NASDAQ false 0001501756 0001501756 2024-11-18 2024-11-18
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
November 18, 2024
Date of Report (Date of earliest event reported)
Adverum Biotechnologies, Inc.
(Exact Name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-36579 |
|
20-5258327 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification Number) |
100 Cardinal Way
Redwood City, CA 94063
(Address of principal executive offices, including zip code)
(650) 656-9323
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title Of Each Class |
|
Trading
Symbol |
|
Name Of Each Exchange On Which Registered |
| Common Stock |
|
ADVM |
|
Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On November 18, 2024, Adverum Biotechnologies, Inc. (the “Company”) announced positive 52-week topline data from the Company’s LUNA Phase 2 clinical trial and new four-year long-term follow-up data from the Company’s OPTIC extension trial of Ixoberogene soroparvovec (“Ixo-vec”) in patients with wet age-related macular degeneration (“AMD”). In connection with the data release, the Company compiled a presentation (the “Presentation”) that includes the data referenced above. A copy of the Presentation is furnished as Exhibit 99.1. For important information about forward-looking statements, see the slide titled “Forward-Looking Statements” in Exhibit 99.1 attached hereto.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01, including Exhibit 99.1, shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission (“SEC”) made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing
As noted in Item 7.01, on November 18, 2024, the Company announced positive 52-week topline data from the Company’s LUNA Phase 2 clinical trial and new four-year long-term follow-up data from the Company’s OPTIC extension trial of Ixo-vec in patients with wet AMD. Ixo-vec is the Company’s clinical-stage gene therapy product candidate for the treatment of wet AMD.
LUNA Phase 2 Trial and OPTIC First-in-Human Trial - Background and Baseline Prior Anti-VEGF Injections
LUNA is an ongoing double-masked, randomized Phase 2 trial. 60 patients with wet AMD were randomized equally across two dose cohorts, 6E10 or 2E11 vg/eye. The trial is evaluating multiple prophylactic regimens, including topical steroid eyedrops (difluprednate) with or without Ozurdex® and with or without oral steroids. LUNA is designed to inform the selection of both the Ixo-vec dose and prophylactic regimen for Phase 3 registrational trials.
OPTIC is an ongoing, open-label, dose-ranging first-in-human trial. 30 patients with wet AMD requiring frequent IVT injections were enrolled equally across two doses, 2E11 or 6E11. Patients received either six weeks of prophylactic topical steroid eye drops or 13 days of prophylactic oral steroids. The OPTIC trial was a two-year study, with an optional 3-year extension.
The LUNA and OPTIC data cutoff dates were August 29, 2024, and August 21, 2024, respectively. At the data cutoff date for LUNA, 57 patients had completed the 52-week study visit, with 3 discontinuations due to adverse events unrelated to study drug. 23 OPTIC patients elected to participate in the OPTIC extension. At the data cutoff date for OPTIC, 21 patients had completed the 4-year study visit, with 2 discontinuations unrelated to study drug.
Both LUNA and OPTIC were designed to assess a broad wet AMD population, including hard-to-treat patients with severe disease who required frequent anti-VEGF injections before enrolling in the trial. At baseline, mean annualized prior anti-VEGF injections in the year prior to enrolling in LUNA and OPTIC were 10.1 (2.6 SD) and 9.9 (1.9 SD), respectively.
LUNA 52-week Analysis Topline Data Summary
| |
- |
Both doses of Ixo-vec maintained visual and anatomic endpoints through 52 weeks. |
| |
• |
|
Best Corrected Visual Acuity (BCVA) - least squares mean BCVA change from baseline at week 52 (95% CI)1: |
1. Excludes 1 participant at each dose with letter loss due to cataract
| |
• |
|
Central Subfield Thickness (CST) - least squares mean CST (µm) change from baseline at week 52 (95% CI): |
| |
• |
|
6E10: -10.2 (-29.0, 8.5) |
| |
• |
|
2E11: -21.9 (-40.4, -3.3) |
| |
- |
Both doses of Ixo-vec achieved an industry leading treatment burden reduction and proportion patients who were injection free through 52 weeks. |
| |
• |
|
Treatment Burden Reduction - % reduction in mean annualized anti-VEGF injections: |
| |
• |
|
6E10: 88% treatment burden reduction |
| |
• |
|
2E11: 92% treatment burden reduction |
| |
• |
|
Proportion of Patients Injection Free: |
| |
• |
|
6E10: 54% injection free, with 75% of patients with ≤1 injection |
| |
• |
|
2E11: 69% injection free, with 79% of patients with ≤1 injection |
| |
- |
Both doses of Ixo-vec were well tolerated, with local steroids effectively managing inflammation when present. |
| |
• |
|
No 6E10 patients had inflammation at week 52 or at any subsequent visit2. |
| |
• |
|
No Ixo-vec-related serious adverse events. All Ixo-vec-related AEs were either mild or moderate: no episcleritis, vasculitis, retinitis, choroiditis, vascular occlusion, or hypotony. |
| |
• |
|
The most common Ixo-vec-related AEs were dose-dependent anterior inflammation responsive to local corticosteroids and anterior pigmentary changes with no impact on vision. |
| |
• |
|
No new onset inflammation after week 30. |
2. Inflammation defined as grade ≥ 1 AC/VC cells
| |
- |
6E10 dose with topical eyedrops as prophylactic regimen selected for pivotal program, providing a predictable long-term favorable safety profile. |
| |
• |
|
No patients at 6E10 with topical eyedrops had inflammation at week 52 or at any subsequent visit. |
| |
• |
|
Only one subject had inflammation, which resolved by year 1. |
| |
- |
LUNA results underscored by sub-group analyses that support potential best-in-class product profile and position Ixo-vec for potential clinical, regulatory and commercial success. |
| |
• |
|
Demonstrated consistent benefit in both patients with ≤300 µm baseline CST (“dry”) and patients with > 300 µm baseline CST (“wet”). |
| |
• |
|
Demonstrated maintenance of visual and anatomic outcomes in injection-free patients. |
| |
• |
|
Demonstrated even more robust clinical activity in patients with less treatment burden (experienced patients with <6 injections in year prior to LUNA). |
| |
- |
Results from our LUNA patient preference survey demonstrate strong preference for Ixo-vec over prior anti-VEGF therapies and acceptability of steroid regimen. |
| |
• |
|
93% (n=56) of LUNA patients at 52 weeks prefer Ixo-vec, including accompanying steroid regimen, over prior treatments. Patient preference for Ixo-vec over prior treatments increased over time, from 88% (n=57) at 26 weeks. |
| |
• |
|
95% (n=56) of LUNA patients would elect to receive Ixo-vec in the other eye if both eyes had wet AMD. |
| |
• |
|
96% (n=56) of LUNA patients would recommend Ixo-vec to their family or friends with wet AMD. |
| |
• |
|
100% (n=10) of patients on the 6E10 pivotal dose and topical eyedrop steroid regimen prefer Ixo-vec over prior treatments for wet AMD. |
| |
• |
|
100% (n=10) of patients on the 6E10 pivotal dose and topical eyedrop steroid regimen would elect to receive Ixo-vec in other eye if both eyes had wet AMD. |
| |
• |
|
100% (n=10) of patients on the 6E10 pivotal dose and topical eyedrop steroid regimen would recommend Ixo-vec to their family or friends with wet AMD. |
| |
• |
|
No patients receiving topical eyedrop alone prophylaxis (n=20) stated it was difficult to manage. |
OPTIC (2E11) 4-year Analysis Topline Data Summary
| |
- |
Patients in OPTIC received 9.9 mean annualized injections prior to receiving Ixo-vec. Despite significant treatment need at baseline, these patients continue to experience long-term benefit from Ixo-vec through at least 4 years of follow up, including maintenance of vision, durability of anatomical improvements and sustained reduction in anti-VEGF treatment burden. Aflibercept levels have been demonstrated up to 5-years post-treatment. |
| |
• |
|
Patients had an 86% reduction in annualized anti-VEGF injections through year 4, with a robust reduction in treatment burden demonstrated in each year following Ixo-vec administration. |
| |
• |
|
Through Year 1: 84% reduction in anti-VEGF injections |
| |
• |
|
Through Year 2: 81% reduction in anti-VEGF injections |
| |
• |
|
Through Year 3: 84% reduction in anti-VEGF injections |
| |
• |
|
Through Year 4: 86% reduction in anti-VEGF injections |
| |
• |
|
4-year OPTIC data underscore Ixo-vec’s reliable long-term benefit. |
| |
• |
|
Nearly 50% of patients were injection free through 4 years following Ixo-vec treatment. |
| |
• |
|
78% of OPTIC participants who were injection free through year 1 remained injection free through year 4. |
| |
• |
|
88% of OPTIC participants who were injection free through year 2 remained injection free through year 4. |
| |
• |
|
Durable aqueous aflibercept protein levels up to 5 years after a single Ixo-vec IVT injection. |
| |
- |
Ixo-vec at 2E11 was generally well tolerated and demonstrated a favorable safety profile. |
| |
• |
|
Inflammation was dose dependent, did not impact vision and, when present, was responsive to local corticosteroids. |
| |
• |
|
Long-term data establish a 10-fold safety margin from highest dose tested in wet AMD. |
Key Design Elements of the Ixo-vec Phase 3 Pivotal Program
| |
- |
The company plans to conduct two, double-masked, randomized Phase 3 clinical trials. |
| |
- |
The initial 284-patient, US-based ARTEMIS Phase 3 study is expected to enroll a broad patient population, including both treatment-naïve and treatment-experienced wet AMD patients. |
| |
- |
The primary endpoint, measured at an average of weeks 52 and 56, is non-inferiority (NI) in mean BCVA change from baseline between Ixo-vec (6E10 vg/eye) and aflibercept (2mg Q8W). The non-inferiority margin for this study is -4.5 letters. |
| |
- |
All patients will receive three monthly loading doses of aflibercept prior to Ixo-vec. |
| |
- |
The study will utilize a sham in the control arm to support masking. Patients in both arms will be eligible for supplemental injections of aflibercept and will receive topical steroid eye drops. |
| |
- |
This trial design is based on our end-of-Phase 2 feedback from the U.S. Food and Drug Administration (FDA). |
| |
- |
ARTEMIS remains on track and is expected to initiate in 1H 2025. |
Estimated Market Opportunity
The Company estimates that the global market opportunity for wet AMD will be approximately $9 billion in 2025 and $13 billion in 2035.
Updated Cash Runway Guidance
As of September 30, 2024, the Company had $153.2 million in cash, cash equivalents and short-term investments. The Company expects to be able to fund operations into the second half of 2025, which does not include completion of the ARTEMIS Phase 3 trial.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include but are not limited to statements regarding: the long-term potential best-in-class product profile of Ixo-vec; potential best-in-class injection-free rates and reduction in injection burden of Ixo-vec; the trial design of the Ixo-vec Phase 3 pivotal program and anticipated initiation timing; the potential of Ixo-vec to be transformative and a best-in-class therapy; the potential life-long therapeutic benefit and predictable safety profile of Ixo-vec; the potential of Ixo-vec to shift the treatment paradigm for patients with wet AMD; the ability to establish gene therapy as a standard of care for wet AMD patients; the likelihood of clinical, regulatory and commercial success of Ixo-vec; the Company’s cash sufficiency and runway; the Company’s estimates regarding the global market opportunity for wet AMD; and other statements that are not historical fact. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including risks inherent to, without limitation: the Company’s novel technology, which makes it difficult to predict the timing of commencement and completion of clinical trials; regulatory uncertainties; enrollment uncertainties; the results of early clinical trials not always being predictive of future clinical trials and results; the potential for future complications or side effects in connection with use of Ixo-vec; and risks associated with market condition. Additional risks and uncertainties facing the Company are set forth under the caption “Risk Factors” and elsewhere in the Company’s SEC filings and reports, including the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024 filed with the SEC on November 4, 2024 and subsequent filings with the SEC. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
|
|
|
| Exhibit No. |
|
Description |
|
|
| 99.1 |
|
Slide presentation. |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Adverum Biotechnologies, Inc. |
|
|
|
|
| Dated: November 18, 2024 |
|
|
|
By: |
|
/s/ Laurent Fischer |
|
|
|
|
|
|
Laurent Fischer, M.D. President and Chief Executive Officer |

Exhibit 99.1 Preserving ® Sight for Life Webcast to Present 52-Week
LUNA and 4- Year OPTIC Results and Pivotal Program th November 18 , 2024

Forward-Looking Statements Statements contained in this document and any
accompanying presentation regarding matters, events, statistics, or clinical or financial results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Such statements include, but are not limited to, statements regarding the potential benefits of Ixo-vec as a treatment of wet AMD, including the potential best-in-class product profile, clinical activity, favorable safety profile and long-term
benefit; plans and milestones related to Adverum’s product candidates, clinical studies and trials (including the trial design of the Ixo-vec Phase 3 pivotal program and anticipated initiation timing), and regulatory filings; the therapeutic
and commercial potential of Adverum’s product candidates; expectations regarding the size of the market for Ixo-vec; the potential of Ixo-vec to transform the treatment paradigm for patients with wet AMD; Adverum’s cash sufficiency and
runway; and other statements containing the words “anticipates,” “may,” “potential,” “will” and similar expressions, all of which are based on certain assumptions made by Adverum on current conditions,
expected future developments and other factors Adverum believes are appropriate under the circumstances. Adverum may not consummate any of these plans or these product, clinical development, process development, manufacturing, or regulatory goals in
a timely manner, or at all, or otherwise carry out the intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results and
the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that Adverum’s resources will not be sufficient
for Adverum to conduct or continue planned development programs and planned clinical trials, the risk that preliminary or interim data from Adverum’s clinical trials may change as more patient data become available, the risk of a delay in the
enrollment of patients in Adverum’s clinical studies or in the manufacturing of products to be used in such clinical studies, risks and uncertainties inherent in the product development and the regulatory approval process, the risk that
Adverum will not be able to successfully develop, manufacture, or commercialize any of its product candidates and the risk that Adverum will be delayed in receiving or fail to receive required regulatory approvals. Additional risks and uncertainties
facing Adverum are set forth under the caption “Risk Factors” and elsewhere in Adverum’s Securities and Exchange Commission (SEC) filings and reports, including Adverum’s most recent Annual Report on Form 10-K for the year
ended December 31, 2023 filed with the SEC on March 18, 2024 and in our Quarterly Reports on Form 10-Q for the quarterly periods ended subsequent to our filing such Annual Report on Form 10-K, as well as any amendments thereto reflected in
subsequent filings with the SEC. All forward-looking statements contained in this document speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to reflect events that occur or circumstances
that exist after the date on which they were made, except as required by law. This document contains, and any accompanying presentation may contain, estimates, projections and other information concerning Adverum’s industry, business and the
markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar
methodologies is inherently subject to uncertainties and actual amounts may differ materially from amounts reflected in this information. Unless otherwise expressly stated, Adverum obtained this industry, business, market and other data from
reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not
guaranteed by, and should not be construed as representations made by, Adverum. 2

Today’s Agenda Welcome & Key Takeaways Patient Preference
Survey 01 05 Laurent Fischer, MD Laurent Fischer, MD President & Chief Executive Officer President & Chief Executive Officer Ixo-vec & Wet AMD Commercial Opportunity 02 06 Laurent Fischer, MD Jason Mitchell President & Chief
Executive Officer Chief Commercial Officer 4-Year OPTIC & 52-week 03 07 KOL Panel LUNA Results Moderator: Star Seyedkazemi, PharmD Star Seyedkazemi, PharmD Chief Development Officer Chief Development Officer Key Pivotal Program Elements 04
Conclusions & Q&A 08 Rabia Gurses Ozden, MD Adverum Management Chief Medical Officer 3

Adverum Participants Laurent Fischer, MD Rabia Gurses Ozden, MD Star
Seyedkazemi, PharmD President and Chief Medical Officer Chief Development Officer Chief Executive Officer Linda Rubinstein Mike Zanoni Jason Mitchell Peter Soparkar Chief Financial Officer Head of Investor Relations Chief Commercial Officer Chief
Operating Officer 4

Key Opinion Leader Participants Szilárd Kiss, MD Charles C. Wykoff,
MD, PhD Mark Barakat, MD Distinguished Professor of Ophthalmology, Director of Research, Director of Clinical Research Director of Retina Service Retina Consultants of Texas Retina Macula Institute of Arizona Cornell University Adverum Board Member
5

Key Takeaways

Ixo-vec’s Derisked Phase 3 and Commercial Profile Clinical updates
underscore Ixo-vec’s potential best-in-class product profile • Potential best-in-class product profile >50% injection free and >80% treatment burden reduction in hard-to-treat patients • 10X safety margin with >4 years
follow-up • No OPTIC 2E11 patients had inflammation at Year 1 and through Year 4 • No LUNA 6E10 patients had inflammation at 52 weeks or any subsequent visit Favorable safety profile with local prophylaxis • LUNA patient survey
demonstrates strong patient preference for Ixo-vec Broad 6E10 EOP2 284 1H25 ARTEMIS Patient population With topical US study, incorporates Patients Expected Phase 3 Phase 3 Trial steroid eyedrops FDA feedback initiation 7 No inflammation: no ≥
1 AC/V cells; OPTIC 2E11 participant with inflammation at year 2.5 underwent a cataract surgery near the start of OPTIC EXT.; EOP2: End of Phase 2 Meeting DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

5+ Years of Clinical Experience Establish 10x Safety Margin Ixo-vec 6E10
with extended prophylaxis de-risks Phase 3 & commercialization 6E11 N=15 2E11 N=15 4+ Years Clinical Data 8 DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024 Dose (vg/eye) 10x Safety Margin

5+ Years of Clinical Experience Establish 10x Safety Margin Ixo-vec 6E10
with extended prophylaxis de-risks Phase 3 & commercialization 6E11 Phase 3 Dose N=15 ü Enhanced Safety Profile ü Extended Steroid Prophylaxis ü Potential Best-in-Class Efficacy Profile 2E11 2E11 N=15 N=30 6E10 N=30 4+ Years
Clinical Data 9 DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024 Dose (vg/eye) 10x Safety Margin

6E10 Dose Selected to Maximize Phase 3 and Commercial Success
Demonstrates potential best-in-class product profile Benefit Safety Treatment Burden % Receiving ≤ 1 Injection Injection Free % With AC ≥1+ Ph3 Go-Forward Dose Reduction Steroids for IOI 6E10 88% 75% 54% 0% 4% (n = 28) N=1 2E11 92% 79%
69% 3% 7% (n = 29) 2E11 84% 73% 60% 0% 27% (n = 15) 6E11 97% 87% 87% 0% 60% (n = 15) Potential best-in-class clinical activity with enhanced safety profile of 6E10 for pivotal studies 10 Benefit comparison provided for outcomes measured over the
initial 52 weeks of LUNA and OPTIC. Safety comparison provided for outcomes measured at 52 weeks for LUNA and OPTIC. DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

Reliable Benefit & Predictable Safety Profile Enable True Paradigm
Shift 4-year OPTIC & 52-week LUNA data underscore Ixo-vec’s profile Demonstrated Reliable Long-Term Benefit 78% of patients who were injection free through year 1 remained injection free through year 4 88% of patients who were injection
free through year 2 remained injection free through year 4 Demonstrated Predictable Safety Profile with Extended Local Prophylaxis NO new onset of inflammation after week 30 100% of inflammation resolved by year 1 11 No inflammation: no ≥ 1
AC/V cells; OPTIC results refer to 2E11 dose DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

Ixo-vec & wet AMD

Large and Growing Global Market Opportunity Wet AMD physicians and
patients embrace innovation Wet Age-Related Macular Degeneration Multi-Billion Dollar Market Opportunity A leading cause of vision loss among older adults Growth driven by aging population and product innovation Global Wet AMD Sales 20M 1.5M ~$13B
US Patients Worldwide ~$9B ~$6B ~200k US patients diagnosed annually Up to 42% of patients develop bilateral disease within 2-3 years of initial diagnosis 2015 2025E 2035E 13 Sources: Bright Focus Foundation. Age-Related Macular Degeneration: Facts
& Figures.; Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:106–16.; Gangnon RE et al. (2015) JAMA
Ophthalmol; 133 (2): 125–132.; 2023 Cowen Equity Research – Therapeutic Categories Outlook.; Company estimates.

Real-World Vision Declines Due to Chronic Undertreatment 10 5 0 IRIS
(N>160,000) -5 HORIZON (N=600) Gilles, al (N=1,212) CATT (N=647) -10 SEVEN-UP (N=65) -15 0 1 2 3 4 5 6 7 Years Up to ~40% STOP anti-VEGF over 2 years and up to 57% stop over 5 years Sources:
https://futureofvision.global/home/the-reality-of-retinal-disease.html. Adapted from Singer MA, et al. Ophthalmology. 2012;119(6):1175-1183. Adapted from Gillies MC, et al. Ophthalmology. 2015;122(9):1837- 14 1845. Adapted from Comparison of
Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Maguire MG, et al. Ophthalmology. 2016;123(8):1751-1761. Adapted from Rofagha S, et al.; Ophthalmology. 2013;120(11):2292-2299. Wykoff CC, Garmo V, Tabano D, et al, Ophthalmol
Sci. 2023;4(2):100421. Mean Letter Change From Baseline

Real-World Vision Declines Due to Chronic Undertreatment Ixo-vec
designed to deliver stable aflibercept to maintain long-term vision Ixo-vec 10 5 0 IRIS (N>160,000) -5 HORIZON (N=600) Gilles, al (N=1,212) CATT (N=647) -10 SEVEN-UP (N=65) -15 0 1 2 3 4 5 6 7 Years Up to ~40% STOP anti-VEGF over 2 years and up
to 57% stop over 5 years 15 *Potential long-term vision outcome with Ixo-vec. Sources: https://futureofvision.global/home/the-reality-of-retinal-disease.html. Adapted from Singer MA, et al. Ophthalmology. 2012;119(6):1175-1183. Adapted from Gillies
MC, et al. Ophthalmology. 2015;122(9):1837-1845. Adapted from Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Maguire MG, et al. Ophthalmology. 2016;123(8):1751-1761. Adapted from Rofagha S, et al.;
Ophthalmology. 2013;120(11):2292-2299. Wykoff CC, Garmo V, Tabano D, et al, Ophthalmol Sci. 2023;4(2):100421. Mean Letter Change From Baseline

Undertreatment Often Results from Barriers to Treatment Physician ASRS
survey underscores need for more durable treatments Barriers to Treatment More Durable Treatment Options Are Needed Physician Survey: Unmet Needs In Treating Wet AMD and DME Needle Anxiety Comorbidities 74% Greater Durability 79% 55% Improved Vision
50% Missed Injections 58% Longer VEGF Suppression 47% 46% Stable Anatomy Life Events Lack of Transportation 30% International US Ixo-vec may address unmet need in wet AMD by delivering stable aflibercept 16 Hahn P, ed. ASRS 2023 Preferences and
Trends Membership Survey. Chicago, IL. American Society of Retina Specialists; 2023.

Ixo-vec Positioned to Transform Treatment Paradigm In contrast, 2nd
generation wet AMD treatments are only incremental advancements Ixo-vec designed to deliver stable and continuous aflibercept 5+ years % Injection Potential 2024 Free US Revenue 4D-150 (Investigational) Paradigm Shift Ixo-vec >50% [Discontinued
Q26W and Relaunched] nd Q8-Q16W $1.6B 2 Generation Q8-Q16W $4.3B 0% Q8W $4.6B st 1 Generation Q4W $23M Q12W Q26W 1Y 2Y 5Y+ 17 Potential 2024 US revenue is extrapolated from Q3 2024 public filings of each company’s reported financial results
and is for all indications

Ixo-vec Delivers Anti-VEGF Aflibercept via Single Intravitreal
Injection 7m8 capsid enables delivery of stable aflibercept levels into the retina AAV.7m8 AAV.7m8 ILM Ganglion Inner Nuclear Photoreceptors RPE IVT injection monomer Cells Membrane Loop IV Retina Loop IV 7m8 insert Promoter Aflibercept PolyA
AAV.7m8: Created by Directed Evolution • Engineered for IVT administration; peptide loop enables Ixo-vec 7m8 to cross the inner limiting membrane (ILM) • AAV.7m8 delivers aflibercept to the retina AAV.7m8 for IVT Gene Therapy CNV •
Validated in 3 clinical programs Aflibercept secretion IVT injection Durable suppression by retina of CNV • Published in peer reviewed journals 18 Khabou et. al. Biotechnology and Bioengineering, 113:12, December 2016

4-Year OPTIC & 52-week LUNA Results

OPTIC First-in-Human Trial Design 6E11 & 2E11 with short
prophylaxis in hard-to-treat population Primary Objective Secondary Objectives • Vision maintenance (BCVA) Long-term safety and efficacy of Ixo-vec IVT in • Anatomy (SD-OCT) treatment experienced patients • Need for supplemental
therapy Day -15 to -7: Day 1: Ixo-vec 2-Year Safety and Efficacy Current Update: 5-Year Safety and Efficacy Baseline 4-Year IVT Aflibercept Screening 2-Year OPTIC Study 3-Year Extension Study Period Corticosteroid Prophylaxis* Corticosteroid
Extension Scheduled Ixo-vec Dose Supplemental Aflibercept (2 mg IVT) Criteria: Prophylaxis Visits Cohort 1 (n=6) 6E11 Oral, 13d • ≥10 letters BCVA (ETDRS) loss from baseline OR, Quarterly assessments • CST >75 μm increase
from baseline OR, Cohort 2 (n=6) Oral, 13d 2E11 following completion of • New Vision-threatening hemorrhage due to AMD 2-year OPTIC parent Cohort 3 (n=9) Eye Drops, 6 wks 2E11 study • After initial supplemental injection subsequent
injections Cohort 4 (n=9) Eye Drops, 6 wks 6E11 administered at investigator discretion 20 Study timelines not to scale. *Participants in Cohorts 1 and 2 received prophylaxis of 60 mg oral prednisone for 6 days starting at Day –3 followed by
7-day taper; participants in Cohorts 3 and 4 received prophylaxis of QID difluprednate eye drops for 3 weeks starting at Day 1 followed by a 3-week taper. QID, four times daily. OPTIC: NCT03748784; OPTIC EXT: NCT04645212. DATA CUT: OPTIC
21AUG2024

Preclinical Data Suggest Best-in-Class Potential of Ixo-vec at 6E10
Dose NHP studies demonstrate consistent aflibercept production with low inflammation Less-than-dose-proportional aflibercept levels across 3 logs Improved inflammation scores with lower doses (no prophylaxis) Aqueous Humor Aflibercept Levels
Inflammation Scores 8 * 6 4 2 0 I I Vehicle 3E10 1E11 2E11 4E11 6E11 2E12 6E12 2E13 NHP 3E10 1E11 2E11 4E11 6E11 2E12 6E12 2E13 NHP Vehicle 3E10 1E11 2E11 4E11 6E11 2E12 6E12 2E13 Dose (vg/eye) Human equivalent Vehicle 6E10 2E11 4E11 8E11 1E12 4E12
1E13 4E13 Human equivalent 6E10 2E11 4E11 8E11 1E12 4E12 1E13 4E13 Dose (vg/eye) Dose (vg/eye) *Scale is cumulative of two parameters for maximum score of 8. 21 Schaefer-Swale K. Non-Clinical Data Support Efficacy and Tolerability of a Human
Equivalent Dose of 6E10 vg/eye of ADVM-022 for the Treatment of Neovascular Age-Related Macular Degeneration. Poster presented at ASGCT 2023. Human Equivalent Dose (HED) is approximately twice the corresponding NHP dose based on eye volume. E.g.,
2E11 is the HED of 1E11 NHP dose. NOAEL established at NHP dose of 1E11 vg/eye. Aflibercept (ng/l) Hackett-McDonald peak score (AC + VC)

Learnings from OPTIC Informed LUNA Phase 2 Trial LUNA goal: optimize
Ixo-vec benefit / risk going into pivotal trials Objective I Objective II Determine the Determine the Optimal Phase 3 Prophylactic Dose for Phase 3 Regimen 22

LUNA Phase 2 Trial Design 6E10 & 2E11 with extended prophylaxis in
hard-to-treat patients Multicenter, double-masked, randomized, parallel-group Phase 2 study Key inclusion criteria: demonstrated response to anti-VEGF therapy and under active treatment for CNV secondary to nAMD (received a minimum of 2 injections
within 4 months of entry), study eye BCVA in the range of 25 – 83 ETDRS letters FIVE YEAR: Day -21 to -14: DAY -7: DAY 1: WEEK 26: WEEK 52: Baseline Randomization IVT Ixo-vec Primary Endpoints EXT Completion Interim Analysis IVT Aflibercept
2mg Long-term 2E11 (n=30) extension ends Screening Period 6E10 (n=30) at year five Corticosteroid prophylaxis (21+ weeks post-dose) Corticosteroid Prophylaxis Regimen Supplemental Injection Criteria • Difluprednate 22 wks ± prednisone
oral 10 wks • Increase in CST > 75 μm from BL confirmed by the CRC OR • Ozurdex IVT + difluprednate after week 4 ± prednisone oral 10 wks* • Loss of ≥ 10 letters in BCVA from BL due to new/worsening IRF or SRF OR
• Randomized 2:1 local versus local + oral • New vision-threatening hemorrhage due to nAMD 23 Study timeline and length of arrows depicted are not to scale. Baseline is defined as the day screening aflibercept is administered. *Protocol
amended early in study to include difluprednate starting at week 4 to match the taper in difluprednate regimens; if initiated after week 4 visit, difluprednate may be adjusted at the discretion of investigator in consult with medical monitors (6
participants did not receive difluprednate as part of prophylaxis). DATA CUT: LUNA 29AUG2024

Demographics and Baseline Characteristics OPTIC & LUNA evaluated
hard-to-treat patients LUNA LUNA LUNA OPTIC Demographics and Baseline 6E10 2E11 Total Total Characteristics N = 30 N = 30 N = 60 N = 30 Mean age, years (SD) 75.4 (8.2) 77.7 (7.4) 76.6 (7.8) 79.0 (7.3) Female, n (%) 16 (53%) 18 (60%) 34 (57%) 15
(50%) Race, n (%) White 27 (90%) 28 (93%) 55 (92%) 30 (100%) Asian 2 (7%) 2 (7%) 4 (7%) 0 Mean years since nAMD diagnosis in the study eye 3.0 (2.9) 3.0 (3.1) 3.0 (2.9) 3.7 (2.8) (SD) Mean annualized anti-VEGF injections in year prior to 10.2 (1.7)
10.0 (3.3) 10.1 (2.6) 9.9 (1.9) Day 1 (SD) Mean BCVA, ETDRS letters (SD) 72.9 (8.8) 71.8 (6.4) 72.3 (7.7) 65.4 (7.2) Mean CST, µm (SD) 360.6 (112.0) 340.5 (119.3) 350.6 (115.2) 397.0 (137.3) Phakic lens status, n (%) 11 (37%) 11 (37%) 22 (37%)
10 (33.3%) 24 Data cut: OPTIC 21AUG2024, LUNA 29AUG2024

Ixo-vec Demonstrates Durable Therapeutic Levels After One Injection
Sustained aqueous aflibercept levels up to 5 years Early aflibercept levels are associated with sustained long-term protein expression 100,000 NHP studies suggest aflibercept levels may be 7x higher in the back of the eye 10,000 1,000 100 10 LUNA
2E11 (n=18) LUNA 6E10 (n=13) OPTIC 6E11 (n=7) OPTIC 2E11 (n=10) 1 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 Years 25 LUNA Week 14 aflibercept levels plotted for 31 of 60 individual participants. LUNA revised to stop collection of AH samples. Measurements
below the level of quantification of the assay (25ng/ml) are not shown. NHP data: Kiss, Szilárd et al, Molecular Therapy Methods & Clinical Development, Volume 18, 345 - 353 Data cut: OPTIC 21AUG2024, LUNA 29AUG2024 Aflibercept in Aqueous
Humor (ng/mL)

Ixo-vec Demonstrates Durable Therapeutic Levels After One Injection
Sustained aflibercept levels measured with 6E10 at year 1 Early aflibercept levels are associated with sustained long-term protein expression 100,000 NHP studies suggest aflibercept levels may be 7x higher in the back of the eye 10,000 1,000 100 10
LUNA 2E11 (n=18) LUNA 6E10 (n=13) OPTIC 6E11 (n=7) OPTIC 2E11 (n=10) 1 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 Years 26 LUNA Week 14 aflibercept levels plotted for 31 of 60 individual participants. LUNA revised to stop collection of AH samples. Measurements
below the level of quantification of the assay (25ng/ml) are not shown. NHP data: Kiss, Szilárd et al, Molecular Therapy Methods & Clinical Development, Volume 18, 345 - 353 Data cut: OPTIC 21AUG2024, LUNA 29AUG2024 Aflibercept in Aqueous
Humor (ng/mL)

Ixo-vec Reduced Treatment Burden Through 4 Years 2E11 shows >80%
reduction in annualized anti-VEGF injections in every year Mean Post Ixo-vec Annual Rate Through Year 1 | 2 | 3 | 4 | | | Mean Prior Annual Rate 11 9.9 10 9 8 7 60% 53% 47% 53% 6 Injection Free Injection Free Injection Free Injection Free 5 81% 84%
84% 4 86% 3 1.9 1.6 1.6 2 1.4 1 0 2E11 Participants N=15 Annualized rate (Prior) = (number of IVTs in 12 months prior to Ixo-vec) / (days from the first IVT in the past 12 months to Ixo-vec / 365.25). 27 Annualized rate (Post) = (number of
aflibercept IVTs since Ixo-vec) / (days from Ixo-vec to the last study follow-up / 365.25). Analysis includes all participants from the OPTIC study. Data cut: OPTIC 21AUG2024 Mean Annualized Number of anti-VEGF Injections (per patient)

Consistent Injection-Free Rates through 4 Years Proportion of
injection-free patients consistent in each year of follow-up Injection Free 60% 53% 67% 73% By Year * OPTIC OPTIC EXT 2E11 (n = 15) -1 0 1 2 3 4 Years Early Termination Aflibercept Bevacizumab Ranibizumab Unknown Visit with no supplemental injection
administered 28 *Supplemental aflibercept injections after the first one could be administered at physician’s discretion. All patients terminated due to loss to follow up, death or other reasons included for calculations across all periods [%
Injection Free = (Total Cohort – Patients Rescued in Period) / (Total Cohort) * 100] Data cut: OPTIC 21AUG2024

52-Week LUNA and OPTIC Injection Burden Reduction are Consistent
>80% reduction in annualized anti-VEGF injections, >50% injection free Mean Prior Annualized Rate Mean Post Ixo-vec Annualized Rate: LUNA OPTIC 11 10.2 10.1 9.9 10 -88% -92% -84% 9 8 75% 79% 73% 7 ≤ 1 Injection ≤ 1 Injection
≤ 1 Injection 6 54% 69% 60% 5 Injection Free Injection Free Injection Free 4 3 1.6 2 1.3 0.8 1 0 6E10 Participants Through Week 52 2E11 Participants Through Week 52 6E10 2E11 2E11 N=30 N=30 N=28 N=29 N=15 29 Annualized rate (Prior) = (number
of IVTs in 12 months prior to Ixo-vec) / (days from the first IVT in the past 12 months to Ixo-vec / 365.25). Annualized rate (Post) = (numbers of aflibercept IVTs since Ixo-vec) / (days from Ixo-vec to last follow-up within the interim analysis
period / 365.25). Data cut: OPTIC 21AUG2024, LUNA 29AUG2024 Mean Annualized Number of anti-VEGF Injections (per patient)

Potential Best-in-Class Injection-Free Rates 52-week LUNA data
demonstrate >50% injection free in hard-to-treat patients LUNA 52 Weeks Overall 6E10 2E11 (N = 57) (n = 28) (n = 29) Injection Free 61% 54% 69% ≤1 Injection 77% 75% 79% ≤2 Injections 89% 89% 90% Week -52 Week -26 Study Week Week 26
Week 52 Week 91 Aflibercept No supplemental injection given Supplemental injection administered out of protocol Ranibizumab Faricimab Bevacizumab Other Early Termination 30 Doses pooled in swim lane plot to preserve investigator masking in an
ongoing double masked study. Early termination patients not included in injection calculations. [% Injection Free = (Total Cohort - Early Termination Patients – Rescued Patients) / (Total Cohort – Early Termination Patients) * 100] Data
cut: LUNA 29AUG2024 All Participants (N=60)

Ixo-vec Maintains BCVA & CST Demonstrated robust and durable
activity through 4 years of follow up OPTIC OPTIC EXT 90 Mean BCVA Mean BCVA (90% CI) by Dose Over Time (letters) change 80 from baseline to last visit (90% CI) 70 -2.9 (-12.3, 6.5) 60 * 2E11 50 40 30 0 0 12 0 24 .5 36 48 1 60 72 1.5 84 96 2108 120
2 1.5 32 144 156 3 168 180 3.5 192 204 4 (N) 15 13 14 13 12 12 12 11 11 Mean CST (90% CI) by Dose Over Time 500 Mean CST (μm) change from 450 baseline to last visit 400 (90% CI) 350 -117.7 (-225.0, -10.5) 300 2E11 250 200 150 100 0 0 4 00 12 24
0.5 36 48 1 60 72 1.584 96 1 208 120 12 32 .5 144 156 3 168 1803.5 192 204 4 (N) 15 13 13 12 12 11 12 11 12 Years 31 *Cataract surgery Data cut: OPTIC 21AUG2024 Mean CST μm (90% CI) Mean BCVA (90% CI)

Ixo-vec Maintains BCVA & CST Both doses demonstrate robust and
durable activity, consistent with OPTIC Least Squares Means Change in Best Corrected Visual Acuity (BCVA) Over Time by Dose* 15 LS Means BCVA Change 6E10 (n=29) 2E11 (n=29) from Baseline at Week 52, 10 Letters (95% CI) 5 -2.1 (-4.8, 0.7) 0 6E10 -5
-1.8 (-4.6, 0.9) 2E11 -10 -15 // BL 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 Week Least Squares Means Change in Central Subfield Thickness (CST) Over Time by Dose 150 LS Means CST Change 6E10 (n=30) 2E11 (n=30)
100 from Baseline at Week 52, μm (95% CI) 50 -10.2 (-29.0, 8.5) 0 6E10 -50 -21.9 (-40.4, -3.3) -100 2E11 -150 // 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 BL Week 32 Least Squares Means are based on Mixed
Model Repeated Measures (MMRM) including dose group, baseline value, visit and visit dose group. *Excludes 1 participant at each dose with letter loss due to cataract Data cut: LUNA 29AUG2024 LS Means Change CST LS Means Change BCVA (95% CI) μm
(95% CI)

OPTIC 2E11 Well Tolerated through 4 Years 100% of patients who had
inflammation resolved by year 1 OPTIC OPTIC EXT OPTIC 2E11 15 AC Cells ≥1+ VC Cells ≥1+ § 2E11 was generally well tolerated through 4 2E11 years of follow up 10 § Inflammation was dose dependent, did not 5 impact vision and,
when present, was responsive to local corticosteroids 0 * 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 18 * 0 192 204 § Long-term safety data with 10-fold safety 0 0.5 1 1.5 2 2.5 3 3.5 4 Years margin from highest dose tested in nAMD Short
prophylactic regimen was 13 days oral prednisone or 6 weeks of topical drops *2E11 participant with inflammation at year 2.5 underwent a cataract surgery near the start of OPTIC EXT. At the next scheduled visit (3 months later—2.5 year visit),
inflammation was detected that was responsive to 33 topical corticosteroids. Cell grades as assessed by slit lamp, Grade categories are based on the Standardization of Uveitis Nomenclature (SUN) criteria for aqueous cells and National Institutes of
Health guidelines for vitreous cells. Data cut: OPTIC 21AUG2024 Count of Participants

Safety Summary: LUNA 52-Week Ixo-vec continues to be well tolerated,
supporting potential best-in-class product profile Ixo-vec had a favorable safety profile and was well tolerated in total LUNA population • No Ixo-vec-related serious adverse events. All Ixo-vec-related adverse events (AEs) were either mild or
moderate • No episcleritis, vasculitis, retinitis, choroiditis, vascular occlusion, or hypotony • Most common Ixo-vec-related AEs were dose-dependent anterior inflammation responsive to 1 local corticosteroid and anterior pigmentary
changes with no impact on vision 2 • 6E10 patients had no inflammation at week 52 and at any subsequent visit 3 Local corticosteroids alone effectively minimized inflammation at both doses • No patients had inflammation at week 52 and at
any subsequent visit 6E10 + topical steroid eyedrops selected for pivotal program 4 • No patients had inflammation at week 52 and at any subsequent visit • Only a single patient had inflammation at any timepoint, which resolved prior to
week 52 34 1 2 3 4 Anterior chamber cell, anterior chamber pigmentation, iris transillumination defect, iritis. No inflammation: no ≥ 1 AC/VC cells. Topical difluprednate with or without IVT dexamethasone (N=34). Topical difluprednate alone
(N=10). Data cut: LUNA 29AUG2024

Extended Local Prophylaxis Effective in Minimizing Inflammation
Inflammation responded to local corticosteroids and resolved over time Initial IVT DEX IVT DEX + DFBA Initial IVT DEX IVT DEX + DFBA Prophylactic Steroid Prophylactic Steroid Injection Injection Usage** Usage** DFBA Prophylaxis Period (DFBA Only)
DFBA Prophylaxis Period (DFBA Only) 0 0 0 0 0 tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 ANTERIOR VITREOUS 6E10 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr tr tr 0 0 0 0 0 6E10 CHAMBER
0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 CELLS 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 CELLS 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 p 0 0 0 1+ tr p 0 p p 0 0 0 0 0 0 0 0 0 0 p p p p 0 0 0 0 0 0 0 0 0 et et 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 None None 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr 1+ 0 0 1+ 0 0 0 0 0 0 0 0 0 0 1+ 1+ tr 0 tr tr 1+ 1+ tr tr tr tr tr tr 1+ 1+ * * * * * 0 0 0 0 0 0 0 0 0 0 0 p p p 0 0 0 p p p p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2+ 2+ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3+ 3+ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 4+ 0 0 0 0 0 0 0 0 0 0
0 0 p 0 p 0 0 p 0 p 4+ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 p p p p p p p p p p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2E11 2E11 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr 0 0 0 0 0 0 0 0 p p p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr tr tr tr tr tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 p p p p p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr 0 0 0 0 0 0 2+ 0 1+ 0 2+ 0 tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1+ 0 0 0 p p p p * * * * 0 0 0 2+ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 p p 0 p 0 0 p p p p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 p p p 0 p 0 p p p p p 0 p 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 tr tr tr tr 0 0 0 0 0 0 0 0 0 0 0 0 0 p tr 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 No
patients had inflammation at week 52 or at any subsequent visit No inflammation: no ≥ 1 AC/V cells. IVT DEX = Ozurdex, DFBA = dilfuprednate, tr = Trace, p = Pigmented Cells, * = Mixed Pigmented / Non-Pigmented Cells, et – Early
Termination. **IVT DEX + DFBA: protocol amended 35 early in study to include difluprednate starting at week 4 to match the taper in the difluprednate only regimen; if initiated after week 4 visit, difluprednate may be adjusted at the discretion of
investigator in consult with medical monitors. Due to this, difluprednate prophylaxis with IVT DEX + DFBA may have lasted up to week 34. Data cut: LUNA 29AUG2024 DFBA IVT DEX + DFBA IVT DEX + DFBA (N=7) DFBA (N=7) (N=10) (N=10) SCRN RAND D1 D3 D8 W2
W4 W6 W8 W10 W14 W18 W22 W26 W30 W34 W38 W42 W48 W52 W65 W78 DFBA IVT DEX + DFBA IVT DEX + (N=10) DFBA (N=7) (N=10) DFBA (N=7) SCRN RAND D1 D3 D8 W2 W4 W6 W8 W10 W14 W18 W22 W26 W30 W34 W38 W42 W48 W52 W65 W78
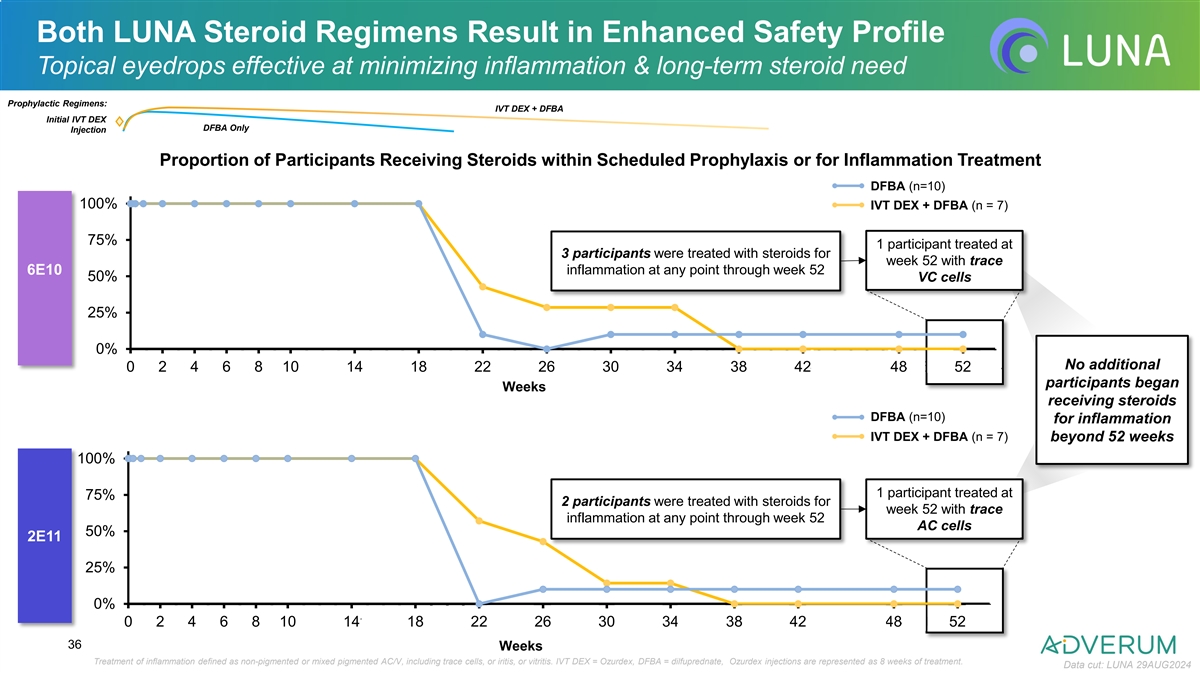
Both LUNA Steroid Regimens Result in Enhanced Safety Profile Topical
eyedrops effective at minimizing inflammation & long-term steroid need Prophylactic Regimens: IVT DEX + DFBA Initial IVT DEX DFBA Only Injection Proportion of Participants Receiving Steroids within Scheduled Prophylaxis or for Inflammation
Treatment DFBA (n=10) 100% IVT DEX + DFBA (n = 7) 75% 1 participant treated at 3 participants were treated with steroids for week 52 with trace 6E10 inflammation at any point through week 52 50% VC cells 25% 0% No additional 0 1 2 3 4 5 6 7 8 9
101112131415161718192021222324252627282930313233343536373839404142434445464748495051525354 participants began Weeks receiving steroids DFBA (n=10) for inflammation IVT DEX + DFBA (n = 7) beyond 52 weeks 100% 1 participant treated at 75% 2
participants were treated with steroids for week 52 with trace inflammation at any point through week 52 AC cells 50% 2E11 25% 0% 0 1 2 3 4 5 6 7 8 9 101112131415161718192021222324252627282930313233343536373839404142434445464748495051525354 36 Weeks
Treatment of inflammation defined as non-pigmented or mixed pigmented AC/V, including trace cells, or iritis, or vitritis. IVT DEX = Ozurdex, DFBA = dilfuprednate, Ozurdex injections are represented as 8 weeks of treatment. Data cut: LUNA
29AUG2024

Extended Prophylaxis in LUNA Improves Upon OPTIC Experience Phase 3
regimen enhances overall safety profile & reduces long-term steroid need LUNA DFBA Prophylaxis Period OPTIC Proportion of Participants Receiving Steroids within Scheduled Prophylaxis or for Treatment of Inflammation 100% 75% 50% 25% 0% 0 1 2 3 4
5 6 7 8 9 101112131415161718192021222324252627282930313233343536373839404142434445464748495051525354 Weeks LUNA: DFBA Only OPTIC: DFBA Only 2E11 (n = 9) 6E10 (n = 10) 6E11 (n = 9) 2E11 (n = 10) 37 Treatment of inflammation defined as non-pigmented
or mixed pigmented AC/V cells, iritis, or vitritis. DFBA = difluprednate. Data cut: OPTIC 21AUG2024, LUNA 29AUG2024

CST Maintained in Patients with High Disease Burden Fluid reduction in
patients with baseline CST >300 μm; maintenance in ≤300 μm 150 LUNA Baseline CST ≤300μm (n=27) LUNA Baseline CST >300μm (n=33) Mean CST Change from 100 Baseline at Week 52, μm (95% CI) 50 2.9 (-14.3, 20.2)
BL ≤300 μm 0 -34.4 (-64.9, -3.9) BL >300 μm -50 -100 -150 // BL 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 Week Baseline Characteristic for Subgroup CST ≤300 µm CST >300 µm
Mean CST, µm (SD) 269.9 (18.7) 416.6 (119.1) 38 Data cut: LUNA 29AUG2024 Mean CST Change from Baseline, μm (95% CI)

Consistent Anatomic Benefit in Broad Population Anatomic stability with
reduction and maintenance of CST in injection-free patients 150 LUNA Baseline CST ≤300μm (n=27) LUNA Baseline CST >300μm (n=33) Mean CST Change from LUNA Baseline CST ≤300μm, Injection-Free (n=16) LUNA Baseline CST
>300μm Injection-Free (n=20) Baseline at Week 52, μm (95% CI) 100 All Participants 2.9 (-14.3, 20.2) 50 BL ≤300 μm, All -34.4 (-64.9, -3.9) BL >300 μm, All 0 Injection Free -50 -7.6 (-20.7, 5.5) BL ≤300 μm,
Inj-Free -100 -38.0 (-67.0, -9.0) BL >300 μm, Inj-Free -150 // BL 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 Week Baseline Characteristic for Subgroup CST ≤300 µm CST >300 µm CST
≤300 µm CST >300 µm All Participants All Participants Injection Free Injection Free Mean CST, µm (SD) 269.9 (18.7) 416.6 (119.1) 273.8 (16.7) 417.6 (142.0) 39 Data cut: LUNA 29AUG2024 Mean CST Change from Baseline, μm
(95% CI)

Ixo-vec Maintained Visual & Anatomic Outcomes BCVA maintenance
demonstrated in overall population and injection-free patients Least Squares Means Change in Best Corrected Visual Acuity (BCVA) Over Time by Dose* 15 LS Means BCVA Change 6E10 (n=29) 2E11 (n=29) from Baseline at Week 52, 10 Letters (95% CI) 5 -2.1
(-4.8, 0.7) 0 6E10 -5 -1.8 (-4.6, 0.9) 2E11 -10 -15 // BL 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 Week Least Squares Means Change in Best Corrected Visual Acuity (BCVA) Over Time by Dose in Supplemental
Injection Free Participants** 15 LS Means BCVA Change 6E10 (n=15) 2E11 (n=20) from Baseline at Week 52, 10 Letters (95% CI) 5 +1.1 (-2.7, 4.9) 0 6E10 -5 -2.2 (-5.5, 1.2) 2E11 -10 -15 // 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44
46 48 50 52 54 BL Week 40 Least Squares Means are based on Mixed Model Repeated Measures (MMRM) including dose group, baseline value, visit and visit dose group *Excludes 1 participant at each dose with letter loss due to cataract, ** Excludes 1
participant with letter loss due to cataract Data cut: LUNA 29AUG2024 LS Means Change BCVA LS Means Change BCVA (95% CI) (95% CI)

75% Injection Free in 6E10 Patients with Less Treatment Burden
Treatment-experienced patients with ≤6 injections in year prior MEAN INJECTION RATE: 4.7 Ixo-vec LUNA 52 Weeks Overall 6E10 2E11 (N = 13) (n = 4) (n = 9) Injection Free 69% 75% 67% ≤1 Injection 92% 100% 89% ≤2 Injections 92% 100%
89% Only one patient required supplemental injection through their Week 52 visit. Week -52 Week -26 Study Week Week 26 Week 52 Week 91 Aflibercept No supplemental injection given Supplemental injection administered out of protocol Ranibizumab
Faricimab Bevacizumab Other Early Termination 41 Doses pooled in swim lane plot to preserve investigator masking in an ongoing double masked study. Early termination patients not included in injection calculations. [% Injection Free = (Total Cohort
- Early Termination Patients – Rescued Patients) / (Total Cohort – Early Termination Patients) * 100]. Experienced patients: diagnosed at least one year prior to administration of Ixo-vec. Data cut: LUNA 29AUG2024 Experienced
Participants w/ ≤ 6 Prior Injections (N=13)

Clinical Updates Underscore Ixo-vec’s Potential Best-in-Class
Profile • Potential best-in-class product profile Maintenance of visual and anatomic endpoints with over 80% reduction in injection burden and greater than 50% injection freedom • 10X safety margin with >4 years follow-up • No
OPTIC 2E11 patients had inflammation at Year 1 and through Year 4 • No LUNA 6E10 patients had inflammation at 52 weeks or any subsequent visit Favorable safety profile with local prophylaxis De-risked dose & prophylactic regimen for
registrational trials 42 No inflammation: no ≥ 1 AC/V cells. OPTIC 2E11 participant with inflammation at year 2.5 underwent a cataract surgery near the start of OPTIC EXT. Data cut: OPTIC 21AUG2024, LUNA 29AUG2024

Ixo-vec Pivotal Program

Ixo-vec Pivotal Program for Wet AMD in Broad Patient Population
Designed to maximize probabilities of clinical, regulatory, and commercial success 5+ Years of Clinical Experience Ixo-vec Global Pivotal Program Broad Wet AMD 10X Safety Population Margin § Two double-masked randomized Phase 3 trials Ixo-vec
§ Noninferiority design vs. on-label aflibercept Phase 3 § ARTEMIS U.S. study based on EOP2 feedback Advised by Regulatory nd § 2 study to be conducted in the U.S. and Ex-U.S. Agreement Global KOLs Addressing Unmet Needs and Patient
Preference 44

ARTEMIS Phase 3 Wet AMD Study Design Noninferiority trial designed to
enable regulatory approval and drive commercial success Randomized, Double Masked, On-Label Aflibercept-Controlled Phase 3 Trial in Broad Population Objective To demonstrate that a single intravitreal injection of Ixo-vec 6E10 achieves comparable
visual outcomes to on-label aflibercept, while significantly reducing the treatment burden Trial Design Endpoints Primary Endpoint • Two-arm noninferiority trial in US • Ixo-vec 6E10 Mean change in BCVA from Baseline to •
Aflibercept control 2mg q8W average of Weeks 52 and 56 • 284 treatment-naïve and previously treated patients with wet AMD • Sham injections for masking Secondary Endpoint • One-year primary endpoint with -4.5 Treatment burden
reduction letter noninferiority margin 45

ARTEMIS Phase 3 Wet AMD Study Design Broad target patient population
enriched for anti-VEGF response Key Inclusion Criteria Treatment-naïve and previously BCVA: Anti-VEGF responsive: treated wet AMD 35 – 78 letters After two aflibercept loading doses -8 -4 D1 1 4 8 12 16 20 24 28 32 36 40 44 48 52 56 104
Week Ixo-vec 6E10 n = 142 Aflibercept 2mg (Q8w) n = 142 Baseline Response Randomize Primary Endpoint Ixo-vec IVT Sham or supplemental injection if criteria met Aflibercept IVT Supplemental injection if criteria met Sham 46

ARTEMIS Phase 3 Wet AMD Study Design Patients randomized after 3
aflibercept loading doses and assessed every 4 weeks for disease activity Key Inclusion Criteria Treatment-naïve and previously BCVA: Anti-VEGF responsive: treated wet AMD 35 – 78 letters After two aflibercept loading doses Difluprednate
prophylaxis -8 -4 D1 1 4 8 12 16 20 24 28 32 36 40 44 48 52 56 LTFU Week Ixo-vec 6E10 n = 142 Aflibercept 2mg (Q8w) n = 142 Baseline Response Randomize Supplemental aflibercept administered based on pre-specified criteria to protect Primary Endpoint
masking, and the primary and secondary endpoints Ixo-vec IVT Sham or supplemental injection if criteria met Aflibercept IVT Supplemental injection if criteria met Sham Designed to Maximize Probability of Clinical, Regulatory, and Commercial Success
47 LTFU: Long-term follow-up

Patient Preference Survey

Pre-specified Patient Preference Survey Designed to understand patient
needs & commercial potential “…Patient preference refers to a patient’s perspective, expectations, and goals for health, as well as the processes involved in evaluating the potential benefits, harms, and costs of each treatment
option offered to the patient…” • Patient preference and DTC advertising has begun to drive adoption of novel therapies for wet AMD, with Vabysmo’s “Open up your World” campaign contributing to >$4B in
annualized sales. • In LUNA, Adverum’s pre-specified patient preference survey asked whether patients prefer Ixo- vec over their prior treatment with IVT injections, whether the prophylaxis was easy to manage and if patients would
recommend Ixo-vec to family or friends. 49 Source: Montori VM, et Al. Decision making and the patient. Manual for Evidence-Based Clinical Practice 2008.

>90% of Patients Preferred Ixo-vec over Today’s Standard of
Care 52-week results from LUNA pre-specified patient preference survey Would you prefer Ixo-vec therapy Would you want to receive Ixo-vec Would you recommend Ixo-vec to over the prior treatment(s) you therapy in your other eye if you had your family
or friends if they had received to treat your wet AMD? wet AMD in both eyes? wet AMD? 5% 4% 7% 95% 96% 93% Total Study Total Study Total Study (n=56) (n=56) (n=56) Yes No 50 Adverum conducted a pre-specified Patient Preference Survey in LUNA Data
cut: LUNA 29AUG2024

Topical Steroid Eyedrops were Easy or Very Easy to Manage 52-week
results from LUNA pre-specified patient preference survey Was the steroid treatment you received easy to manage? 5% 5% 13% 35% 60% 88% 95% Difluprednate Ozurdex + Oral Prednisone + Only Difluprednate Difluprednate +/- Ozurdex (n=20) (n=16) (n=20)
Easy or Very Easy to Manage Undecided Difficult or Very Difficult to Manage 51 Adverum conducted a pre-specified Patient Preference Survey in LUNA Data cut: LUNA 29AUG2024

100% of Ixo-vec 6E0 + Steroid Drops Preferred it over Standard of Care
52-week results from LUNA pre-specified patient preference survey Would you prefer Ixo-vec therapy Would you want to receive Ixo-vec Would you recommend Ixo-vec to over the prior treatment(s) you therapy in your other eye if you had your family or
friends if they had received to treat your wet AMD? wet AMD in both eyes? wet AMD? 95% 93% 100% 100% 100% 6E10 + 6E10 + 6E10 + Difluprednate Difluprednate Difluprednate (n=10) (n=10) (n=10) 52 Yes No Adverum conducted a pre-specified Patient
Preference Survey in LUNA Data cut: LUNA 29AUG2024

Commercial Opportunity

Large and Growing Global Market Opportunity Wet AMD physicians and
patients embrace innovation Wet Age-Related Macular Degeneration Multi-Billion Dollar Market Opportunity A leading cause of vision loss among older adults Growth driven by aging population and product innovation Global Wet AMD Sales 20M 1.5M ~$13B
US Patients Worldwide ~$9B ~$6B ~200k US patients diagnosed annually Up to 42% of patients develop bilateral disease within 2-3 years of initial diagnosis 2015 2025E 2035E 54 Sources: Bright Focus Foundation. Age-Related Macular Degeneration: Facts
& Figures.; Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:106–16.; Gangnon RE et al. (2015) JAMA
Ophthalmol; 133 (2): 125–132.; 2023 Cowen Equity Research – Therapeutic Categories Outlook.; Company estimates.

Ixo-vec is Poised to Transform How wAMD is Treated Potential product
profile designed to fit needs of key stakeholders Seamless Patient Powerful and Emerging Predictable Practice Preference Durable Safety Profile Product Profile Efficacy Profile Integration & Impact • Sustained vision • No new onset
of • IVT administration • Reduced patient drop off inflammation past week 30 • Durable anatomic • Integrates into clinical • Sustained long-term vision Providers improvements • Inflammation is infrequent,
practice, operations, and for patients resolves and does not economic model • Substantial reduction in impact vision treatment burden • >50% chance of injection • Easy to manage • Fewer office visits • 93% preference
over prior freedom steroid eye drops IVT injections Patients & • Routine retina visit • Sustained vision for life with • 100% said topical steroids Caregivers few injections were Easy or Very Easy to manage • Potential to
reduce healthcare system burden in a cost-effective manner Payers • Maximize payer return on investment through preserving long-term vision with as little as one injection 55 Projected outcomes based on LUNA and OPTIC data presented and
company expectations of how practices and payers will evolve to a changed paradigm.

Market Leadership in Wet AMD May Require Best-in-Class Profile that
First Demonstrates Success in Hard-to-Treat Patients Annual Injection Burden (IRIS Registry, Weighted Average) Adoption over time >9 Injections 7-9 Injections 4-6 Injections 1-3 Injections Early Adopters Subsequent Expansion Hard-to-Treat
Patients Less Severe Patients 56 Adapted from Wykoff CC, Garmo V, Tabano D, et al. Ophthalmol Sci. 2023;4(2):100421. Proportion of Patients

Wet AMD May be the First Large-Market Indication for Gene Therapy
Market Epidemiology Economics Environment HIGH MASS MARKET URGENCY FOR ADOPTION PREVALENCE PRICING Lack of maintained vision in the Large addressable Lower cost per patient real-world suggest early adoption market at launch than orphan diseases
after initial vision gain LARGE ANNUAL LOWER VALIDATED INCIDENCE COGS TREATMENT New patients, bilateral >1000x lower dose than Aflibercept sustained delivery, disease, growing with systemic gene therapies a mega-blockbuster anti-VEGF population
ages 1 used in over 70M injections 57 1 https://investor.regeneron.com/news-releases/news-release-details/eylea-hdr-aflibercept-injection-8-mg-data-euretina-reinforce

CMC Positioned to Support Pivotal Program and Commercial Scale Up
Ixo-vec currently manufactured at commercial scale Commercial-scale manufacturing in place at global CDMO Supported by robust regulatory CMC input All Ixo-vec Phase 3 material has been manufactured Cost-efficient Sf9 suspension process enables
biologics-like COGS 58

KOL Panel

KOL Panel with Leading Retinal Specialists Discussing LUNA and OPTIC
Results, and Pivotal Trial Design M O DE RA T O R Charles C. Wykoff, MD, PhD Director of Research, Retina Consultants of Texas Star Seyedkazemi, PharmD Chief Development Officer Szilárd Kiss, MD Mark Barakat, MD Distinguished Professor of
Ophthalmology, Director of Clinical Research Director of Retina Service Macula Institute of Arizona Cornell University Adverum Board Member 60

Conclusion & Takeaways

Largest Treatment Burden Reduction in Hard-to-Treat Patients from 6
Months to > 4 Years % Annualized Reduction in Injection Burden 95% 92% 90% 89% 88% 86% 86% 85% 84% 84% 81% 81% 80% OPTIC (2E11) 76% 71% LUNA (6E10) 68% LUNA (2E11) RGX-314 SCS (2.5E11) RGX-314 SCS (5E11) RGX-314 SCS (1E12) 4D-150 (1E10) 4D-150
(3E10) Month 6 Year: 1 2 3 4 Among IVT and suprachoroidal gene therapy for wet AMD; excludes ABBV-RGX-314 subretinal. ABBV-RGX-314 SCS in AAVIATE (NB: 21 of 56 subjects at 1E12 dose received booster shot at wk 4, not included in post RGX-314 mean
annualized injections). RGX-314 data as of 3Feb2024. Latest available 4D-150 PRISM Phase 2a Dose Expansion Cohort data as of 18Sep2024 (Phase 1/2a for 1E10 dose as Phase 2a wasn’t reported 62 separately). Data are based on a cross-trial
comparison and are not based on head-to-head clinical trials. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those
set forth herein. Data cut: OPTIC 21AUG2024, LUNA 29AUG2024
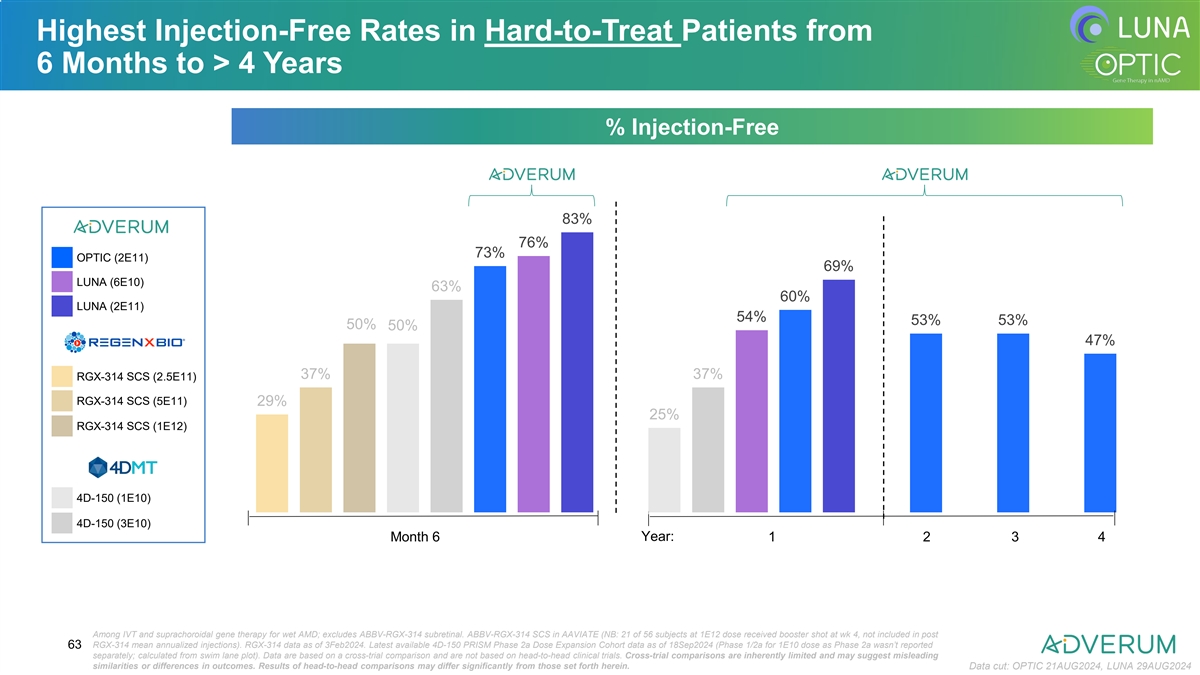
Highest Injection-Free Rates in Hard-to-Treat Patients from 6 Months to
> 4 Years % Injection-Free 83% 76% 73% OPTIC (2E11) 69% LUNA (6E10) 63% 60% LUNA (2E11) 54% 53% 53% 50% 50% 47% 37% 37% RGX-314 SCS (2.5E11) RGX-314 SCS (5E11) 29% 25% RGX-314 SCS (1E12) 4D-150 (1E10) 4D-150 (3E10) Month 6 Year: 1 2 3 4 Among IVT
and suprachoroidal gene therapy for wet AMD; excludes ABBV-RGX-314 subretinal. ABBV-RGX-314 SCS in AAVIATE (NB: 21 of 56 subjects at 1E12 dose received booster shot at wk 4, not included in post RGX-314 mean annualized injections). RGX-314 data as
of 3Feb2024. Latest available 4D-150 PRISM Phase 2a Dose Expansion Cohort data as of 18Sep2024 (Phase 1/2a for 1E10 dose as Phase 2a wasn’t reported 63 separately; calculated from swim lane plot). Data are based on a cross-trial comparison and
are not based on head-to-head clinical trials. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth
herein. Data cut: OPTIC 21AUG2024, LUNA 29AUG2024

5+ Years of Clinical Experience Establish 10x Safety Margin Ixo-vec
6E10 with extended prophylaxis de-risks Phase 3 & commercialization 6E11 Phase 3 Dose N=15 ü Enhanced Safety Profile ü Extended Steroid Prophylaxis ü Potential Best-in-Class Efficacy Profile 2E11 2E11 N=15 N=30 6E10 N=30 4+ Years
Clinical Data 64 DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024 Dose (vg/eye) 10x Safety Margin

Reliable Benefit & Predictable Safety Enable True Paradigm Shift
4-year OPTIC & 52-week LUNA data underscore Ixo-vec’s profile Demonstrated Reliable Long-Term Benefit 78% of patients who were injection free through year 1 remained injection free through year 4 88% of patients who were injection free
through year 2 remained injection free through year 4 Demonstrated Predictable Safety Profile with Local Prophylaxis NO new onset of inflammation after week 30 100% of inflammation resolved by year 1 65 No inflammation: no ≥ 1 AC/VC cells;
OPTIC results refer to 2E11 dose DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

Ixo-vec’s Derisked Phase 3 and Commercial Profile Clinical
updates underscore Ixo-vec’s potential best-in-class product profile • Potential best-in-class product profile >50% injection free and >80% treatment burden reduction in hard-to-treat patients • 10X safety margin with >4
years follow-up • No OPTIC 2E11 patients had inflammation at Year 1 and through Year 4 • No LUNA 6E10 patients had inflammation at 52 weeks or any subsequent visit Favorable safety profile with local prophylaxis • LUNA patient
survey demonstrates strong patient preference for Ixo-vec Broad 6E10 EOP2 284 1H25 ARTEMIS Patient population With topical US study, incorporates Patients Expected Phase 3 Phase 3 Trial steroid eyedrops FDA feedback initiation 66 No inflammation: no
≥ 1 AC/VC cells; OPTIC 2E11 participant with inflammation at year 2.5 underwent a cataract surgery near the start of OPTIC EXT.; EOP2: End of Phase 2 Meeting DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

Q&A

Question & Answer Session Laurent Fischer, MD Rabia Gurses Ozden,
MD Star Seyedkazemi, PharmD President and Chief Medical Officer Chief Development Officer Chief Executive Officer Linda Rubinstein Mike Zanoni Jason Mitchell Peter Soparkar Chief Financial Officer Head of Investor Relations Chief Commercial Officer
Chief Operating Officer Mark Barakat, MD Szilárd Kiss, MD Charles C. Wykoff, MD, PhD Director of Clinical Research Distinguished Professor of Ophthalmology, Director of Research, Macula Institute of Arizona Director of Retina Service Retina
Consultants of Texas Cornell University Adverum Board Member

www.adverum.com For additional questions reach out to: IR@adverum.com
® Preserving Sight for Life

Appendix IVT Gene Therapy for the Treatment of wet AMD

LUNA & OPTIC Study Disposition 90 patients enrolled across LUNA
& OPTIC trials LUNA OPTIC PARTICIPANT DISPOSITION Total Total Number of participants enrolled and dosed with Ixo-vec 60 30 Number of participants who completed 52-week visit 57 30 Number of participants enrolled in open-label extension - 23
Number of participants who completed year 4 visit - 21 Number of participants who discontinued after Ixo-vec dosing 3 7 By week 52 visit 3 0 By year 4 visit - 7 Reason for discontinuation (not related to Ixo-vec) Unrelated adverse event (incl.
death) 3 3 Lost to follow-up / withdrew consent - 4 71 DATA CUT: OPTIC 21AUG2024, LUNA 29AUG2024

Response to Anti-VEGF in Treatment Naïve vs Treatment Experienced
Wet AMD: Similar BCVA Trajectories After Initial 2 Loading Doses Treatment Naive Treatment Experienced 10 15 8 10 5 6 0 -5 4 -10 2 -15 0 4 8 12 16 20 24 28 32 36 40 0 0 4 8 12 16 20 24 28 32 36 40 44 48 Weeks Weeks 6 15 5 4 10 3 2 5 1 0 0 -1 4 8 12
16 20 24 28 32 36 40 44 48 Baseline 4 8 12 16 20 24 28 32 36 40 44 48 52 Weeks Weeks 72 Heier et al, Phase 3, Multicenter, Randomized, Double-Masked, Active Comparator–Controlled Studies to Evaluate the Efficacy and Safety of Faricimab in
Patients With Neovascular Age-Related Macular Degeneration. Angiogenesis 2021 Virtual. EyleaHD (aflibercept injection) [package insert) 2023. Holekamp et al.. Ophthalmology.2022; 129(3):295-307. Khanani et al. Ophthalmology. 2022;129(9):974-985
PULSAR LUCERNE (EYELEA HD) (VABYSMO) Adjusted Mean BCVA Change Adjusted Mean BCVA Change from Baseline (ETDRS Letters) from Baseline (Letters) MERLIN ARCHWAY (BEOVU) (SUSVIMO) LS Mean BCVA Change from Adjusted Mean BCVA Change from Baseline (ETDRS
Letters) Baseline (Letters)

CST Maintained in Patients with High Disease Burden Fluid reduction in
patients with baseline CST >300 μm; maintenance in ≤300 μm Mean CST Change from Baseline at Week 52, μm (95% CI) 150 LUNA Baseline CST ≤300μm (n=27) LUNA Baseline CST >300μm (n=33) 2.9 (-14.3, 20.2) BL
≤300 μm 100 6E10 9.2 (-24.6, 43.0) BL ≤300 μm 50 2E11 -3.4 (-17.9, 11.1) 0 BL ≤300 μm -50 -34.4 (-64.9, -3.9) BL >300 μm 6E10 -100 -35.5 (-83.3, 12.3) BL >300 μm -150 // 2E11 BL 0 2 4 6 8 10 12 14 16
18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 -33.3 (-77.6, 10.9) BL >300 μm Week Baseline Characteristic for Subgroup CST ≤300 µm CST >300 µm Mean CST, µm (SD) 269.9 (18.7) 416.6 (119.1) 73 Data cut: LUNA
29AUG2024 Mean CST Change from Baseline, μm (95% CI)

Stable Intraocular Pressure at Both Doses Mean IOP Over Time by Dose
6E10 30 6E10 Study Eye 6E10 Fellow Eye 25 20 15 10 5 0 // 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 BL Week 2E11 2E11 Study Eye 2E11 Fellow Eye 30 25 20 15 10 5 0 // BL 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32
34 36 38 40 42 44 46 48 50 52 Week 74 Data cut: LUNA 29AUG2024 Mean (SD) IOP mm Mean (SD) IOP mm Hg Hg

LUNA Overall Population: Frequency of Inflammation No patients at 6E10
had inflammation at week 52 or at any subsequent visit Frequency of Inflammation Over Time by Dose 6E10 2E11 LUNA LUNA 30 30 25 25 20 20 15 15 10 10 5 5 0 0 0 2 4 6 8 1012141618202224262830323436384042444648505254565860626466 0 2 4 6 8
10121416182022242628303234363840424446485052545658606264 65 66 65 * Weeks Weeks AC Cells ≥1+ VC Cells ≥1+ 75 AC, aqueous cells; VC, vitreous cells. Cell grades as assessed by slit lamp, Grade categories are based on the Standardization
of Uveitis Nomenclature (SUN) criteria for aqueous cells and National Institutes of Health guidelines for vitreous cells. AC: 0.5+: 1-5 cells 1+: 6-15 cells 2+: 16-25 cells 3+: 26-50 cells 4+: >50 cells; VC: 0.5+: 1-10 cells 1+: 11-20 cells 2+:
21-30 cells 3+: 31-100 cells 4+: >100 cells; Rare cells are captured as 0.5+ for analysis, * . *Protocol amended early in study to include difluprednate starting at week 4 to match the taper in difluprednate regimens; if initiated after week 4
visit, difluprednate may be adjusted at the discretion of investigator in consult with medical monitors (6 participants did not receive difluprednate as part of prophylaxis Data cut: LUNA 29AUG2024 Count of Participants Count of Participants

Case Study: Ixo-vec 2E11 Reduces Fluctuations in Fluid and Central
Subfield Thickness (CST) 90-Year-Old Female with 9 IVTs in the 12 Months Prior to Ixo-vec -56 weeks Aflibercept Q5W prior to Ixo-vec -2 wks (baseline) 90 BCVA: 53 letters CST: 358 µm 80 70 Day 0 60 Ixo-vec administration 50 40 0 1 2 3 Years 600
500 400 300 200 100 -1 2 3 0 1 -55 -43 -31 -19 -7 5 17 29 41 53 65 77 89 101 113 125 137 149 161 173 Years Baseline Anti-VEGF injection Study visit, no supplemental injection 76 Data cut: OPTIC 21AUG2024 CST (µm) BCVA (ETDRS)

Case Study: Ixo-vec 2E11 Reduces Fluctuations in Fluid and Central
Subfield Thickness (CST) 90-Year-Old Female with 9 IVTs in the 12 Months Prior to Ixo-vec -56 weeks Aflibercept Q5W prior to Ixo-vec 100% anti-VEGF injection free following Ixo-vec Ixo-vec -2 wks (baseline) 90 BCVA: 53 letters CST: 358 µm 80 70
Day 0 60 Ixo-vec administration 50 9 Months 40 0 1 2 3 BCVA ∆: +18 letters Years CST ∆: -127 µm Ixo-vec 1 Year 600 BCVA ∆: +16 letters 500 CST ∆: -132 µm 400 2 Years 300 BCVA ∆: +5 letters 200 CST ∆: -93
µm 100 -1 2 3 2.2 Years 0 1 -55 -43 -31 -19 -7 5 17 29 41 53 65 77 89 101 113 125 137 149 161 173 Years BCVA ∆: +13 letters CST ∆: -135 µm 77 Baseline Anti-VEGF injection Study visit, no supplemental injection Data cut: OPTIC
21AUG2024 CST (µm) BCVA (ETDRS)

v3.24.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Adverum Biotechnologies (NASDAQ:ADVM)
過去 株価チャート
から 12 2024 まで 1 2025
Adverum Biotechnologies (NASDAQ:ADVM)
過去 株価チャート
から 1 2024 まで 1 2025