Form 8-K - Current report
2023年12月14日 - 10:05PM
Edgar (US Regulatory)
false000185672500018567252023-12-142023-12-14
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): December 14, 2023 |
Rani Therapeutics Holdings, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
Delaware |
001-40672 |
86-3114789 |
(State or other jurisdiction
of incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
2051 Ringwood Avenue |
|
San Jose, California |
|
95131 |
(Address of principal executive offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (408) 457-3700 |
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Class A common stock, par value $0.0001 per share |
|
RANI |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On December 14, 2023, Rani Therapeutics Holdings, Inc. issued a press release to announce preclinical data regarding a unimolecular triagonist molecule delivered via endoscope and an update regarding timing of the initiation of its RT-102 Phase 2 clinical study.
Data Highlights
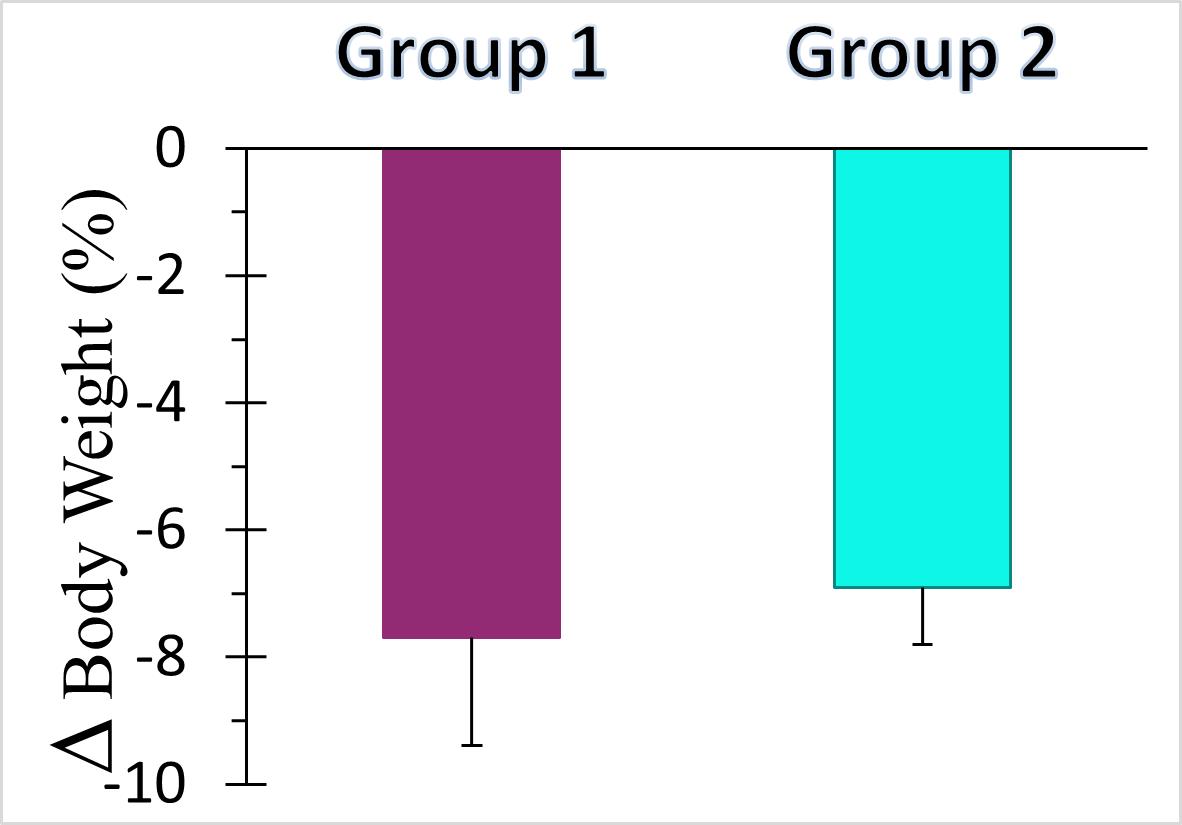
The recent preclinical study evaluated the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of an incretin triagonist (GLP-1, GIP, glucagon receptors) when delivered via endoscope-guided transenteric administration to mimic the RaniPill route of administration, versus the traditional administration route of subcutaneous (SC) injection. The study was conducted in canines separated into two groups. In Group 1 (N=5), 0.12 mg/kg of drug was administered via transenteric delivery by endoscope. In Group 2 (N=5), 0.12 mg/kg of drug was administered by subcutaneous injection. Blood samples were collected over 2 weeks for analysis of serum drug concentrations and various PD and safety biomarkers.
A single dose of drug delivered via either transenteric or SC routes elicited rapid decreases in body weight and serum lipids. Weight loss is believed to be due to early satiety leading to reduced caloric intake. The drug was well tolerated in both groups with no serious adverse events (SAEs) observed or changes in safety markers examined.
Peak Decreases in Body Weights
|
|
|
|
Group 1: Endoscopic (N=5) Group 2: SC (N=5) |
|
All data are Means ± SE |
|
Near-Term Milestone Expectations
•Initiation of Phase 2 clinical trial of RT-102, a RaniPill GO containing teriparatide for osteoporosis, expected in 2024.
•Topline results of Phase 1 clinical trial of RT-111, a RaniPill GO containing ustekinumab biosimilar CT-P43, expected in the first quarter of 2024.
•Development of RaniPill HC to be ready for potential Phase 1 clinical trials in the second half of 2024.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the expected initiation of a Phase 2 clinical trial of RT-102 in 2024, the potential opportunity for the RaniPill platform to support oral delivery of multiple obesity drug products, the expected timing of topline results from the RT-111 Phase 1 clinical trial in the first quarter of 2024, the expected readiness of the RaniPill HC for clinical development in the second half of 2024, the potential for the tranenteric delivery via endoscope of an incretin triagonist to mimic the RaniPill route of delivery, the likelihood that weight loss in the preclinical study of the incretin triagonist was caused by early satiety leading to reduced caloric intake, the belief that the preclinical data are reflective of the potential contributions the RaniPill capsule can make to the GLP-1 receptor agonist space and the broader obesity market, and the potential for Rani to use the RaniPill platform to create oral alternatives for single and multiagonist drugs with differentiated dosing flexibility. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as
“potential,” “expected,” “believed,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2022, and subsequent filings and reports by Rani. All forward-looking statements contained in this Form 8-K speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
Rani Therapeutics Holdings, Inc. |
|
|
|
|
Date: |
December 14, 2023 |
By: |
/s/ Svai Sanford |
|
|
|
Svai Sanford
Chief Financial Officer |
v3.23.3
Document And Entity Information
|
Dec. 14, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 14, 2023
|
| Entity Registrant Name |
Rani Therapeutics Holdings, Inc.
|
| Entity Central Index Key |
0001856725
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-40672
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
86-3114789
|
| Entity Address, Address Line One |
2051 Ringwood Avenue
|
| Entity Address, City or Town |
San Jose
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
95131
|
| City Area Code |
(408)
|
| Local Phone Number |
457-3700
|
| Entity Information, Former Legal or Registered Name |
N/A
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Class A common stock, par value $0.0001 per share
|
| Trading Symbol |
RANI
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Rani Therapeutics (NASDAQ:RANI)
過去 株価チャート
から 11 2024 まで 12 2024

Rani Therapeutics (NASDAQ:RANI)
過去 株価チャート
から 12 2023 まで 12 2024
