Exhibit 99.1
Inhibitor Therapeutics, Inc. Exclusive License with Johns Hopkins University
A critical milestone completed on the mission path of commercializing Itraconazole in numerous oncology indications.
Inhibitor Therapeutics, Inc. (“Inhibitor”) (OTCQB:INTI) has entered into an exclusive, worldwide licensing agreement (the “License”) with
Johns Hopkins University (JHU) for their U.S Patent 8,980,930 (Canada Patent 2,572,223) “New Angiogenesis Inhibitors”. Angiogenesis Inhibitors, as described by the National Cancer Institute, are unique cancer fighting agents as they block
the growth of blood vessels that support tumor growth rather than blocking the growth of the tumor cells themselves. Inventors affiliated with JHU developed this patent, listing Itraconazole as an Active Pharmaceutical Ingredient (API) that has
anti-angiogenic properties.
The License notes field of use is for use in Prostate Cancer, Basal Cell Carcinoma including Basal Cell Carcinoma Nevus
Syndrome (an orphan oncology disease), and Lung Cancer, all of which emphasize a significant unmet need. Inhibitor believes the License is a mutually beneficial agreement, yielding a modest annual royalty rate with milestone payments typical to such
a license.
Within the literature Head et al1 explains the long-term recognition that
angiogenesis is a requirement for tumor growth and metastasis and that growing tumors can promote angiogenesis by secreting proangiogenic factors such as VEGF, basic FGF, EGF and others. These proangiogenic factors stimulate the proliferation,
migration, and differentiation of the endothelial cells that make up the inner layer of all blood vessels, causing them to form new vessels that grow towards the source of these factors, this process termed “tumor angiogenesis”. It is
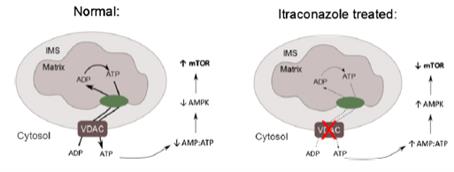
identified that the major target of itraconazole in endothelial cells is VDAC1. VDAC1 knockdown profoundly inhibits mTOR activity and cell proliferation in HUVEC revealing a previously unknown connection. Inhibition of VDAC1 by itraconazole leads to
an increase in cellular AMP:ATP ratio and activation of the AMP-activated protein kinase (AMPK), an upstream regulator of mTOR. VDAC1-knockout cells are resistant to AMPK activation and mTOR inhibition by
itraconazole, demonstrating that VDAC1 is the mediator of this activity. In their testing it was found that by using a phenotypical approach starting with the effect of itraconazole the G1-S cell cycle
transition of the endothelial cells, itraconazole specifically inhibited the mTOR signaling pathway by downregulating the kinase activity of mTORC1.
Chow Et Al2 completed a study utilizing RNA
sequencing to decipher the biological pathway propelling BCC growth. From the assay results, it was observed that BCCs exhibited a considerable amplification in the expression of the mTOR pathway. This pathway plays an essential role in regulating
angiogenesis, the growth of new blood vessels from pre-existing ones. Thus, this indicates that it is the heightened activity of the mTOR pathway, and the consequent enhancement of angiogenesis, that
stimulates the growth.
Data from Inhibitor’s completed Phase 2b SCORING Trial complements the literature with reference to the Lesion Response of
the study which shows that across the 477 target lesions the investigators reported reductions of any size from baseline for 399 (83.65%) lesions, 64 (13.42%) had no change and 14 increased in size. A
pre-determined reduction of 30% or greater was seen in 275 of the 477 (57.7%) target lesions, including 130 lesions which resolved completely (27.3%). A total of 13 new ‘surgically eligible’ lesions
across 8 of the 38 patients developed over the duration of the study.