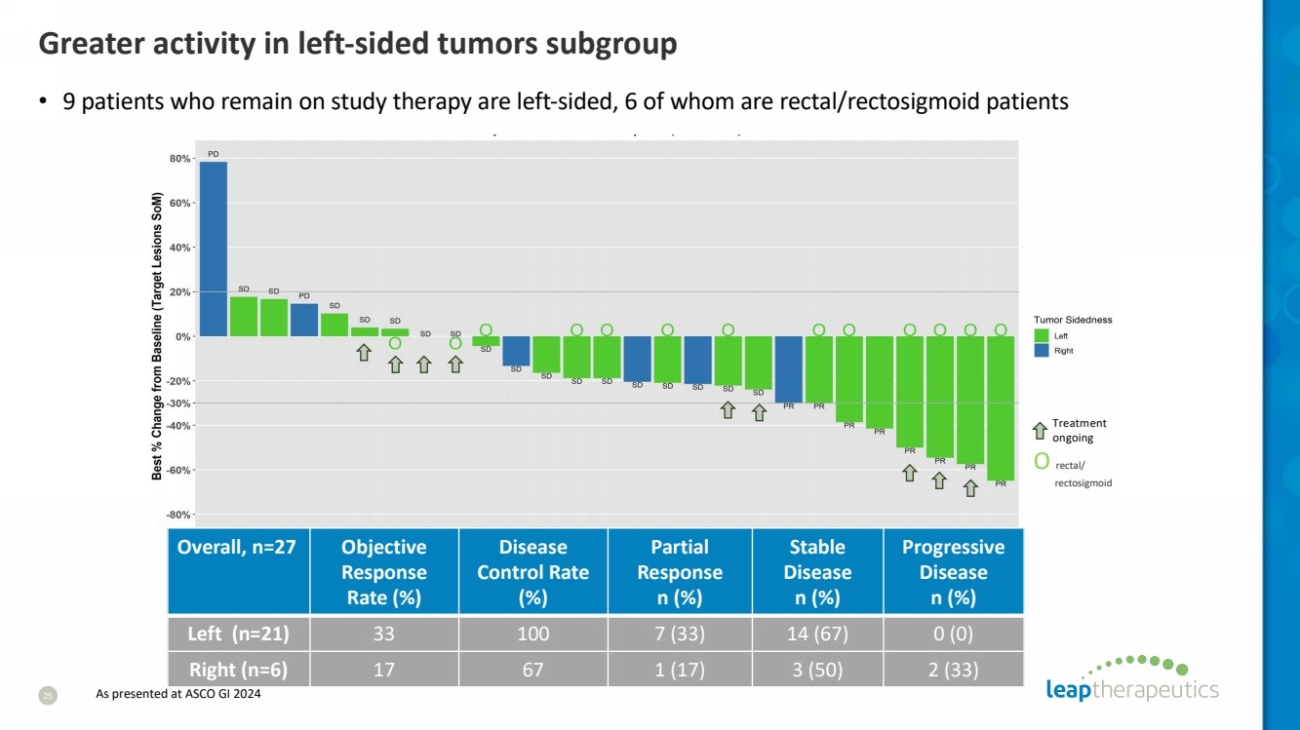
| Greater activity in left-sided tumors subgroup
25
• 9 patients who remain on study therapy are left-sided, 6 of whom are rectal/rectosigmoid patients
Treatment
ongoing
Overall, n=27 Objective
Response
Rate (%)
Disease
Control Rate
(%)
Partial
Response
n (%)
Stable
Disease
n (%)
Progressive
Disease
n (%)
Left (n=21) 33 100 7 (33) 14 (67) 0 (0)
Right (n=6) 17 67 1 (17) 3 (50) 2 (33)
O rectal/
rectosigmoid
As presented at ASCO GI 2024 |
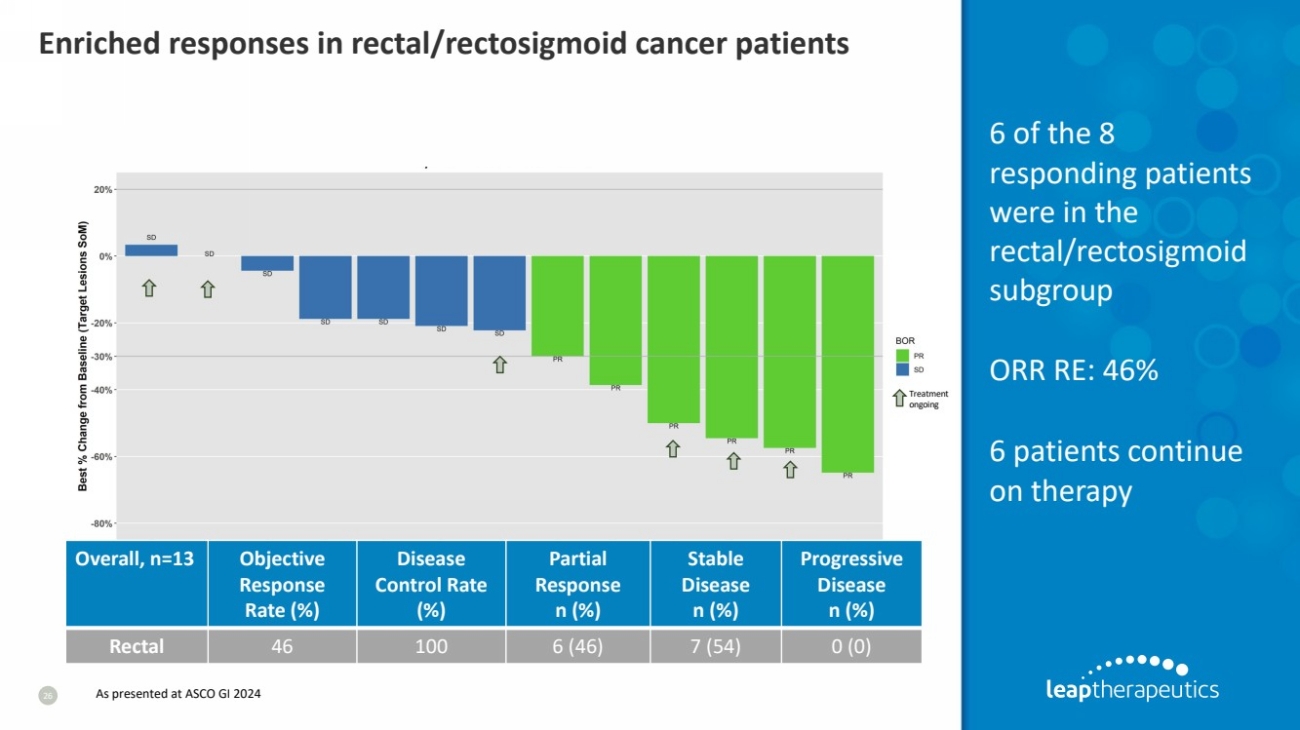
| Enriched responses in rectal/rectosigmoid cancer patients
26
Overall, n=13 Objective
Response
Rate (%)
Disease
Control Rate
(%)
Partial
Response
n (%)
Stable
Disease
n (%)
Progressive
Disease
n (%)
Rectal 46 100 6 (46) 7 (54) 0 (0)
6 of the 8
responding patients
were in the
rectal/rectosigmoid
subgroup
ORR RE: 46%
6 patients continue
on therapy
Treatment
ongoing
As presented at ASCO GI 2024 |

| 27
Duration of clinical benefit
Tumor sidedness subgroup
As presented at ASCO GI 2024 |
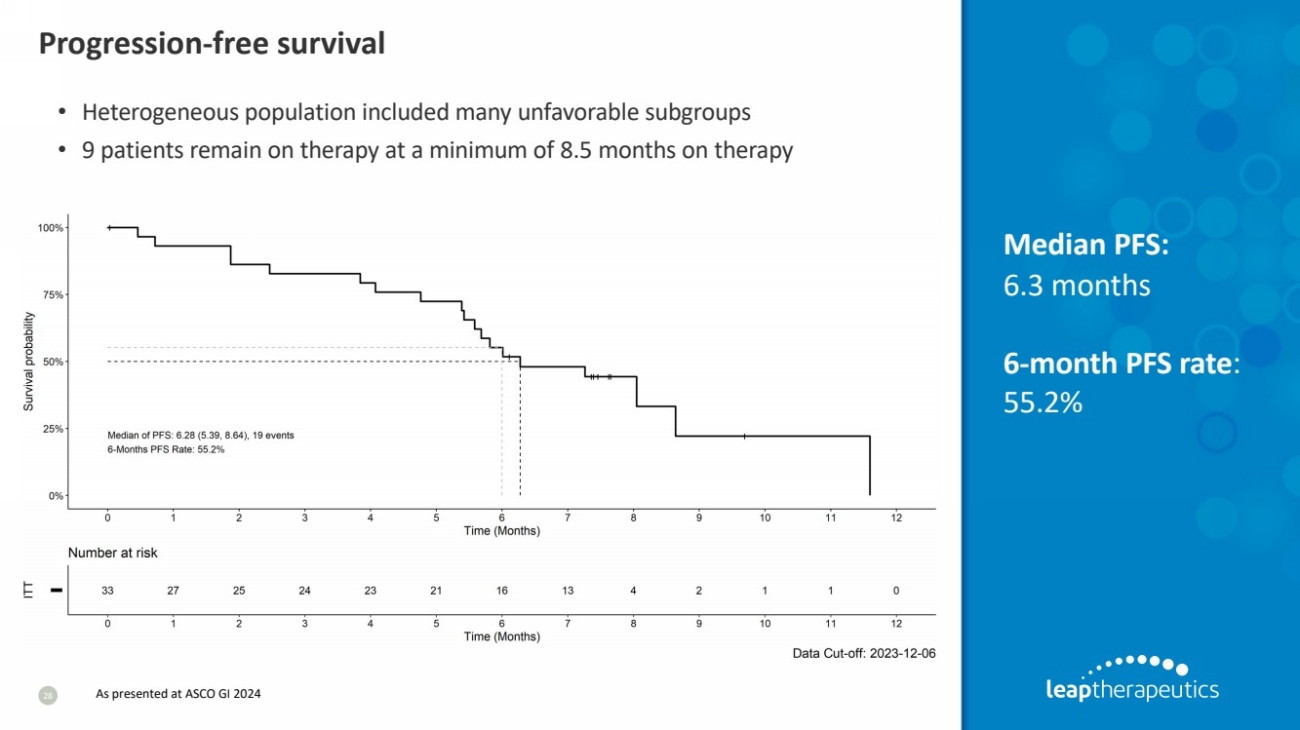
| Progression-free survival
28
Median PFS:
6.3 months
6-month PFS rate:
55.2%
• Heterogeneous population included many unfavorable subgroups
• 9 patients remain on therapy at a minimum of 8.5 months on therapy
As presented at ASCO GI 2024 |
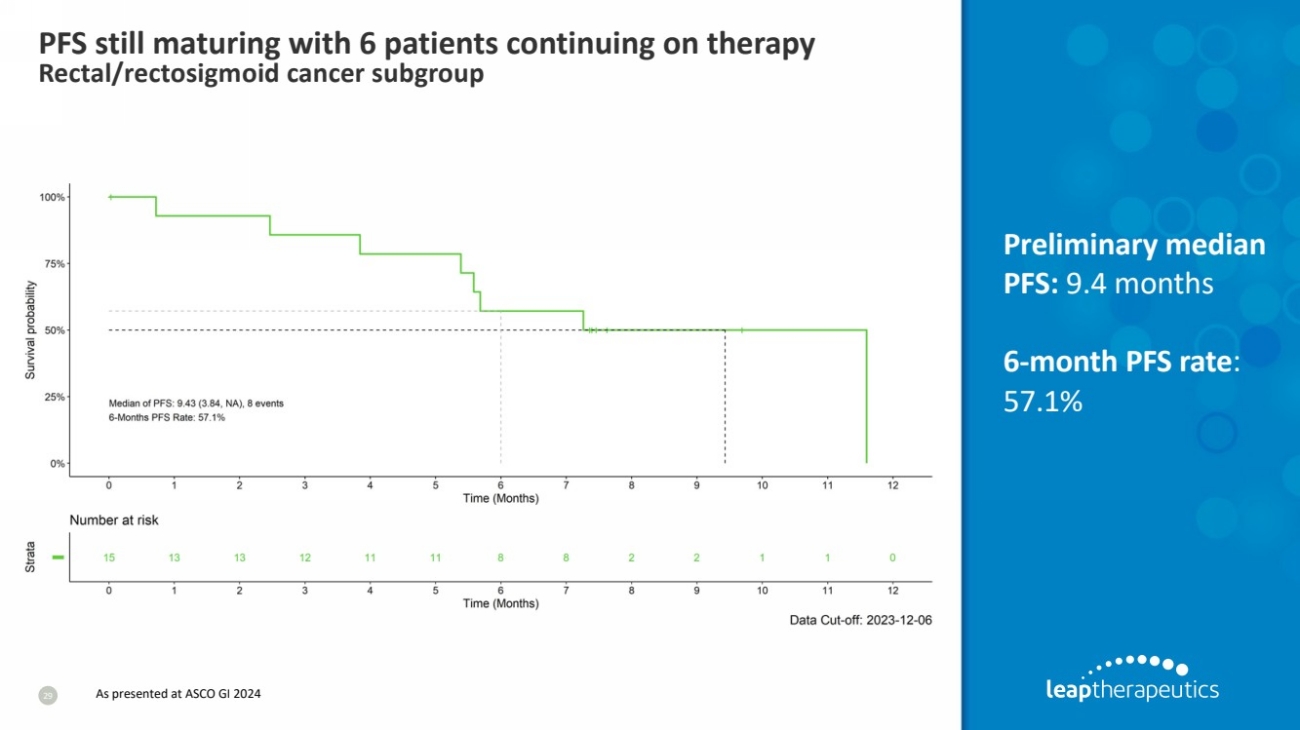
| PFS still maturing with 6 patients continuing on therapy
Rectal/rectosigmoid cancer subgroup
29
Preliminary median
PFS: 9.4 months
6-month PFS rate:
57.1%
As presented at ASCO GI 2024 |
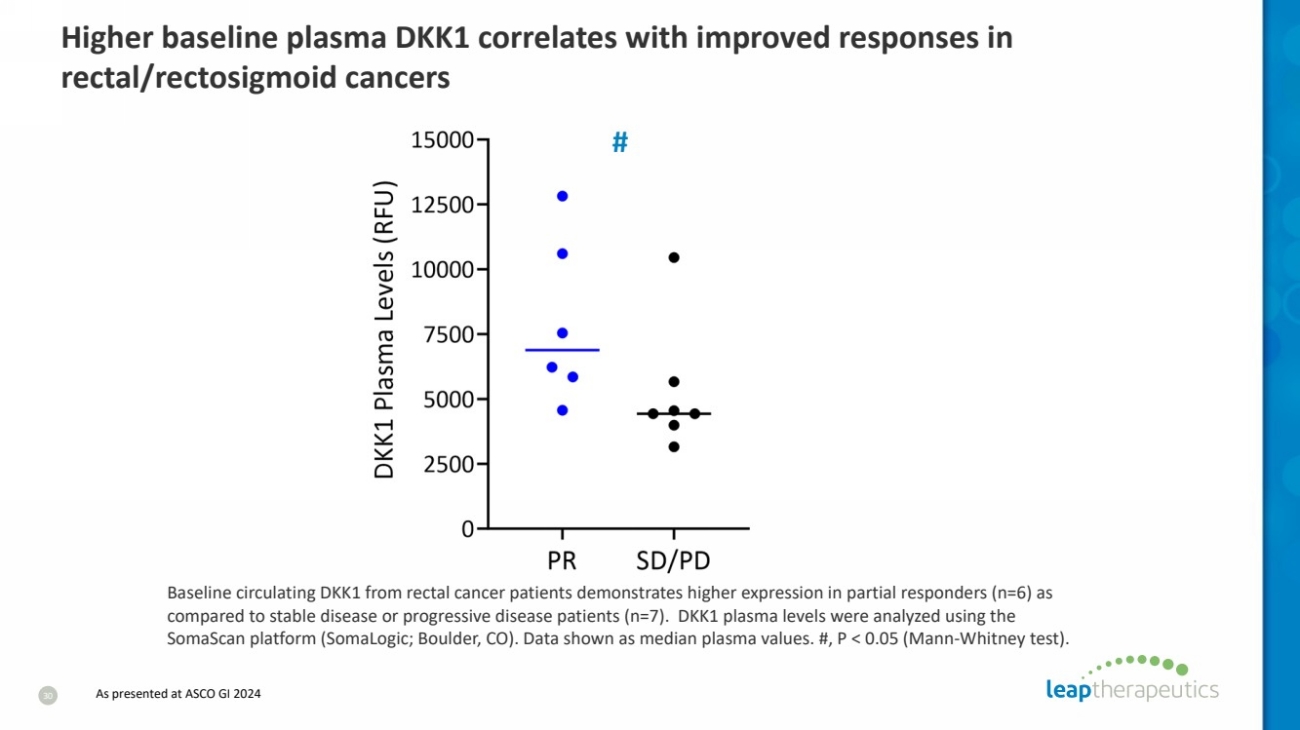
| Baseline circulating DKK1 from rectal cancer patients demonstrates higher expression in partial responders (n=6) as
compared to stable disease or progressive disease patients (n=7). DKK1 plasma levels were analyzed using the
SomaScan platform (SomaLogic; Boulder, CO). Data shown as median plasma values. #, P < 0.05 (Mann-Whitney test).
#
Higher baseline plasma DKK1 correlates with improved responses in
rectal/rectosigmoid cancers
30 As presented at ASCO GI 2024 |

| DKN-01
Endometrial cancer development |
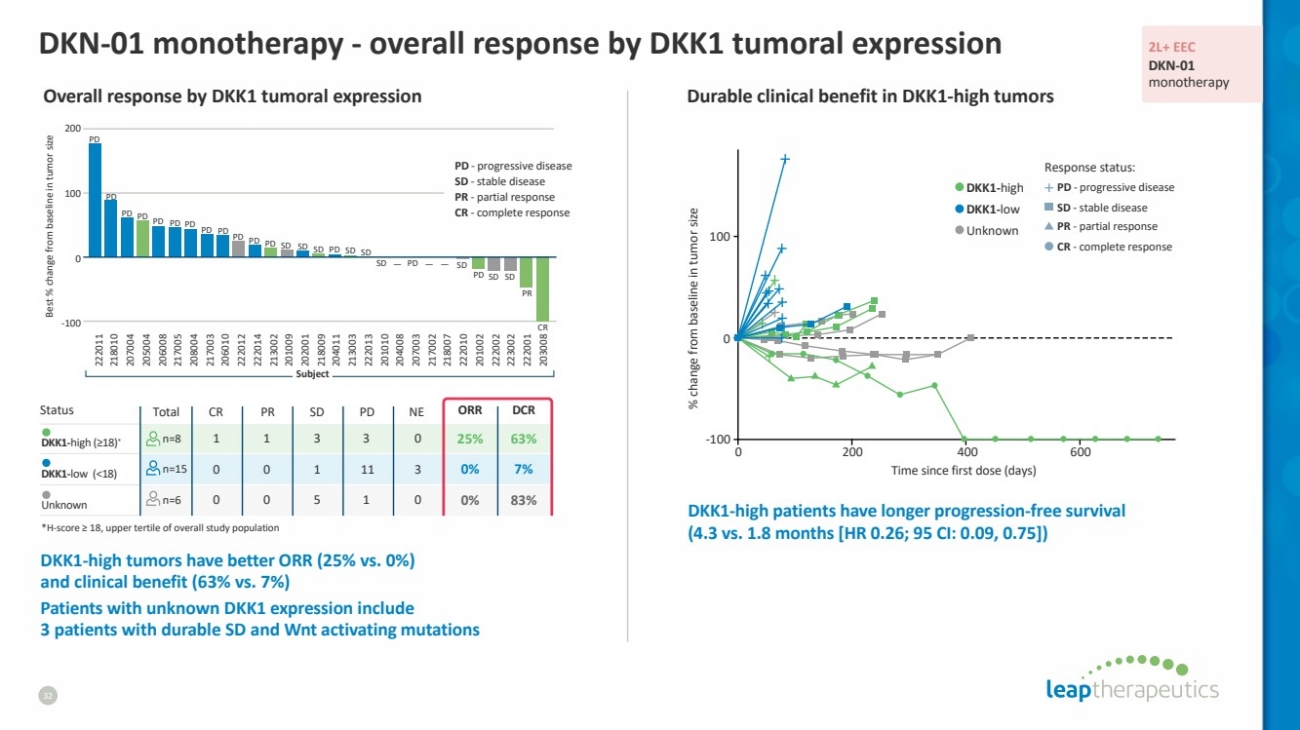
| Status Total CR PR SD PD NE ORR DCR
DKK1-high (≥18)* n=8 1 1 3 3 0 25% 63%
DKK1-low (<18) n=15 0 0 1 11 3 0% 7%
Unknown n=6 0 0 5 1 0 0% 83%
DKN-01 monotherapy - overall response by DKK1 tumoral expression
32
CR
PR
SD SD
PD
PD
PD PD PD PD
PD
PD PD PD PD PD PD
SD
SD SD
— PD —
PD SD SD SD
SD —
*H-score ≥ 18, upper tertile of overall study population
DKK1-high tumors have better ORR (25% vs. 0%)
and clinical benefit (63% vs. 7%)
Patients with unknown DKK1 expression include
3 patients with durable SD and Wnt activating mutations
Overall response by DKK1 tumoral expression
100
0
-100
200
Best
% change from baseline in tumor size
Subject
PD - progressive disease
SD - stable disease
PR - partial response
CR - complete response
Durable clinical benefit in DKK1-high tumors
222011
218010
207004
205004
206008
217005
208004
217003
206010
222012
222014
213002
201009
202001
218009
204011
213003
222013
201010
204008
207003
217002
218007
222010
201002
222002
223002
222001
203008
DKK1-high patients have longer progression-free survival
(4.3 vs. 1.8 months [HR 0.26; 95 CI: 0.09, 0.75])
0
-100
0 200 600
Time since first dose (days)
100
% change from baseline in tumor size
DKK1-high
DKK1-low
Unknown
Response status:
PD - progressive disease
SD - stable disease
PR - partial response
CR - complete response
400
2L+ EEC
DKN-01
monotherapy |
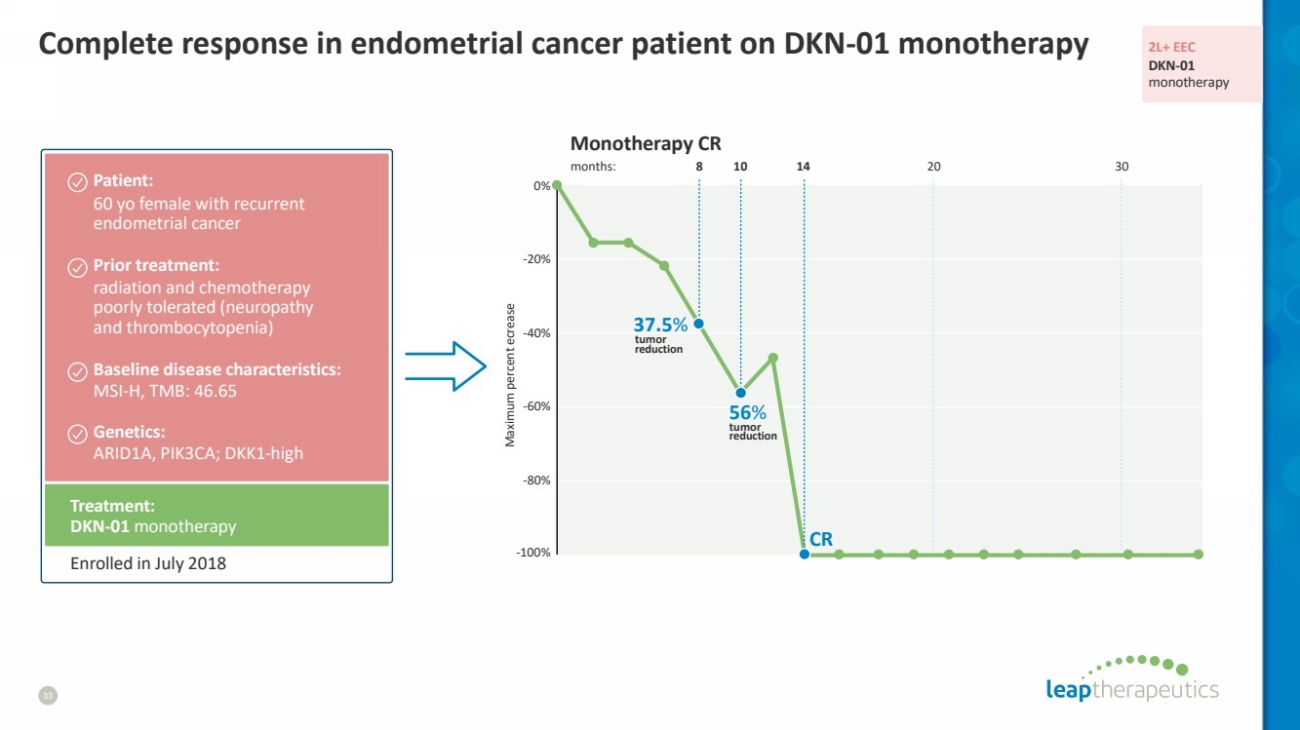
| Complete response in endometrial cancer patient on DKN-01 monotherapy
33
Maximum percent ecrease -100%
-60%
-20%
-80%
-40%
0%
37.5%
56%
tumor
reduction
CR
Treatment:
DKN-01 monotherapy
Patient:
60 yo female with recurrent
endometrial cancer
Prior treatment:
radiation and chemotherapy
poorly tolerated (neuropathy
and thrombocytopenia)
Baseline disease characteristics:
MSI-H, TMB: 46.65
Genetics:
ARID1A, PIK3CA; DKK1-high
Enrolled in July 2018
months: 30
Monotherapy CR
tumor
reduction
8 10 14 20
2L+ EEC
DKN-01
monotherapy |
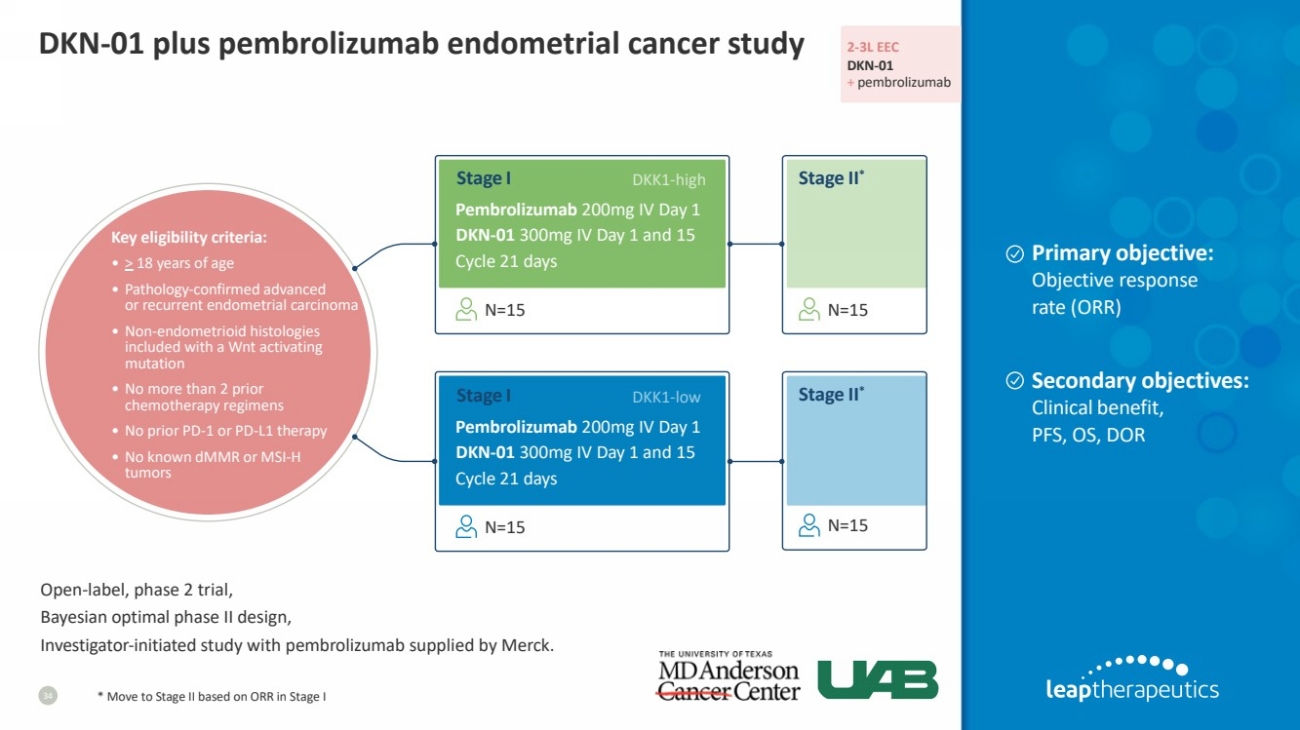
| DKN-01 plus pembrolizumab endometrial cancer study
34 * Move to Stage II based on ORR in Stage I
2-3L EEC
DKN-01
+ pembrolizumab
Key eligibility criteria:
• > 18 years of age
• Pathology-confirmed advanced
or recurrent endometrial carcinoma
• Non-endometrioid histologies
included with a Wnt activating
mutation
• No more than 2 prior
chemotherapy regimens
• No prior PD-1 or PD-L1 therapy
• No known dMMR or MSI-H
tumors
Primary objective:
Objective response
rate (ORR)
Secondary objectives:
Clinical benefit,
PFS, OS, DOR
Open-label, phase 2 trial,
Bayesian optimal phase II design,
Investigator-initiated study with pembrolizumab supplied by Merck.
Pembrolizumab 200mg IV Day 1
DKN-01 300mg IV Day 1 and 15
Cycle 21 days
Pembrolizumab 200mg IV Day 1
DKN-01 300mg IV Day 1 and 15
Cycle 21 days
DKK1-high
DKK1-low
N=15 N=15
N=15 N=15
Stage I Stage II*
Stage II* Stage I |

| FL-301 (NBL-015)
FL-302 (NBL-016)
Anti-Claudin18.2
antibodies |
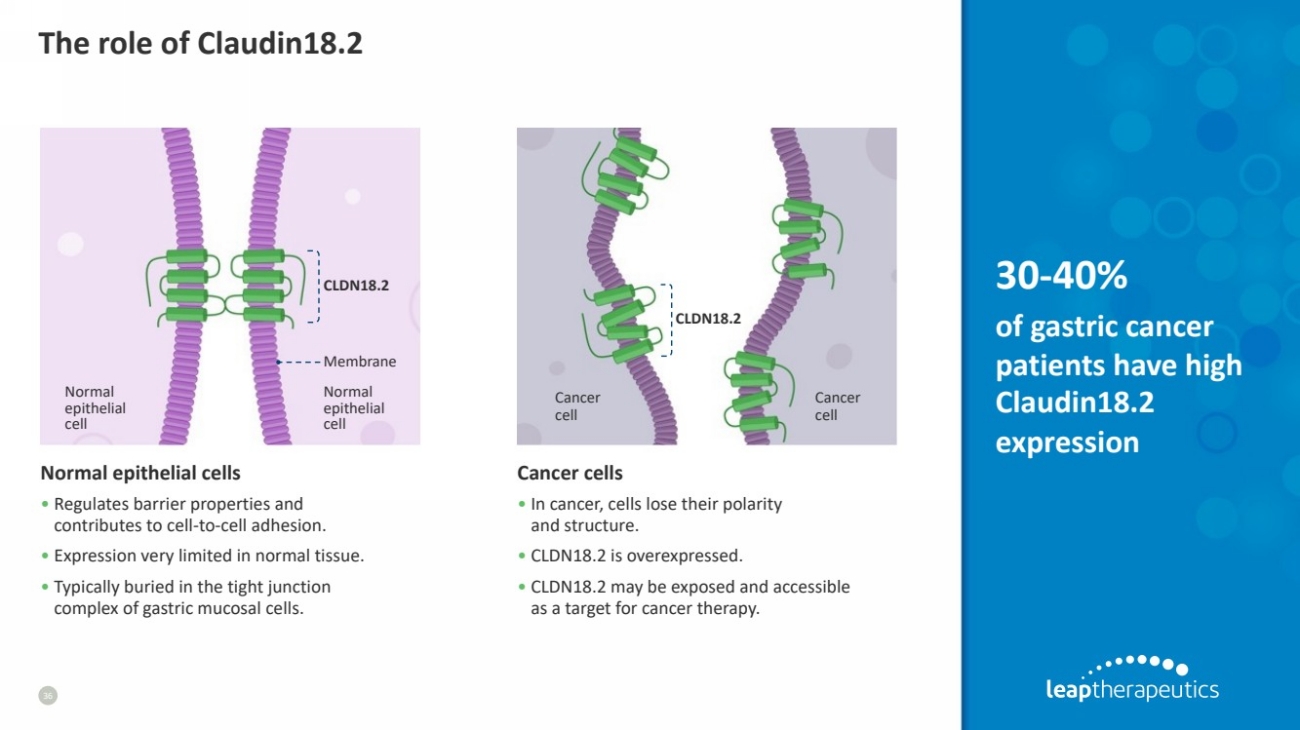
| The role of Claudin18.2
36
Normal epithelial cells
• Regulates barrier properties and
contributes to cell-to-cell adhesion.
• Expression very limited in normal tissue.
• Typically buried in the tight junction
complex of gastric mucosal cells.
Cancer cells
• In cancer, cells lose their polarity
and structure.
• CLDN18.2 is overexpressed.
• CLDN18.2 may be exposed and accessible
as a target for cancer therapy.
CLDN18.2
CLDN18.2
Membrane
Normal
epithelial
cell
Normal
epithelial
cell
Cancer
cell
Cancer
cell
30-40%
of gastric cancer
patients have high
Claudin18.2
expression |
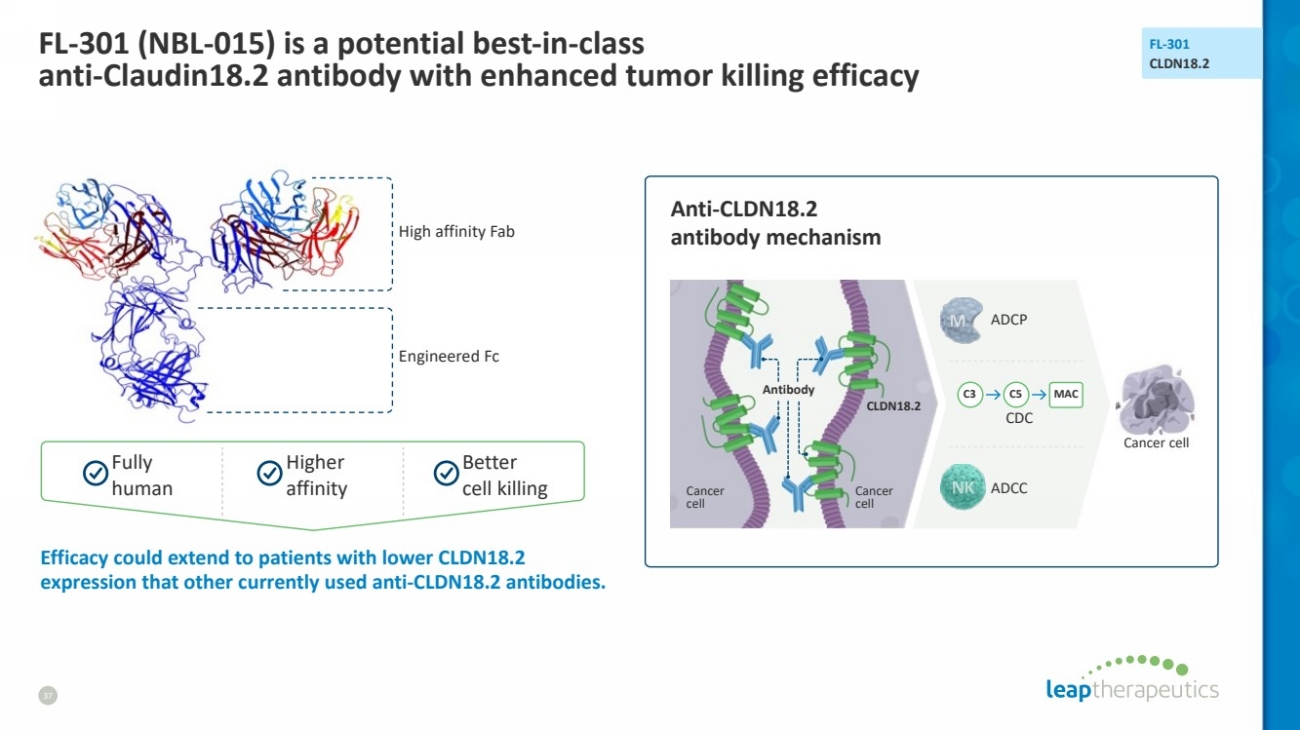
| FL-301 (NBL-015) is a potential best-in-class
anti-Claudin18.2 antibody with enhanced tumor killing efficacy
37
FL-301
CLDN18.2
Efficacy could extend to patients with lower CLDN18.2
expression that other currently used anti-CLDN18.2 antibodies.
Engineered Fc
High affinity Fab
Higher
affinity
Fully
human
Anti-CLDN18.2
antibody mechanism
CLDN18.2
Antibody
Cancer cell
Cancer
cell
Better
cell killing
CDC
ADCP
C3 C5 MAC
Cancer ADCC
cell |
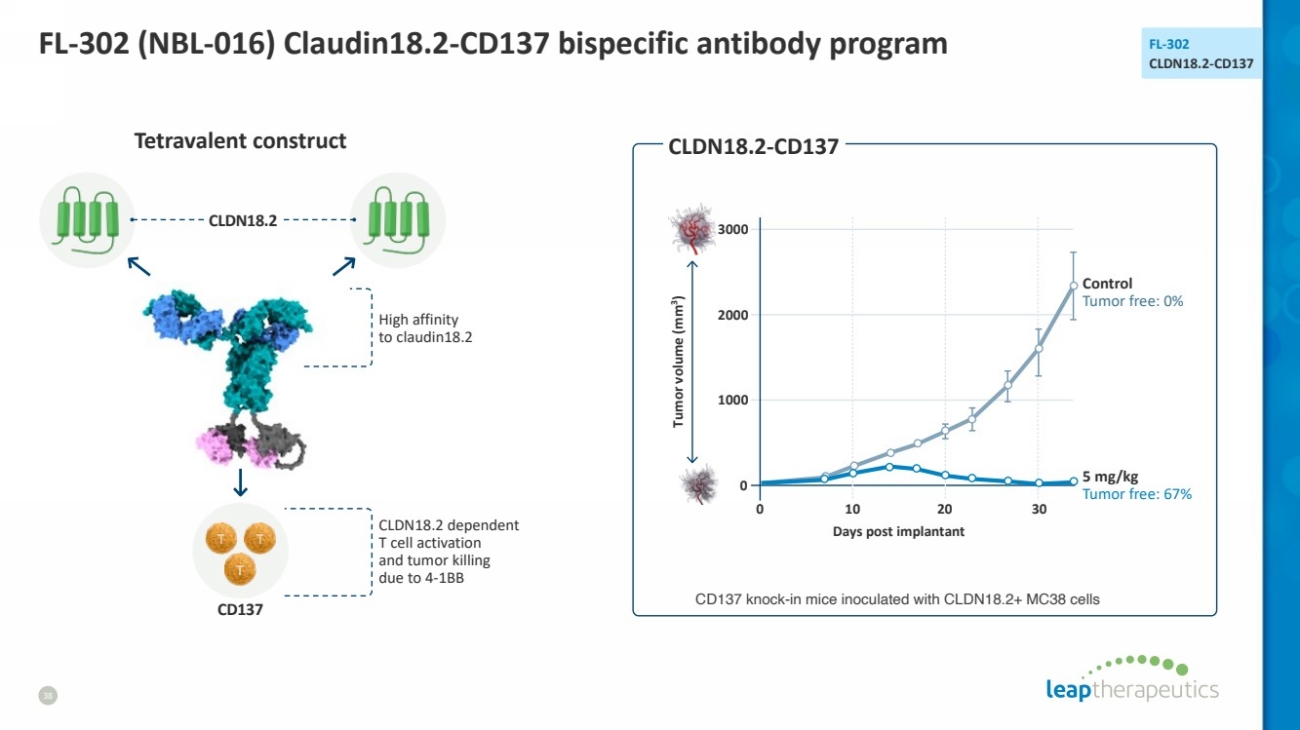
| FL-302 (NBL-016) Claudin18.2-CD137 bispecific antibody program
38
FL-302
CLDN18.2-CD137
0 30
3000
2000
1000
0
Tumor volume (mm3
)
Days post implantant
Control
Tumor free: 0%
5 mg/kg
Tumor free: 67%
Tetravalent construct СLDN18.2-CD137
High affinity
to claudin18.2
СLDN18.2
CD137
CLDN18.2 dependent
T cell activation
and tumor killing
due to 4-1BB
10 20
CD137 knock-in mice inoculated with CLDN18.2+ MC38 cells |

| FL-501
Anti-GDF15 monoclonal
antibody |
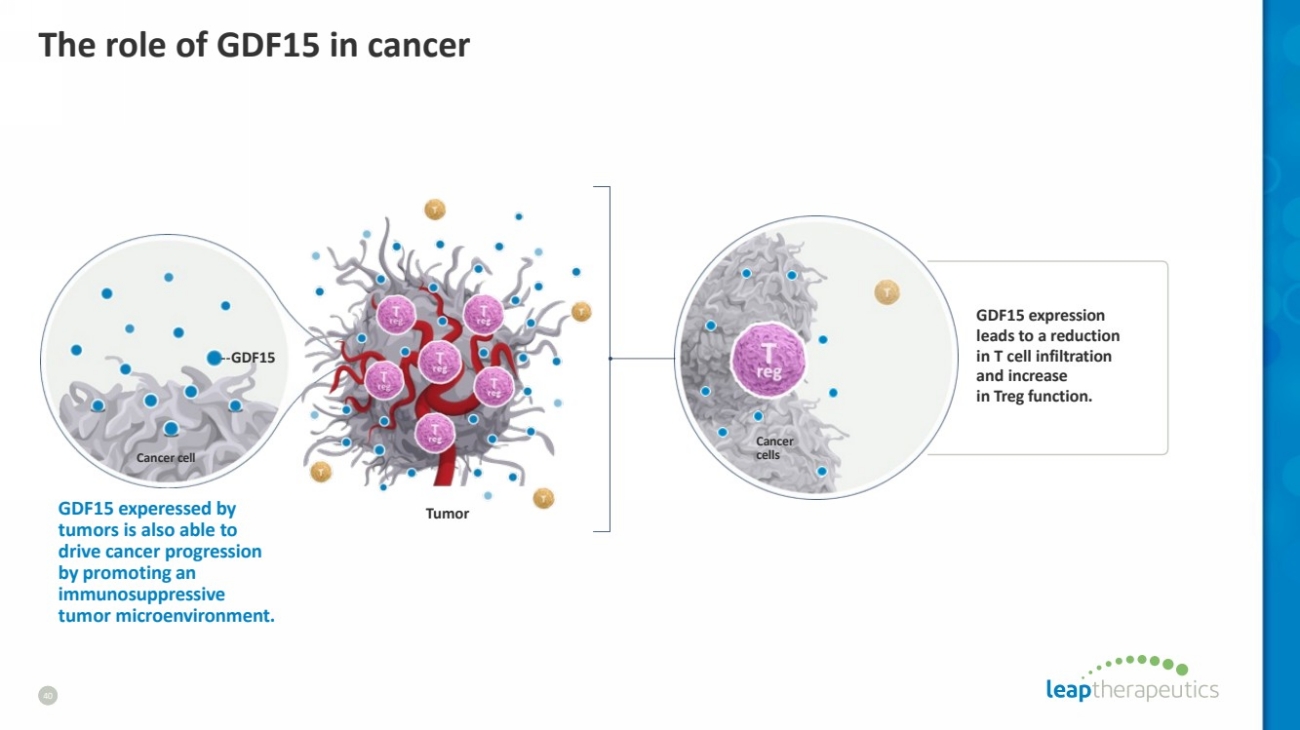
| The role of GDF15 in cancer
40
Cancer
cells
GDF15 expression
leads to a reduction
in T cell infiltration
and increase
in Treg function.
Cancer cell
GDF15
GDF15 experessed by
tumors is also able to
drive cancer progression
by promoting an
immunosuppressive
tumor microenvironment.
Tumor |
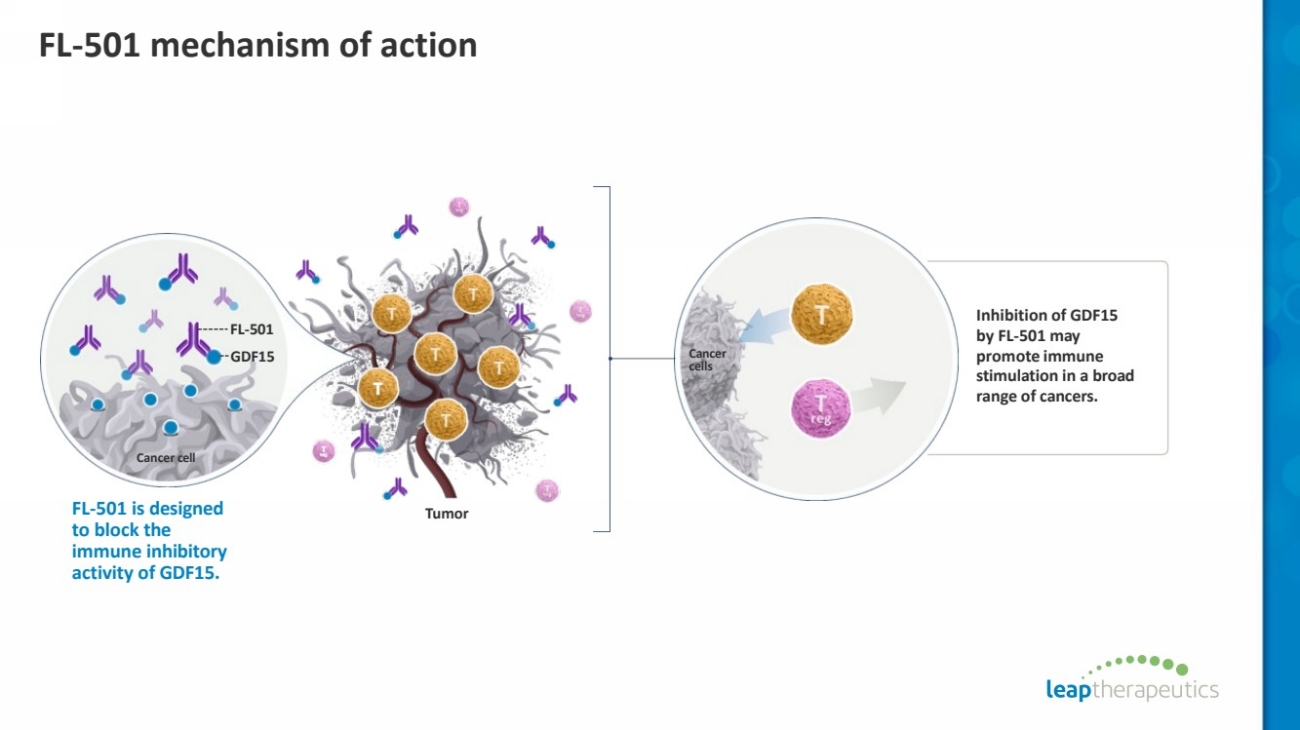
| FL-501 mechanism of action
Cancer
cells
Inhibition of GDF15
by FL-501 may
promote immune
stimulation in a broad
range of cancers. T
T
reg
FL Tumor -501 is designed
to block the
immune inhibitory
activity of GDF15.
Cancer cell
FL-501
GDF15 |
| CORPORATE |
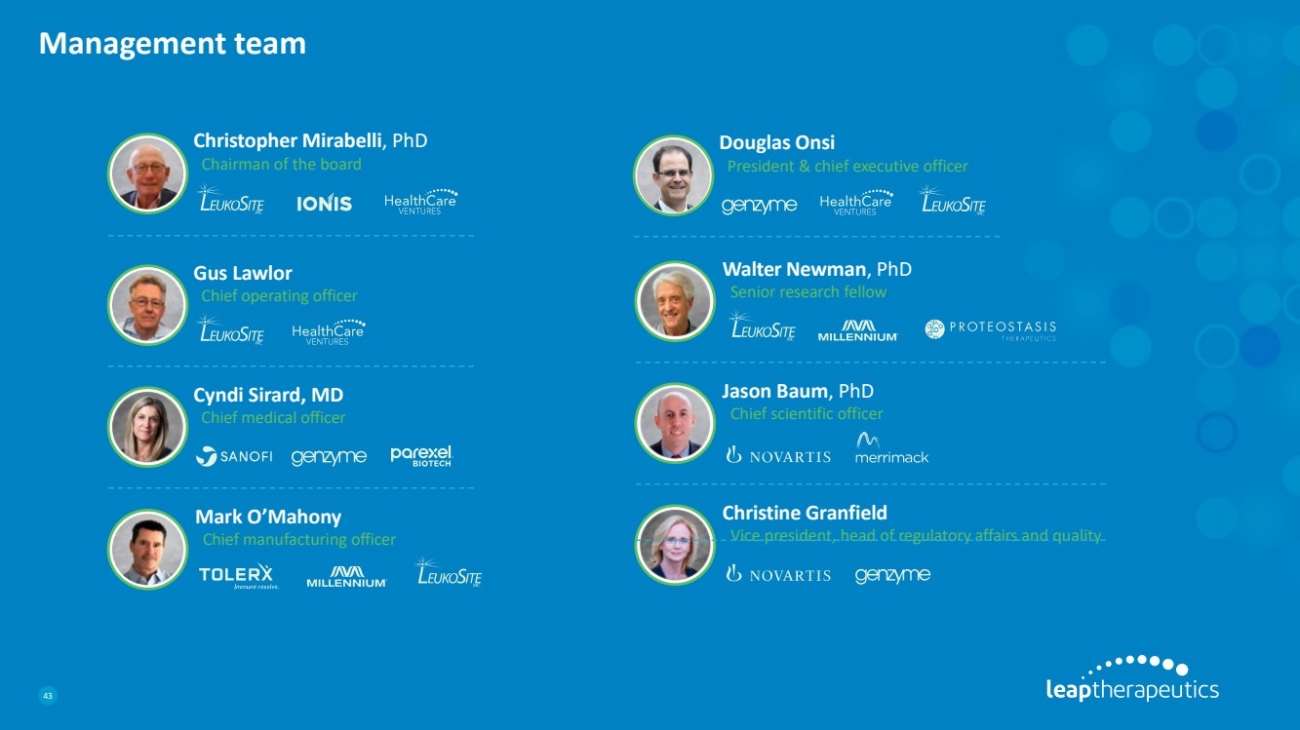
| Management team
43
Christine Granfield
Vice president, head of regulatory affairs and quality
Cyndi Sirard, MD
Chief medical officer
Jason Baum, PhD
Chief scientific officer
Walter Newman, PhD
Senior research fellow
Douglas Onsi
President & chief executive officer
Mark O’Mahony
Chief manufacturing officer
Christopher Mirabelli, PhD
Chairman of the board
Gus Lawlor
Chief operating officer |
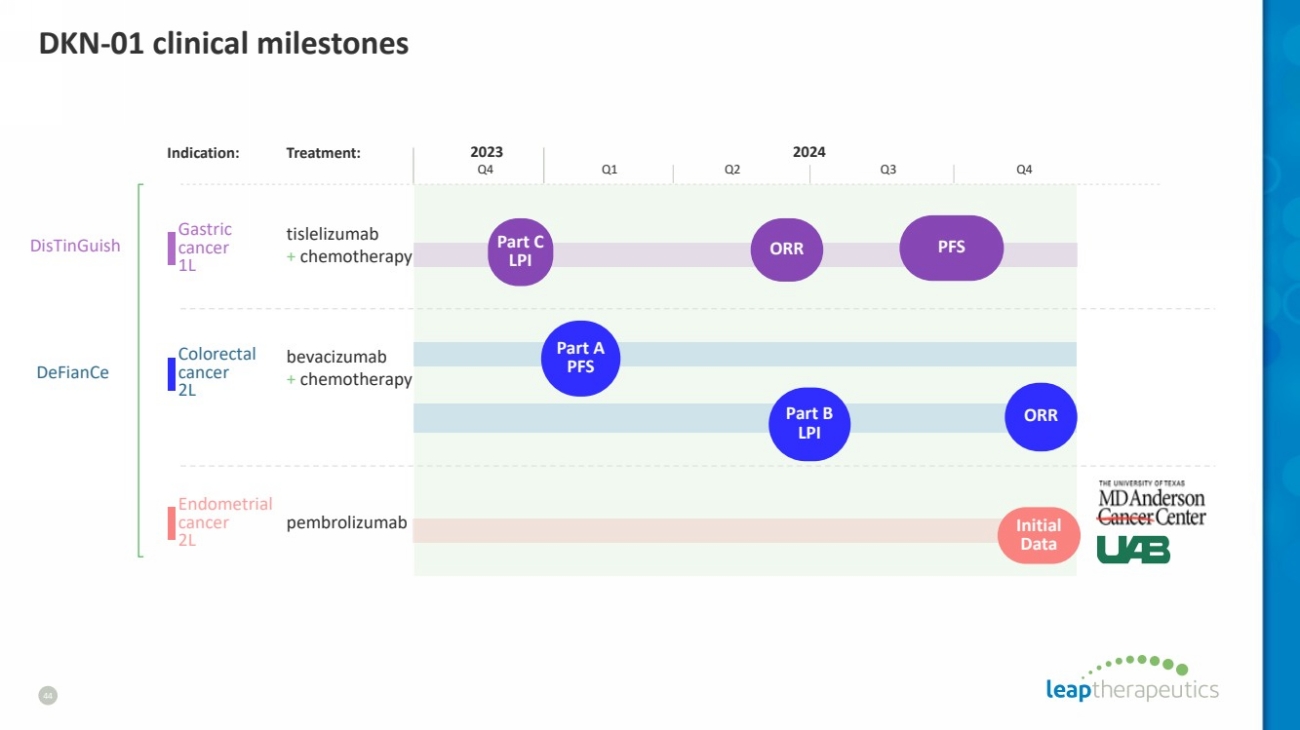
| DKN-01 clinical milestones
44
Indication: Treatment:
tislelizumab
+ chemotherapy
bevacizumab
+ chemotherapy
pembrolizumab
Gastric
cancer
1L
Colorectal
cancer
2L
Endometrial
cancer
2L
Q4
2023 2024
Q1 Q2 Q3 Q4
ORR PFS
Initial
Data
Part A
PFS
Part B
LPI
ORR
Part C
LPI
DisTinGuish
DeFianCe |
Cover
|
Jan. 23, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 23, 2024
|
| Entity File Number |
001-37990
|
| Entity Registrant Name |
Leap Therapeutics, Inc.
|
| Entity Central Index Key |
0001509745
|
| Entity Tax Identification Number |
27-4412575
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
47 Thorndike Street
|
| Entity Address, Address Line Two |
Suite B1-1
|
| Entity Address, City or Town |
Cambridge
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02141
|
| City Area Code |
617
|
| Local Phone Number |
714-0360
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.001
|
| Trading Symbol |
LPTX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Leap Therapeutics (NASDAQ:LPTX)
過去 株価チャート
から 10 2024 まで 11 2024

Leap Therapeutics (NASDAQ:LPTX)
過去 株価チャート
から 11 2023 まで 11 2024




