As filed with the Securities and Exchange
Commission on December 4, 2023.
Registration No. 333-260338
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
POST-EFFECTIVE AMENDMENT
NO. 3 TO
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
NeuroSense Therapeutics
Ltd.
(Exact Name of Registrant as Specified in Its Charter)
Not Applicable
(Translation of Registrant’s name into English)
| State of Israel |
|
2834 |
|
Not Applicable |
(State or Other Jurisdiction of
Incorporation or Organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
11 HaMenofim Street,
Building B
Herzliya 4672562 Israel
+972-9-799-6183
(Address, Including Zip Code, and Telephone Number,
Including Area Code, of Registrant’s Principal Executive Offices)
Cogency Global Inc.
122 East 42nd Street,
18th Floor, New York, NY 10168
(212) 947-7200
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
Brian K. Rosenzweig
Sarah C. Griffiths
Covington & Burling LLP
The New York Times Building
620 Eighth Avenue
New York, New York 10018
(212) 841-1000 |
|
Perry Wildes
Goldfarb Gross Seligman & Co.
One Azrieli Center
Tel Aviv 6702100, Israel
+972 (3) 607-4444 |
Approximate date of commencement of proposed
sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this
form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this form is filed to register additional securities
for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is
an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒
If an emerging growth company that prepares its
financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standards† provided pursuant to Section 7(a)(2)(B)
of the Securities Act. ☐
| † | The term “new or revised financial accounting standard”
refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5,
2012. |
The Registrant hereby amends this Registration
Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which
specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to
such Section 8(a), may determine.
EXPLANATORY NOTE
NeuroSense
Therapeutics Ltd. (the “Company”) filed with the Securities and Exchange Commission (the “SEC”) a Registration
Statement on Form F-1 (Registration No. 333-260338) on October 18, 2021, as subsequently amended by Post-Effective Amendment No. 1, which
was initially declared effective by the Securities and Exchange Commission, or the SEC, on December 8, 2021 and by Post-Effective Amendment
No. 2 filed with the SEC on November 21, 2023 (the “Registration Statement”). The Registration Statement originally registered
the offer and sale by the Company in its initial public offering (the “IPO”) of (i) 2,000,000 units (the “Units”),
with each Unit consisting of one ordinary share and a warrant to purchase one ordinary share (the “Base Warrants”), (ii)
an option by the underwriters to purchase up to 300,000 additional ordinary shares and/or 300,000 warrants to purchase up to an additional
300,000 ordinary shares (the “Option Warrants,” and together with the together with the Base Warrants, the “Public
Warrants”), and (iii) warrants granted to the underwriters to purchase up to 100,000 ordinary shares (the “Underwriter Warrants,”
and together with the Public Warrants, the “Warrants”).
On
December 13, 2021, the Company closed its IPO in which it sold the Units. In addition, the underwriters exercised their option to purchase
the Option Warrants. As of the date of this prospectus, we had (i) 1,655,000 outstanding Public Warrants and (ii) 100,000 Underwriter
Warrants.
On
April 28, 2022, the Company filed a post-effective amendment to the Registration Statement, to file a consent of Somekh Chaikin, Member
Firm of KPMG International, with respect to its report dated April 14, 2022 relating to the Company’s financial statements contained
in its Annual Report on Form 20-F for the year ended December 31, 2021.
On
November 21, 2023, the Company filed Post-Effective Amendment No. 2 to the Registration Statement to update and supplement information
contained in the Registration Statement and to incorporate by reference the Company’s Annual Report on Form 20-F for the year ended
December 31, 2022, filed with the SEC on March 22, 2023 and subsequent submissions made by the Registration with the SEC.
This
Post-Effective Amendment No. 3 is being filed by the Company to update the information contained in the Registration Statement to reflect
the Company’s financial results for the third quarter ending September 30, 2023.
No additional
securities are being registered under this Post-Effective Amendment No. 3. This Post-Effective Amendment No. 3 concerns only the offer
and sale of ordinary shares issuable from time to time upon exercise of the Warrants that remain unexercised.
All
filing fees payable in connection with the registration of these securities were previously paid in connection with the initial filing
of the Registration Statement.
The information in
this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed
with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities nor does
it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PROSPECTUS |
|
SUBJECT TO COMPLETION DATED DECEMBER
4, 2023 |
Up to 1,755,000 Ordinary Shares Underlying Previously
Issued Warrants

NeuroSense Therapeutics Ltd.
This prospectus relates to
the issuance of up to (i) 1,655,000 of our ordinary shares, no par value per share (the “ordinary shares”) issuable upon the
exercise of warrants that were issued as part of an initial public offering (the “Public Warrants”), at an exercise price
of $6.00 per ordinary share and (ii) 100,000 ordinary shares issuable upon the exercise of warrants that were issued to the underwriters
of our initial public offering at an exercise price of $7.50 per ordinary share (the “Underwriter Warrants”).
Our ordinary shares trade
on the Nasdaq Capital Market, or Nasdaq, under the symbol “NRSN”, and our Public Warrants trade on the Nasdaq under the symbol
“NRSNW”. On November 30, 2023, the last reported sale price of our ordinary shares on Nasdaq was $1.29 per ordinary share.
We are an “emerging growth
company” and a “foreign private issuer” as defined under the U.S. federal securities laws, and as such, are eligible
for reduced public company disclosure requirements. See “Prospectus Summary—Implications of Being an Emerging Growth Company
and a Foreign Private Issuer” for additional information.
Investing in our securities
is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 5 to read about factors you
should consider before buying any of our ordinary shares.
Neither the Securities and Exchange Commission
nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of the disclosures
in this prospectus. Any representation to the contrary is a criminal offense.
Prospectus dated ,
2023
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of
the registration statement on Form F-1 that we filed with the Securities and Exchange Commission (the “SEC”) for the
issuance of the ordinary shares covered by this prospectus.
You should not assume that
the information contained in, or incorporated by reference into, this prospectus is accurate on any date subsequent to the date set forth
on the front cover of this prospectus, even though this prospectus is delivered or ordinary shares covered by this prospectus are sold
or otherwise disposed of on a later date. It is important for you to read and consider all information contained in, or incorporated by
reference into, this prospectus in making your investment decision. You should also read and consider the information in the documents
to which we have referred you under the caption “Where You Can Find Additional Information” in this prospectus.
We have not authorized anyone
to provide any information or to make any representation other than those contained in, or incorporated by reference into, this prospectus.
You must not rely upon any information or representation not contained in, or incorporated by reference into, this prospectus. This prospectus
does not constitute an offer to sell or the solicitation of an offer to buy any of our securities other than the securities covered hereby,
nor does this prospectus constitute an offer to sell or the solicitation of an offer to buy any securities of the Company in any jurisdiction
to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
This prospectus, including
the information incorporated by reference herein, contains forward-looking statements that are subject to a number of risks and uncertainties,
many of which are beyond our control. See “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.”
Unless otherwise indicated or the context otherwise
requires, all references in this prospectus to the terms “NeuroSense,” “NeuroSense Therapeutics,” the “Company,”
“we,” “us” and “our” refer to NeuroSense Therapeutics Ltd. In this prospectus, any reference to any
provision of any legislation shall include any amendment, modification, re-enactment or extension thereof. Words importing the singular
shall include the plural and vice versa, and words importing the masculine gender shall include the feminine or neutral gender.
TRADEMARKS, SERVICE MARKS AND TRADENAMES
The NeuroSense Therapeutics
logo and other trademarks and service marks of NeuroSense Therapeutics Ltd. appearing in this prospectus or the information incorporated
by reference herein are the property of the Company. Solely for convenience, some of the trademarks, service marks, logos and trade names
referred to in this prospectus are presented without the® and™ symbols, but such references are not intended
to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks, service
marks and trade names. This prospectus, including the information incorporated by reference herein, contains additional trademarks, service
marks and trade names of others. All trademarks, service marks and trade names appearing in this prospectus or the information incorporated
by reference herein are, to our knowledge, the property of their respective owners. We do not intend our use or display of other companies’
trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
MARKET AND INDUSTRY DATA
This prospectus, including
the information incorporated by reference herein, contains industry, market and competitive position data that are based on industry publications
and studies conducted by third parties as well as our own internal estimates and research. These industry publications and third-party
studies generally state that the information that they contain has been obtained from sources believed to be reliable, although they do
not guarantee the accuracy or completeness of such information. While we believe that each of these publications and third-party studies
is reliable, we have not independently verified the market and industry data obtained from these third-party sources. Forecasts and other
forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward-looking
statements included in, or incorporated by reference into, this prospectus. While we believe our internal research is reliable and the
definition of our market and industry are appropriate, neither such research nor these definitions have been verified by any independent
source.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and certain
information incorporated by reference herein contain forward-looking statements within the meaning of Section 27A of the Securities
Act and Section 21E of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”),
and other securities laws. Many of the forward-looking statements contained in, or incorporated by reference into, this prospectus can
be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “estimate,”
“expect,” “intend,” “may,” “might,” “plan,” “potential,” “should,”
“target,” “would” and other similar expressions that are predictions of or indicate future events and future trends,
although not all forward-looking statements contain these identifying words.
Forward-looking statements
are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements
are subject to substantial risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking
statements due to a variety of factors, including, but not limited to, those identified under the section titled “Risk Factors”
in this prospectus or the documents incorporated herein. These risks and uncertainties include factors relating to:
| ● | the going concern reference in our financial statements and
our need for substantial additional financing to achieve our goals; |
| ● | our limited operating history and history of incurring significant
losses and negative cash flows since our inception, which we anticipate will continue for the foreseeable future; |
| ● | our dependence on the success of our lead product candidate,
PrimeC, including our obtaining of regulatory approval to market PrimeC in the United States; |
| ● | our limited experience in conducting clinical trials and
reliance on clinical research organizations and others to conduct them; |
| ● | our ability to advance our preclinical product candidates
into clinical development and through regulatory approval; |
| ● | the results of our clinical trials, which may fail to adequately
demonstrate the safety and efficacy of our product candidates; |
| ● | our ability to achieve the broad degree of physician adoption
and use and market acceptance necessary for commercial success; |
| ● | our reliance on third parties in marketing, producing or
distributing products and research materials for certain raw materials, compounds and components necessary to produce PrimeC for clinical
trials and to support commercial scale production of PrimeC, if approved; |
| ● | our receipt of regulatory clarity and approvals for our therapeutic
candidates and the timing of other regulatory filings and approvals; |
| ● | estimates of our expenses, revenues, capital requirements
and our needs for additional financing; |
| ● | our efforts to obtain, protect or enforce our patents and
other intellectual property rights related to our product candidates and technologies; |
| ● | our ability
to maintain the listing of our ordinary shares on Nasdaq; |
| ● | the impact
of the public health, political and security situation in Israel, the U.S. and other
countries in which we may obtain approvals for our products or our business; and |
| |
● |
the
impacts on our ongoing and planned trials and manufacturing as a result of the war in Israel. |
The preceding list is not intended
to be an exhaustive list of all of our risks and uncertainties. As a result of these factors, we cannot assure you that the forward-looking
statements contained in, or incorporated by reference into, this prospectus will prove to be accurate. Furthermore, if our forward-looking
statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking
statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our
objectives and plans in any specified time frame, or at all.
In addition, statements that
“we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon
information available to us as of the date of this prospectus or any document incorporated herein or therein, and while we believe such
information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not
be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information.
PROSPECTUS SUMMARY
This summary highlights
selected information about us and the ordinary shares being offered. It may not contain all of the information that may be important to
you. Before investing in the ordinary shares, you should read this entire prospectus and other information incorporated by reference from
our other filings with the SEC carefully for a more complete understanding of our business and this offering, including our consolidated
financial statements and the section entitled “Risk Factors” included or incorporated by reference in this prospectus.
Overview
We are a clinical-stage
biotechnology company focused on discovering and developing treatments for patients suffering from neurodegenerative diseases, including
Amyotrophic Lateral Sclerosis (“ALS”), Alzheimer’s disease (“AD”) and Parkinson’s disease (“PD”).
We believe these diseases represent some of the most significant unmet medical needs of our time, with limited effective therapeutic options
available. The burden of these diseases on both patients and society is substantial. For example, the average annual cost of ALS alone
is $180,000 per patient, and its estimated annual burden on the U.S. healthcare system is greater than $1 billion. Due to the
complexity of neurodegenerative diseases, our strategy is utilizing a combined therapeutic approach to target multiple disease-related
pathways.
Our lead therapeutic candidate,
PrimeC, is a novel extended-release oral formulation, fixed-dose combination of two FDA-approved drugs, ciprofloxacin and celecoxib. PrimeC
is designed to treat ALS by modulating microRNA synthesis, iron accumulation, and neuroinflammation, all of which are hallmarks of ALS
pathology. The U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”) have granted
PrimeC orphan drug designation for the treatment of ALS. In addition, the EMA has granted PrimeC the Small and Medium-Sized Enterprise
(SME) status, which offers significant potential benefits leading up to and following drug regulatory approval. We believe PrimeC’s
multifactorial mechanism of action has the potential to significantly prolong lifespan and improve ALS patients’ quality of life,
thereby reducing the burden of this debilitating disease on both patients and healthcare systems.
PrimeC is currently being evaluated
in PARADIGM (“NST003”), a Phase IIb randomized, multi-center, multinational, prospective, double-blind, placebo-controlled
study, to evaluate safety, tolerability, and efficacy of PrimeC in 69 people living with ALS. Participants are being administered
PrimeC or placebo at a 2:1 ratio, respectively. Study participants are allowed to continue standard of care treatment of approved products.
The primary endpoints of the study are an evaluation of ALS-biomarkers as well as safety and tolerability assessment. Secondary and exploratory
endpoints are the evaluation of clinical efficacy (ALS Functional Rating Scale — Revised and slow vital capacity), survival,
and improvement in quality of life. All subjects who complete the six-month double-blind, placebo-controlled dosing period are transferred
to the PrimeC active arm for a 12-month open label extension. The study completed enrollment in May 2023, enrolling 69 participants
living with ALS in Israel, Italy, and Canada. On November 6, 2023, we announced that we had completed dosing of the last patient in the
double-blind segment of the study. We anticipate reporting clinical efficacy (secondary endpoints) and safety and tolerability (primary
endpoints) results of the double-blind segment of the trial in December 2023. We also expect to report on another primary endpoint, the
assessment of ALS-biomarkers, TDP-43 and Prostagladin2, in the first half of 2024.
Since the FDA had requested
additional non-clinical data to support the overall duration of the PARADIGM trial, as PrimeC is intended for long-term administration
in the treatment of ALS, we withdrew the protocol from its investigational new drug application (“IND”) in order to align
our clinical strategy with the FDA, and we intend to discuss a potential trial design for an ALS pivotal trial with the FDA. Subject to
agreement with FDA on trial design, the results of the PARADIGM trial and results of additional studies we intend to conduct in the interim,
we believe we could be in a position to initiate a pivotal clinical trial of PrimeC for the treatment of ALS as early as 2024. On November
13, 2023, we announced that we had concluded a successful Type D meeting with the FDA regarding chemistry, manufacturing, and controls
(“CMC”) development plans in advance of an expected Phase 3 pivotal study and potential subsequent marketing approval. The
FDA agreed with our proposed CMC development plan.
PrimeC was previously evaluated
in a Phase IIa clinical trial (“NST002”) in 15 people living with ALS, conducted at the Tel Aviv Sourasky Medical Center,
Israel. The primary endpoint of the NST002 trial, which was safety and tolerability, was met. In this trial, the safety profile observed
was consistent with known safety profiles of ciprofloxacin and celecoxib. Side effects were mild and transient in nature. There were no
new or unexpected safety signals detected during the trial.
Additionally, we observed positive
clinical signals in comparison to virtual controls, and a serum biomarker analysis showed significant changes following treatment, indicating
biological activity of the drug in comparison to untreated matched ALS patients. All 12 patients who completed the NST002 trial elected
to continue into an extension study with PrimeC, that was conducted as an Investigator Initiated Study. To date, the Company is still
supporting the drug supply for a few of the participants in this study, which is over than 40 months since NST002 was initiated.
We completed three additional
studies in 2022 as part of our drug development program to further support our future regulatory submissions. In April 2022, we initiated
a pharmacokinetic (“PK”) study (“NCT05232461”) of PrimeC. The PK open-label, randomized, single-dose, three-treatment,
three-period crossover study evaluated the effect of food on the bioavailability of PrimeC as compared to the bioavailability of co-administered
ciprofloxacin tablets and celecoxib capsules in adult subjects in the U.S. under an FDA cleared IND protocol.
In August 2022, we completed
enrollment and dosing of all subjects in a multi-dose PK study (“NCT05436678”). On September 28, 2022, we released the
results of the NCT05436678 study. Based on results, we believe the PK profile of PrimeC supports the formulation’s extended-release
properties, as the concentrations of the active components have been synchronized, aiming to potentially maximize the synergism between
the two compounds. In June 2022, we reported the successful completion of the “in-life” phase of its 90-day GLP toxicology
study. In this study, the components of PrimeC, celecoxib and ciprofloxacin, were administered to rodents at doses 4x the maximal clinical
dose. All animals appeared normal, with no significant findings observed. The Company intends to present the data from these studies to
the FDA as part of PrimeC’s drug development plan.
We believe we have a strong
patent estate, including patents on method of use, combination, and formulation. We secured U.S. Patent 10,980,780 relating to methods
for treatment of ALS using ciprofloxacin and celecoxib, the components of PrimeC, which expires in 2038. This patent also been issued
in the European Patent Office, Canada, Australia, Israel and Japan. We also expect to take advantage of orphan drug exclusivity for PrimeC,
if approved, for seven years in the United States and ten years in the European Union. In addition, U.S. patent application
16/623,467, which relates to methods of treatment of neurodegenerative disease using combinations of ciprofloxacin and celecoxib, is currently
pending. This patent application is expected to expire on June 20, 2038.
Our organization is built around
a management team with extensive experience in the pharmaceutical industry, with a particular focus on ALS research and clinical trials.
We believe that our leadership team is well-positioned to lead us through clinical development, regulatory approval and commercialization
of our product candidates.
In addition to PrimeC, we have
conducted research and development efforts in AD and PD, with a similar strategy of combined products. The following chart represents
our current product development pipeline:

In May 2023, we entered
into a Collaborative Evaluation Agreement with Biogen MA Inc. (“Biogen”), a subsidiary of Biogen Inc., under which Biogen
will evaluate the impact of PrimeC on neurofilament levels in the plasma of participants in PARADIGM. Biogen will fund the neurofilament
biomarker study and conduct the analysis. Biogen also received a right of first refusal for a definitive licensing agreement to co-develop
and/or commercialize PrimeC for the treatment of ALS. We expect to report the results of the studies conducted pursuant to the Collaborative
Evaluation Agreement with Biogen in the first quarter of 2024.
Corporate Information
Our legal and commercial name
is NeuroSense Therapeutics Ltd. We were incorporated on February 13, 2017 and were registered as a private company limited by shares
under the laws of the State of Israel. We completed our initial public offering on the Nasdaq in December 2021. The ordinary shares
and warrants to purchase ordinary shares are traded on the Nasdaq under the symbol “NRSN” and “NRSNW,” respectively.
Our principal executive offices
are located at 11 HaMenofim Street, Building B, Herzliya, 4672562 Israel, and our telephone number is +972-9-7996183. Our website address
is www.neurosense-tx.com. The information on our website does not constitute a part of this prospectus. Our agent for service of
process in the United States is Cogency Global Inc., 122 East 42nd Street, 18th Floor, New York, NY 10168.
Implications of Being an Emerging Growth Company
As a company with less than
$1.235 billion in revenue during our last fiscal year, we are an “emerging growth company” as defined in the Jumpstart
Our Business Startups Act of 2012 (the “JOBS Act”). As such, we may take advantage of certain exemptions from various
reporting requirements that are applicable to other publicly traded entities that are not emerging growth companies. These exemptions
include:
| ● | not being required to have our registered independent public
accounting firm attest to management’s assessment of our internal control over financial reporting; |
| ● | not being required to comply with any requirement that may
be adopted by the Public Company Accounting Oversight Board (“PCAOB”), regarding mandatory audit firm rotation or a supplement
to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion
and analysis); |
| ● | not being required to submit certain executive compensation
matters to stockholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “say-on-golden parachutes”; |
| | ● | the audit reports of our independent registered public accounting firm do not require communication of critical audit matters; and |
| ● | not being required to disclose certain executive compensation
related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s
compensation to median employee compensation. |
As a result, the information
contained in this prospectus and the documents incorporated by reference herein may be different from the information you receive from
other public companies in which you hold shares. We may take advantage of these provisions for up until we are no longer an emerging growth
company. We would cease to be an emerging growth company upon the earliest to occur of: (i) the last day of the first fiscal
year in which our annual gross revenues exceed $1.235 billion; (ii) the date on which we have issued more than $1 billion
in non-convertible debt securities during the previous three years; (iii) the date on which we are deemed to be a “large
accelerated filer” as defined in Rule 12b-2 under the Exchange Act; or (iv) the last day of the fiscal year
following the fifth anniversary of our initial public offering.
Implications of Being a Foreign Private Issuer
We are also a non-U.S. company
with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we continue to qualify as
a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable
to U.S. domestic public companies, including:
| ● | the rules under the Exchange Act requiring domestic
filers to issue financial statements prepared under U.S. generally accepted accounting principles (“U.S. GAAP”); |
| ● | the sections of the Exchange Act regulating the solicitation
of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| ● | the sections of the Exchange Act requiring insiders
to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short
period of time; and |
| ● | the rules under the Exchange Act requiring the filing
with the SEC of quarterly reports on Form 10-Q containing unaudited financial statements and other specified information, and current
reports on Form 8-K upon the occurrence of specified significant events. |
Notwithstanding these exemptions,
we will file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC,
an Annual Report on Form 20-F containing financial statements audited by an independent registered public accounting firm.
We may take advantage of these
exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as
more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies:
(i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets
are located in the United States; or (iii) our business is administered principally in the United States.
Both foreign private issuers
and emerging growth companies also are exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no
longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent
compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
THE OFFERING
| Securities offered by us |
|
Up to 1,755,000 ordinary shares, upon the exercise of Warrants |
| |
|
|
Ordinary
shares outstanding prior to this offering |
|
13,666,042 ordinary shares |
| |
|
|
| Ordinary shares outstanding immediately after this offering |
|
15,421,042 ordinary shares
if the Warrants offered in this offering are exercised in full. |
| |
|
|
| Use of Proceeds |
|
We will receive the exercise price upon any exercise of the Warrants, to the extent exercised on a cash basis. We will receive gross proceeds of approximately $10.7 million assuming the exercise of all 1,755,000 Warrants for cash. However, the holders of the Warrants are not obligated to exercise the Warrants, and we cannot predict whether or when, if ever, the holders of the Warrants will choose to exercise the Warrants, in whole or in part. The exercise price of the Warrants may exceed the trading price of our ordinary shares. Accordingly, we currently intend to use the proceeds received upon such exercise, if any, for general corporate purposes and working capital. |
| |
|
|
| Listing |
|
Our ordinary shares trade on the Nasdaq under the symbol “NRSN”, and our Public Warrants trade on the Nasdaq under the symbol “NRSNW”. |
| |
|
|
| Risk factors |
|
You should carefully read the section titled “Risk Factors” and other information included in this prospectus for a discussion of factors that you should consider before deciding to invest in our securities. |
Unless
otherwise indicated, the number of ordinary shares outstanding prior to this offering is based on 13,666,042 ordinary shares outstanding
as of September 30, 2023, and excludes:
| ● | 1,093,128 ordinary
shares issuable upon the exercise of options outstanding as of September 30, 2023, at
a weighted average exercise price of $2.30 under our 2018 Employee Share Option Plan; |
| ● | 360,000
ordinary shares issuable upon the vesting of restricted share units outstanding under our
2018 Share Incentive Plan; |
| ● | 209,543 ordinary
shares reserved for issuance and available for future grant under our 2018 Share Incentive
Plan; |
| ● | 1,670,000 ordinary shares underlying pre-funded warrants
with an exercise price of $0.0001 per share issued in connection with our registered direct offering in June 2023 (all of which have
been exercised as of the date of this prospectus); |
| ● | 3,000,000 ordinary shares underlying warrants with an exercise
price of $1.50 per share issued in connection with our registered direct offering in June 2023; and |
| ● | 1,755,000 ordinary shares underlying the Warrants to which
this prospectus relates. |
Unless
otherwise stated, all information in this prospectus assumes no exercise of the outstanding options or warrants into ordinary shares
as described above.
RISK FACTORS
Investing in our securities
involves significant risks. Before making an investment decision, you should carefully consider the risks described below and under the
section titled “Item 3. Key Information — D. Risk Factors” in our Annual Report on Form 20-F
for the year ended December 31, 2022 which is incorporated by reference herein, as well as any other information included or incorporated
by reference in this prospectus, together with all of the other information appearing in this prospectus or incorporated by reference
herein, including in light of your particular investment objectives and financial circumstances. The risks so described are not the only
risks we face. Additional risks not presently known to us or that we currently deem immaterial may also impair our business operations
and become material. Our business, financial condition and results of operations could be materially adversely affected by any of these
risks. The trading price of our securities could decline due to any of these risks, and you may lose all or part of your investment. The
discussion of risks includes or refers to forward-looking statements; you should read the explanation of the qualifications and limitations
on such forward-looking statements discussed elsewhere in this prospectus under the caption “Cautionary Note Regarding Forward-Looking
Statements” above.
Risk Related to our Ordinary Shares
Our failure to maintain
compliance with Nasdaq’s continued listing requirements could result in the delisting of our ordinary shares.
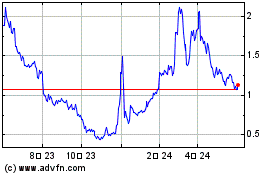
On September 25, 2023, we
received a notice from the Listing Qualifications Department of Nasdaq notifying us that, because the closing bid price for our ordinary
shares had fallen below $1.00 per share for 30 consecutive business days, we no longer meet the minimum bid price requirement for continued
listing on Nasdaq under Nasdaq Listing Rule 5550(a)(2). The notice provided a grace period of 180
calendar days, or until March 25, 2024, to regain compliance with the minimum closing bid price requirement for continued listing.
In order to regain compliance,
the closing bid price of the ordinary shares must meet or exceed $1.00 per share for at least ten consecutive business days during this
180-day grace period. If we do not regain compliance with Rule 5550(a)(2) by March 25, 2024, we may be eligible for an additional 180-calendar
day compliance period. To qualify for the additional 180-calendar day compliance period, we must meet the continued listing requirement
for market value of publicly held shares and all other initial listing standards for the Nasdaq, except for the minimum bid price requirement,
and provide written notice to Nasdaq of our intent to cure the deficiency during this second compliance period, by effecting a reverse
share split, if necessary. However, if it appears to the Nasdaq staff that we will not be able to cure the deficiency, or if we are otherwise
not eligible, Nasdaq will provide notice to us that the ordinary shares will be subject to delisting.
No assurance can be given
that we will be able to comply with the other standards that we are required to meet in order to maintain a listing on such exchange,
such as the minimum stockholders’ equity and minimum market value of publicly held shares, and no assurance can be given that the
price of the ordinary shares will not again be in violation of Nasdaq’s minimum bid price rule in the future. Our failure to meet
these requirements may result in our securities being delisted from Nasdaq.
If the ordinary shares are
delisted from Nasdaq, we may seek to list them on other markets or exchanges or the ordinary shares may trade on the pink sheets. In the
event of such delisting, our shareholders’ ability to trade, or obtain quotations of the market value of, our ordinary shares would
be severely limited because of lower trading volumes and transaction delays. These factors could contribute to lower prices and larger
spreads in the bid and ask prices for our securities. In addition, the substantially decreased trading in the ordinary shares and decreased
market liquidity of the ordinary shares as a result of the loss of market efficiencies associated with Nasdaq and the loss of federal
preemption of state securities laws, which could materially adversely affect our ability to obtain financing on acceptable terms, if at
all, and may result in the potential loss of confidence by investors, suppliers, customers and employees and fewer business development
opportunities. Additionally, the market price of the ordinary shares may decline further and stockholders may lose some or all of their
investment. There can be no assurance that the ordinary shares, if delisted from the Nasdaq in the future, would be listed on another
national or international securities exchange or on a national quotation service, the Over-The-Counter Markets or the pink sheets.
We may effect a
reverse stock split of our ordinary shares, but it may not result in us obtaining the intended benefits.
We may be required to seek
shareholder approval to effect a reverse share split in order to regain compliance with Nasdaq’s minimum
closing bid price requirement. However, if we do effect a reverse share split, there can be no assurance that the market price
per new ordinary share after the reverse share split will remain unchanged or increase in proportion to the reduction in the number of
old shares outstanding before the reverse share split. Other factors, such as our financial results, market conditions and the market
perception of our business may adversely affect the market price of our ordinary shares, and there can be no assurance that a reverse
share split, if completed, will result in the intended benefits, that the market price of our ordinary shares will increase in proportion
to the reduction in the number of shares outstanding before the reverse share split or that the market price of our ordinary shares will
not decrease in the future. If the market price of our ordinary shares does not increase the price per share above Nasdaq’s minimum
bid price threshold of $1.00 per share or if the market price of our ordinary shares does not remain above Nasdaq’s minimum bid
price threshold of $1.00 per share, our ordinary shares may still be delisted from Nasdaq.
If we do effect a reverse
stock split, the liquidity of our ordinary shares may be affected adversely by any such reverse share split given the reduced number of
shares that will be outstanding following the reverse share split, especially if the market price of our ordinary shares does not increase
as a result of the reverse share split.
Risk Related to this Offering
The price of our ordinary shares may be volatile.
The market price of the ordinary shares has fluctuated
in the past. Consequently, the current market price of our ordinary shares may not be indicative of future market prices, and we may be
unable to sustain or increase the value of your investment in our ordinary shares.
Risks Related to our Operations
in Israel
We conduct some of our
operations in Israel. Conditions in Israel, including the recent attack by Hamas and other terrorist organizations from the Gaza Strip
and Israel’s war against them, may affect our operations.
Because we are incorporated
under the laws of the State of Israel, and most of our officers and fourteen out of sixteen of our employees are residents of Israel,
our business and operations are directly affected by economic, political, geopolitical and military conditions in Israel. Since the establishment
of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its neighboring countries and terrorist organizations
active in the region. These conflicts have involved missile strikes, hostile infiltrations and terrorism against civilian targets in various
parts of Israel, which have negatively affected business conditions in Israel.
In October 2023, Hamas terrorists
infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas
also launched extensive rocket attacks on Israeli population and industrial centers located along Israel’s border with the Gaza
Strip and in other areas within the State of Israel. These attacks resulted in thousands of deaths and injuries, and Hamas additionally
kidnapped many Israeli civilians and soldiers. Following the attack, Israel’s security cabinet declared war against Hamas
and a military campaign against these terrorist organizations commenced in parallel to their continued rocket and terror attacks. In parallel,
border clashes between Israel and the Hezbollah terrorist group on Israel’s northern border with Lebanon intensified and may escalate
into a greater regional conflict.
All of our clinical and
pre-clinical research and development is currently being conducted outside of Israel, other than our 12-month open label-extension
(“OLE”) study of the PARADIGM trial, partially conducted in Tel Aviv, and a planned Phase 2 trial with
PrimeC for Alzheimer’s disease that we plan to conduct in Haifa, Israel. The OLE has not been affected by the war,
although the quality of the study may be adversely affected if as a result of the war patients are unable to visit the study center or
the study coordinator is not able to conduct home visits and monitor the patients. In addition, in the event of a significant escalation
of hostilities in northern Israel, there may be a delay in the planned Alzheimer trial. We do not believe the planned
Alzheimer’s trial will be materially affected by the war and do not anticipate that any such delay as a result of the war would
have a material impact on the Company. We may also elect to set up a site in Israel for a Phase 3 pivotal ALS trial of PrimeC, but this
would be in addition to numerous other sites in Europe and the U.S., and as a result we do not expect the timeline or quality of this
trial to be adversely affected by the war. Our manufacturing is conducted in India. We do not currently anticipate any disruption to
the supply chain relevant to our ongoing clinical trials and believe there are alternative sources of supply from whom we could obtain
the necessary finished drug product to conduct our clinical trials. In addition, we believe we have sufficient finished product in inventory
to continue our ongoing clinical trials for at least the next few months.
The Israel Defence Force
(the “IDF”), the national military of Israel, is a conscripted military service, subject to certain exceptions. Since October
7, 2023, the IDF has called up several hundred thousand of its reserve forces to serve. Fourteen out of our current 16 employees are
resident in Israel. Three of our five executive officers and four out of 11 other employees who we believe are performing critical functions
reside in Israel. Two of our non-management employees in Israel who do not perform critical functions have been called, and additional
employees may be called, for service in the current or future wars or other armed conflicts with Hamas, and such persons may be absent
for an extended period of time. As a result, our operations in Israel may be disrupted by such absences, which disruption may materially
and adversely affect our business, prospects, financial condition and results of operations.
It is currently not possible to predict the duration
or severity of the ongoing conflict or its effects on our business, operations and financial conditions. The ongoing conflict is rapidly
evolving and developing, and could disrupt our business and operations, and hamper our ability to raise additional funds or sell our securities,
among others.
USE OF PROCEEDS
We
will receive gross proceeds of approximately $10.7 million assuming the exercise of all 1,755,000 Warrants for cash. We will not receive
any proceeds from the sale of the ordinary shares underlying the Warrants.
However, the holders of the
Warrants are not obligated to exercise the Warrants, and we cannot predict whether or when, if ever, the holders of the Warrants will
choose to exercise the Warrants, in whole or in part. The exercise price of the Warrants may exceed the trading price of our ordinary
shares. If the trading price of the ordinary shares is below $6.00 ($7.50 in the case of the Underwriter Warrants), we believe that holder
of the Warrants will be unlikely to exercise their warrants, resulting in little to no cash proceeds to us. Accordingly, we currently
intend to use the proceeds received upon such exercise, if any, for general corporate purposes and working capital.
MARKET FOR ORDINARY SHARES AND DIVIDEND POLICY
The ordinary shares are
traded on the Nasdaq Capital Market under the symbol “NRSN.” The last reported sale price of the ordinary shares on November
30, 2023 on the Nasdaq Capital Market was $1.29 per share. As of November 30, 2023, there were 1,389 shareholders of record of the
ordinary shares.
We have never declared or paid any cash dividends
on the ordinary shares, and we anticipate that, for the foreseeable future, we will retain any future earnings to support operations and
to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends for at least the next several years.
The distribution of dividends
may also be limited by the Israeli Companies Law, 5759-1999 (the “Companies Law”), which permits the distribution
of dividends only out of retained earnings or earnings derived over the two most recent fiscal years, whichever is higher, provided
that there is no reasonable concern that payment of a dividend will prevent a company from satisfying its existing and foreseeable obligations
as they become due. As of September 30, 2023, we did not have distributable earnings pursuant to the Companies Law. Dividend distributions
may be determined by our board of directors, as our amended and restated articles of association do not provide that such distributions
require shareholder approval.
CAPITALIZATION
The table below sets forth
our total capitalization as of September 30, 2023:
| |
● |
on an as adjusted basis to give effect to the issuance of 1,755,000 ordinary shares upon the exercise of the Warrants for aggregate gross proceeds of approximately $10.7 million. |
The financial data in the
following table should be read in conjunction with the “Use of Proceeds” section herein, our financial statements and notes
thereto incorporated by reference herein. Our historical results do not necessarily indicate our expected results for any future periods.
| |
|
As of
September 30, 2023 |
|
| |
|
Actual
(in thousands) |
|
|
As
Adjusted |
|
| Cash |
|
$ |
4,759 |
|
|
|
15,439 |
|
| |
|
|
|
|
|
|
|
|
| Liability in respect to warrants and pre-funded warrants |
|
|
2,689 |
|
|
|
2,479 |
|
| Shareholders’ equity: |
|
|
|
|
|
|
|
|
| Ordinary shares, no par value per share |
|
|
— |
|
|
|
|
|
| Share premium and capital reserve |
|
|
28,920 |
|
|
|
39,810 |
|
| Accumulated deficit |
|
|
(29,116 |
) |
|
|
(29,116 |
) |
| Total shareholders’ equity |
|
|
(196 |
) |
|
|
10,694 |
|
| Total capitalization |
|
$ |
2,493 |
|
|
|
13,173 |
|
DILUTION
If you invest in our ordinary shares in this offering,
your ownership interest will be diluted to the extent of the difference between the exercise price per ordinary share and the as adjusted
net tangible book value per ordinary share immediately after this offering.
Our net tangible book
value as of September 30, 2023 was approximately $(0.2) million, or $(0.01) per share. Net tangible book value per ordinary share is
determined by dividing our total tangible assets (including our right-of-use lease assets), less total liabilities, by the number of
ordinary shares outstanding as of September 30, 2023. Dilution with respect to net tangible book value per ordinary share represents
the difference between the amount per ordinary share paid by the purchaser of ordinary shares in this offering and the net tangible book
value per ordinary share immediately after this offering.
After giving effect to
the issuance of 1,655,000 ordinary shares upon the exercise of the Public Warrants at an exercise price of $6.00 per ordinary share and
the issuance of 100,000 ordinary shares upon the exercise of the Underwriter Warrants at an exercise price of $7.50 per ordinary share,
our as adjusted net tangible book value as of September 30, 2023 would have been approximately $10.7 million, or $0.69 per ordinary share.
This amount represents an increase in the net tangible book value of $0.70 per ordinary share to our existing shareholders and an immediate
dilution in net tangible book value of $5.31 per ordinary share and $6.81 per ordinary share to new investors exercising Public Warrants
and Underwriter Warrants, respectively. The following table illustrates this per ordinary share dilution:
| Exercise price per ordinary share underlying Public Warrants |
|
|
|
|
|
$ |
6.00 |
|
| Exercise price per ordinary share underlying Underwriter Warrants |
|
|
|
|
|
$ |
7.50 |
|
| Net tangible book value per ordinary share as of September 30, 2023 |
|
$ |
(0.01 |
) |
|
|
|
|
| Net increase in net tangible book value per
ordinary share attributable to existing shareholders |
|
$ |
0.70 |
|
|
|
|
|
| As adjusted net tangible book value per ordinary share after this offering |
|
|
|
|
|
$ |
0.69 |
|
| Dilution in net tangible book value per ordinary share to new investors exercising Public Warrants |
|
|
|
|
|
$ |
5.31 |
|
| Dilution in net tangible book value per ordinary share to new investors exercising Underwriter
Warrants |
|
|
|
|
|
$ |
6.81 |
|
Unless otherwise indicated,
the number of ordinary shares outstanding prior to this offering is based on 13,666,042 ordinary shares outstanding as of September 30,
2023, and excludes:
| |
● |
1,093,128 ordinary shares issuable upon the exercise
of options outstanding as of September 30, 2023, at a weighted average exercise price of $2.30 under our 2018 Employee Share
Option Plan; |
| |
● |
360,000 ordinary shares issuable upon the vesting
of restricted share units outstanding under our 2018 Share Incentive Plan; |
| |
● |
209,543 ordinary shares reserved for issuance
and available for future grant under our 2018 Share Incentive Plan; |
| ● | 1,670,000 ordinary shares underlying pre-funded warrants
with an exercise price of $0.0001 per share issued in connection with our registered direct offering in June 2023 (all of which have
been exercised as of the date of this prospectus); and |
| ● | 3,000,000 ordinary shares underlying warrants with an exercise
price of $1.50 per share issued in connection with our registered direct offering in June 2023. |
TAXATION
The following description is
not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our securities
offered hereby. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax
consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Material United States Federal Income
Tax Considerations
The following discussion describes
material United States federal income tax considerations relating to the acquisition, ownership, and disposition of ordinary shares
covered by this prospectus by a U.S. Holder (as defined below) that acquires the ordinary shares in this offering and holds them
as a capital asset. This discussion is based on the tax laws of the United States, including the Internal Revenue Code of 1986, as
amended (the “Code”), the United States Department of the Treasury (“Treasury”) regulations promulgated or
proposed thereunder, and administrative and judicial interpretations thereof, all as in effect on the date hereof. These tax laws are
subject to change, possibly with retroactive effect, and subject to differing interpretations that could affect the tax consequences described
herein. This discussion does not address the tax consequences to a U.S. Holder under the laws of any state, local or foreign taxing
jurisdiction, estate tax consequences, alternative minimum tax consequences, potential application of the Medicare contribution tax on
net investment income, and tax consequences applicable to U.S. Holders subject to special rules, as discussed below.
For purposes of this discussion,
a “U.S. Holder” is a beneficial owner of the ordinary shares that, for United States federal income tax purposes,
is:
| ● | an individual who is a citizen or resident of the United States; |
| ● | a domestic corporation (or other entity taxable as a corporation); |
| ● | an estate the income of which is subject to United States
federal income taxation regardless of its source; or |
| ● | a trust if (1) a court within the United States
is able to exercise primary supervision over the trust’s administration and one or more United States persons have the authority
to control all substantial decisions of the trust or (2) a valid election under the Treasury regulations is in effect for the trust
to be treated as a United States person. |
This discussion does not address
all aspects of United States federal income taxation that may be applicable to U.S. Holders in light of their particular circumstances
or status (including, for example, banks and other financial institutions, insurance companies, broker and dealers in securities or currencies,
traders in securities that have elected to mark securities to market, regulated investment companies, real estate investment trusts, partnerships
or other pass-through entities and arrangements, corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt
organizations, pension plans, persons that own or who are treated as constructively owning 10 percent or more of our stock by vote or
by value, persons that hold the ordinary shares as part of a straddle, hedge or other integrated investment, and persons subject to alternative
minimum tax or whose “functional currency” is not the U.S. dollar).
If a partnership (including
any entity or arrangement treated as a partnership for United States federal income tax purposes) holds the ordinary shares the tax
treatment of a person treated as a partner in the partnership for United States federal income tax purposes generally will depend
on the status of the partner and the activities of the partnership. Partnerships (and other entities or arrangements so treated for United States
federal income tax purposes) and their partners should consult their own tax advisors.
This discussion addresses
only U.S. Holders and does not discuss any tax considerations other than United States federal income tax considerations. Prospective
investors are urged to consult their own tax advisors regarding the United States federal, state, and local, and non-United States
tax consequences of the purchase, ownership, and disposition of the ordinary shares.
Dividends
We do not expect to make any distribution with respect
to the ordinary shares. However, if we make any such distribution, under the United States federal income tax laws, and subject to
the passive foreign investment company (“PFIC”) rules discussed below, the gross amount of any dividend we pay out of our
current or accumulated earnings and profits (as determined for United States federal income tax purposes) will be includible in income
for a U.S. Holder and subject to United States federal income taxation. Dividends paid to a noncorporate U.S. Holder that
constitute qualified dividend income will be taxable at a preferential tax rate applicable to long-term capital gains of, currently, 20
percent, provided that the U.S. Holder holds the ordinary shares for more than 60 days during the 121-day period beginning 60 days
before the ex-dividend date and meets other holding period requirements. If we are treated as a PFIC, dividends paid to a U.S. Holder
will not be treated as qualified dividend income. If we are not treated as a PFIC, dividends we pay with respect to the ordinary shares
generally will be qualified dividend income, provided that the holding period requirements are satisfied by the U.S. Holder and in
the year that the U.S. Holder receives the dividend, the ordinary shares are readily tradable on an established securities market
in the United States.
A U.S. Holder must include any Israeli tax
withheld from the dividend payment in the gross amount of the dividend even though the holder does not in fact receive it. The dividend
is taxable to the holder when the holder of ordinary shares receives the dividend, actually or constructively. Because we are not a United States
corporation, the dividend will not be eligible for the dividends-received deduction generally allowed to United States corporations
in respect of dividends received from other United States corporations. The amount of the dividend distribution includible in a U.S. Holder’s
income will be the U.S. dollar value of the NIS payments made, determined at the spot NIS/U.S. dollar rate on the date the dividend
distribution is includible in income, regardless of whether the payment is in fact converted into U.S. dollars. Generally, any gain
or loss resulting from currency exchange fluctuations during the period from the date the dividend payment is included in income to the
date the payment is converted into U.S. dollars will be treated as ordinary income or loss and will not be eligible for the special
tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States
for foreign tax credit limitation purposes.
Subject to certain limitations, the Israeli tax
withheld in accordance with the United States Israel Tax Treaty and paid over to Israel will be creditable or deductible against
a U.S. Holder’s United States federal income tax liability, if the U.S. Holder satisfies certain minimum holding
period requirements. Dividends that we distribute generally should constitute “passive category income,” or, in the case of
certain U.S. Holders, “general category income” for foreign tax credit limitation purposes. The rules relating to the
determination of the foreign tax credit limitation are complex, and U.S. Holders should consult their tax advisor to determine whether
and to what extent they will be entitled to a credit for Israeli withholding taxes imposed in respect of any dividend we distribute.
To the extent a
distribution with respect to the ordinary shares exceeds our current or accumulated earnings and profits, as determined under
United States federal income tax principles, the distribution will be treated, first, as a tax-free return of the
U.S. Holder’s investment, up to the holder’s adjusted tax basis in its ordinary shares, and, thereafter, as capital
gain, which is subject to the tax treatment described below in “— Gain on Sale, Exchange or Other Taxable
Disposition of the ordinary shares.”
Gain on Sale, Exchange or Other Taxable Disposition of the ordinary
shares
Subject to the PFIC
rules described below under “— Passive Foreign Investment Company Considerations,” a U.S. Holder that
sells, exchanges or otherwise disposes of ordinary shares in a taxable disposition generally will recognize capital gain or loss for
United States federal income tax purposes equal to the difference between the U.S. Dollar value of the amount realized and
the holder’s tax basis, determined in U.S. Dollars, in the ordinary shares. Gain or loss recognized on such a sale,
exchange or other disposition of ordinary shares generally will be long-term capital gain if the U.S. Holder’s holding
period in the ordinary shares exceeds one year. Long-term capital gains of non-corporate U.S. Holders are generally taxed at
preferential rates. The gain or loss generally will be income or loss from sources within the United States for foreign tax
credit limitation purposes. A U.S. Holder’s ability to deduct capital losses is subject to limitations.
Passive Foreign Investment Company Considerations
Based on our income and assets, we believe that
we were a PFIC for the preceding taxable year and expect that we will be a PFIC for the current taxable year. Because the determination
of our PFIC status is made annually based on the factual tests described below, however, we cannot provide any assurances regarding our
PFIC status for the current or future taxable years or that the IRS will agree with our conclusion regarding our PFIC status. If
we were classified as a PFIC in any taxable year, a U.S. Holder would be subject to special rules with respect to distributions on
and sales, exchanges and other dispositions of the ordinary shares. We will be treated as a PFIC for any taxable year in which at least
75 percent of our gross income is “passive income” or at least 50 percent of our gross assets during the taxable year (based
on the average of the fair market values of the assets determined at the end of each quarterly period) are assets that produce or are
held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest,
rents, royalties, gains from commodities and securities transactions, and gains from assets that produce passive income. However, rents
and royalties received from unrelated parties in connection with the active conduct of a trade or business are not considered passive
income for purposes of the PFIC test. In determining whether we are a PFIC, a pro rata portion of the income and assets of each corporation
in which we own, directly or indirectly, at least a 25% interest (by value) is taken into account.
Excess Distribution Rules
If we were a PFIC with respect to a U.S. Holder,
then unless the holder makes one of the elections described below under “— QEF Election” or “— Mark-to-Market
Election,” a special tax regime would apply to the U.S. Holder with respect to (a) any “excess distribution”
(generally, aggregate distributions in any year that are greater than 125% of the average annual distribution received by the holder in
the shorter of the three preceding years or the holder’s holding period for the ordinary shares) and (b) any gain realized
on the sale or other disposition of the ordinary shares. Under this regime, any excess distribution and realized gain will be treated
as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over the U.S. Holder’s
holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest
marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would
be subject to tax at the U.S. Holder’s regular ordinary income rate for the current year and would not be subject to the interest
charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed
to have been payable in those years. In addition, dividend distributions would not qualify for the lower rates of taxation applicable
to long-term capital gains discussed above under “— Dividends.” For ordinary shares acquired pursuant to the exercise
of the Warrants, a U.S. Holder’s holding period will for this purpose include the holding period of such warrants.
A U.S. Holder that holds the ordinary shares
at any time during a taxable year in which we are classified as a PFIC generally will continue to treat such ordinary shares, as well
as ordinary shares acquired pursuant to an exercise of the Warrants, as ordinary shares in a PFIC, even if we no longer satisfy the income
and asset tests described above, unless the U.S. Holder elects to recognize gain, which will be taxed under the excess distribution
rules as if such ordinary shares had been sold on the last day of the last taxable year for which we were a PFIC.
Certain elections by a U.S. Holder would alleviate
some of the adverse consequences of PFIC status and would result in an alternative treatment of the ordinary shares, as described below.
However, under U.S. Treasury Regulations applying to warrants with respect to stock in a PFIC, these elections are not available
with respect to the Warrants.
QEF Election
If we were a PFIC, the rules above would not apply
to a U.S. Holder that timely makes an election to treat the ordinary shares as stock of a “qualified electing fund” (“QEF”).
A U.S. Holder that makes a QEF election is required to include in income its pro rata share of the ordinary earnings and net capital
gain as ordinary income and long-term capital gain, respectively, subject to a separate election to defer payment of taxes, which deferral
is subject to an interest charge.
The timely QEF election also allows the electing
U.S. Holder to: (i) generally treat any gain recognized on the disposition of its shares of the PFIC as capital gain; (ii) treat its share
of the PFIC’s net capital gain, if any, as long-term capital gain instead of ordinary income; and (iii) either avoid interest charges
resulting from PFIC status altogether, or make an annual election, subject to certain limitations, to defer payment of current taxes on
its share of the PFIC’s annual realized net capital gain and ordinary earnings subject, however, to an interest charge on the deferred
tax computed by using the statutory rate of interest applicable to an extension of time for payment of tax. In addition, net losses (if
any) of a PFIC will not pass through to an electing U.S. Holder and may not be carried back or forward in computing such PFIC’s
ordinary earnings and net capital gain in other taxable years. Consequently, a U.S. Holder may over time be taxed on amounts that as an
economic matter exceed our net profits.
A U.S. Holder’s
tax basis in ordinary shares will be increased to reflect QEF income inclusions and will be decreased to reflect distributions of amounts
previously included in income as QEF income inclusions. No portion of the QEF income inclusions attributable to ordinary income will
be treated as qualified dividend income. Amounts included as QEF income inclusions with respect to direct and indirect investments generally
will not be taxed again when distributed. U.S. Holders should consult their tax advisors as to the manner in which QEF income inclusions
affect their allocable share of our income and their basis in their ordinary shares.
A U.S. Holder makes a QEF election generally by attaching a completed
IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) (“IRS
Form 8621”) to a timely filed United States federal income tax return for the year beginning with which the QEF election
is to be effective (taking into account any extensions). A QEF election can be revoked only with the consent of the IRS. In order
for a U.S. Holder to make a valid QEF election, we must annually provide or make available to the holder certain information. While
we intend to provide to U.S. Holders the information required to make a valid QEF election, we cannot provide any assurances that
we will in fact provide such information. A QEF election under the proposed U.S. Treasury Regulations is not available for the Warrants
regardless of whether we provide such information
Mark-to-Market Election
If we were a PFIC, the rules above also would not
apply to a U.S. Holder that makes a “mark-to-market” election with respect to the ordinary shares, but this election
will be available with respect to the ordinary shares only if they meet certain minimum trading requirements to be considered “marketable
stock” for purposes of the PFIC rules. A mark-to-market election under the U.S. Treasury Regulations is not available for the
Warrants.
Ordinary shares will be marketable stock if they
are regularly traded on a national securities exchange that is registered with the SEC or on a non-U.S. exchange or market that meets
certain requirements under the Treasury regulations. Ordinary shares generally will be considered regularly traded during any calendar
year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter.
Any trades that have as their principal purpose meeting this requirement will be disregarded.
A U.S. Holder that makes a valid mark-to-market
election for the first tax year in which the holder holds (or is deemed to hold) the ordinary shares and for which we are a PFIC will
be required to include each year an amount equal to the excess, if any, of the fair market value of such shares the U.S. Holder owns
as of the close of the taxable year over their adjusted tax basis in such shares. The U.S. Holder will be entitled to a deduction
for the excess, if any, of the U.S. Holder’s adjusted tax basis in the ordinary shares over the fair market value of such shares
as of the close of the taxable year, but only to the extent of any net mark-to-market gains with respect to such shares included by the
U.S. Holder under the election for prior taxable years. The U.S. Holder’s basis in such ordinary shares will be adjusted
to reflect the amounts included or deducted pursuant to the election. Amounts included in income pursuant to a mark-to-market election,
as well as gain on the sale, exchange or other taxable disposition of such ordinary shares, will be treated as ordinary income. The deductible
portion of any mark-to-market loss, as well as loss on a sale, exchange or other disposition of the ordinary shares to the extent that
the amount of such loss does not exceed net mark-to-market gains previously included in income, will be treated as ordinary loss.
The mark-to-market election applies to the taxable
year for which the election is made and all subsequent taxable years, unless the ordinary shares cease to be treated as marketable
stock for purposes of the PFIC rules or the IRS consents to its revocation. The excess distribution rules described above generally will
not apply to a U.S. Holder for tax years for which a mark-to-market election is in effect. However, if we were a PFIC for any
year in which the U.S. Holder owns the ordinary shares but before a mark-to-market election is made, the interest charge rules described
above would apply to any mark-to-market gain recognized in the year the election is made.
PFIC Reporting Obligations
If we were, or in the future are, a PFIC. a U.S. Holder
of the ordinary shares must generally file an annual information return on IRS Form 8621 containing such information as the Treasury
may require. A U.S. Holder’s failure to file the annual information return will cause the statute of limitations for such U.S. Holder’s
U.S. federal income tax return to remain open with regard to the items required to be included in such report until three years after
the U.S. Holder files the annual information return, and, unless such failure is due to reasonable cause and not willful neglect, the
statute of limitations for the U.S. Holder’s entire U.S. federal income tax return will remain open during such period. U.S. Holders
should consult their tax advisors regarding the requirements of filing such information returns under these rules.
U.S. Holders are urged to consult their
tax advisors as to our status as a PFIC, and the tax consequences to them if we were a PFIC, including the reporting requirements and
the desirability of making, and the availability of, a QEF election or a mark-to-market election with respect to the ordinary shares .
Medicare Tax
Non-corporate U.S. Holders that are individuals,
estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment
income, which may include their gross dividend income and net gains from the disposition of ordinary shares. A United States person
that is an individual, estate or trust is encouraged to consult its tax advisors regarding the applicability of this Medicare tax to its
income and gains in respect of any investment in the ordinary shares.
Information Reporting with Respect to Foreign Financial Assets
Individual U.S. Holders (and, under regulations,
certain entities) may be subject to certain reporting obligations on IRS Form 8938 (Statement of Specified Foreign Financial Asset)
with respect to the ordinary shares for any taxable year during which the U.S. Holder’s aggregate value of these and certain
other “specified foreign financial assets” exceed a threshold amount that varies with the filing status of the individual
or entity. This reporting obligation also applies to domestic entities formed or availed of to hold, directly or indirectly, specified
foreign financial assets, including the ordinary shares. Significant penalties can apply if U.S. Holders are required to make this
disclosure and fail to do so. Such U.S. Holders who fail to timely furnish the required information may be subject to a penalty. Additionally,
if a U.S. Holder does not file the required information, the statute of limitations with respect to tax returns of the U.S. Holder to
which the information relates may not close until three years after such information is filed. U.S. Holders should consult their tax advisors
regarding their reporting obligations with respect to their ownership and disposition of the ordinary shares .
Information Reporting and Backup Withholding
In general, information reporting, on IRS Form 1099,
will apply to dividends in respect of ordinary shares and the proceeds from the sale, exchange or redemption of ordinary shares that are
paid to a holder of ordinary shares within the United States (and in certain cases, outside the United States), unless such
holder is an exempt recipient such as a corporation. Backup withholding (currently at a 24% rate) may apply to such payments if a holder
of ordinary shares fails to provide a taxpayer identification number (generally on an IRS Form W-9) or certification of other exempt
status or fails to report in full dividend and interest income.
Backup withholding is not an additional tax. A U.S. Holder
generally may obtain a refund or credit of any amounts withheld under the backup withholding rules that exceed the U.S. Holder’s
income tax liability by timely filing a refund claim with the IRS.
Israeli Tax Considerations
The following is a brief summary of certain material
Israeli tax laws applicable to us, and certain Israeli government programs that benefit us. This section also contains a discussion of
certain material Israeli tax consequences concerning the ownership and disposition of our securities offered hereby. This summary does
not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment
circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents
of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. To the extent that the discussion
is based on tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the
appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion below is not intended, and
should not be construed, as legal or professional tax advice and is not exhaustive of all possible tax considerations. The discussion
is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations
of Israeli law, which change could affect the tax consequences described below, possibly with a retroactive effect.
THEREFORE, YOU ARE URGED TO CONSULT YOUR OWN TAX
ADVISORS AS TO THE ISRAELI OR OTHER TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR SECURITIES OFFERED HEREBY, INCLUDING,
IN PARTICULAR, THE EFFECT OF ANY FOREIGN, STATE OR LOCAL TAXES.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate
tax at a flat rate. In December 2016, the Israeli Parliament approved the Economic Efficiency Law (Legislative Amendments for Applying
the Economic Policy for the 2017 and 2018 Budget Years) (“EEL”) which reduced the corporate income tax rate from 25%
to 24% effective from January 1, 2017, and to 23% effective from January 1, 2018 and thereafter. However, the effective tax
rate payable by a company that derives income from an Approved Enterprise, a Preferred Enterprise, a Benefited Enterprise or a Technological
Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli company are generally subject to corporate
tax rate.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969,
generally referred to as the Industry Encouragement Law (“IEL”) provides several tax benefits for “Industrial Companies.”
We may qualify as an Industrial Company within the meaning of the IEL.
The IEL defines an “Industrial Company”
as an Israeli resident-company, of which 90% or more of its income in any tax year, other than income from certain government loans, capital
gains, interest and dividends, is derived from an “Industrial Enterprise” owned by it and located in Israel or in the “Area,”
in accordance with the definition under Section 3A of the Israeli Income Tax Ordinance (New Version) 1961 (the “Ordinance”).
An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following are the main tax benefits available
to Industrial Companies:
| ● | Amortization of the cost of purchased patent, rights to use
a patent, and know-how, which are used for the development or advancement of the Industrial Enterprise, over an eight-year period, commencing
on the year in which such rights were first exercised; |
| ● | Under limited conditions, an election to file consolidated
tax returns with controlled Israeli Industrial Companies; and |
| ● | Expenses related to a public offering are deductible in equal
amounts over three years commencing on the year of the offering. |
Eligibility for benefits under the Industry Encouragement
Law is not contingent upon approval of any governmental authority.
Tax Benefits and Grants for Research and Development
Israeli tax law allows, under certain conditions,
a tax deduction for expenditures related to scientific research and development projects, including capital expenditures, for the year
in which they are incurred. Expenditures are deemed related to scientific research and development projects, if:
| ● | The research and expenditures are approved by the relevant
Israeli government ministry, determined by the field of research; |
| ● | The research and development must be for the promotion of
the company; and |
| ● | The research and development is carried out by or on behalf
of the company seeking such tax deduction. |
The amount of such deductible expenses is reduced
by the sum of any funds received through government grants for the finance of such scientific research and development projects. No deduction
under these research and development deduction rules is allowed if such deduction is related to an expense invested in an asset depreciable
under the general depreciation rules of the Ordinance. Expenditures that are unqualified under the conditions above are deductible, under
certain conditions, in equal amounts over three years.
From time to time we may apply to the Israel Innovation
Authority (“IIA”) for approval to allow a tax deduction for all or most of research and development expenses during the year
incurred. There can be no assurance that such application will be accepted. If we will not be able to deduct research and development
expenses during the year of the payment, we may be able to deduct research and development expenses in equal amounts over a period of
three years commencing in the year of the payment of such expenses.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959
(“the Investment Law”) provides certain incentives for capital investments in production facilities (or other eligible assets).
Generally, an investment program that is implemented in accordance with the provisions of the Investment Law, referred to as an Approved
Enterprise, a Beneficiary Enterprise, a Preferred Enterprise, a Preferred Technological Enterprise, or a Special Preferred Technological
Enterprise, is entitled to benefits as discussed below. These benefits may include cash grants from the Israeli government and tax benefits,
based upon, among other things, the geographic location in Israel of the facility in which the investment is made. In order to qualify
for these incentives, the Company is required to comply with the requirements of the Investment Law.
The Investment Law was significantly amended effective
as of April 1, 2005 (“the 2005 Amendment”), as of January 1, 2011 (the “2011 Amendment”), and as of
January 1, 2017 (the “2017 Amendment”). Pursuant to the 2005 Amendment, tax benefits granted in accordance with the provisions
of the Investment Law prior to its revision by the 2005 Amendment remain in force but any benefits granted subsequently are subject to
the provisions of the amended Investment Law. Similarly, the 2011 Amendment introduced new benefits to replace those granted in accordance
with the provisions of the Investment Law in effect prior to the 2011 Amendment. However, companies entitled to benefits under the Investment
Law as in effect prior to January 1, 2011 were entitled to choose to continue to enjoy such benefits, provided that certain conditions
are met, or elect instead, irrevocably, to forego such benefits and have the benefits of the 2011 Amendment apply. The 2017 Amendment
introduces new benefits for Technological Enterprises, alongside the existing tax benefits.
Tax Benefits Under the 2011 Amendment
The 2011 Amendment cancelled
the availability of the benefits granted to Industrial Companies under the Investment Law prior to 2011 and, instead, introduced new benefits
for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in
the Investment Law) as of January 1, 2011. The definition of a Preferred Company includes a company incorporated in Israel that is
not fully owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed
from Israel. Pursuant to the 2011 Amendment, a Preferred Company is entitled to a reduced corporate tax rate of 15% with respect to its
income derived by its Preferred Enterprise in 2011 and 2012, unless the Preferred Enterprise is located in a specified development zone,
in which case the rate will be 10%. Under the 2011 Amendment, such corporate tax rate was reduced from 15% and 10%, respectively, to 12.5%
and 7%, respectively, in 2013, 16% and 9% respectively, in 2014, 2015 and 2016, and 16% and 7.5%, respectively, in 2017 and thereafter.
Income derived by a Preferred Company from a “Special Preferred Enterprise” (as such term is defined in the Investment Law)
would be entitled, subject to certain conditions and during a benefits period of 10 years, to further reduced tax rates of 8%, or
5% if the Special Preferred Enterprise is located in a certain development zone.
Dividends distributed from
income which is attributed to a “Preferred Enterprise” will be subject to tax at the following rates: (i) Israeli resident
corporations–0% (although, if such dividends are subsequently distributed to individuals or a non-Israeli company the below rates
detailed in sub sections (ii) and (iii) shall apply); (ii) Israeli resident individuals–20%; or (iii) non-Israeli
residents (individuals and corporations)–20%, subject to a reduced tax rate under the provisions of any applicable tax treaty. The
withholding tax rate applicable to distribution of dividend from such income to non-Israeli residents is 25% (or 30% if distributed to
a “substantial shareholder” at the time of the sale or at any time during the preceding twelve months period, as defined
below), which may be reduced under an applicable tax treaty by applying in advance for a withholding certificate from the Israel Tax Authority
(“ITA”). A “substantial shareholder” is generally a person who alone or together with such person’s relative
or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “Means
of Control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a
director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act,
regardless of the source of such right.
The 2011 Amendment also provided
transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional provisions
provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended in 2011
with respect to income to be derived as of January 1, 2011, a Beneficiary Enterprise can elect to continue to benefit from the benefits
provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
New Tax Benefits Under the 2017 Amendment
That Became Effective on January 1, 2017
The 2017 Amendment was enacted
as part of the EEL that was published on December 29, 2016 and is effective as of January 1, 2017. The 2017 Amendment provides
new tax benefits for two types of “Technological Enterprises,” as described below, and is in addition to the other existing
tax beneficial programs under the Investment Law.
The 2017 Amendment provides
that a Preferred Company satisfying certain conditions will qualify as having a “Preferred Technological Enterprise” and will
thereby enjoy a reduced corporate tax rate of 12% on income that qualifies as “Preferred Technological Income,” as defined
in the Investment Law. The corporate tax rate is further reduced to 7.5% with respect to a Preferred Technological Enterprise located
in development zone “A.” In addition, a Preferred Company who qualifies as having a “Preferred Technological Enterprise”
will enjoy a reduced corporate tax rate of 12% on capital gain derived from the sale of certain “Benefitted Intangible Assets”
(as defined in the Investment Law) to a related foreign company if the Benefitted Intangible Assets were acquired from a foreign company
on or after January 1, 2017 for at least NIS 200 million, and the sale receives prior approval from the IIA.
The 2017 Amendment further
provides that a Preferred Company satisfying certain conditions (including group consolidated revenues of at least NIS 10 billion)
will qualify as having a “Special Preferred Technological Enterprise” and will thereby enjoy a reduced corporate tax rate
of 6% on “Preferred Technological Income” regardless of the company’s geographic location within Israel. In addition,
a Special Preferred Technological Enterprise will enjoy a reduced corporate tax rate of 6% on capital gain derived from the sale of certain
“Benefitted Intangible Assets” to a related foreign company if the Benefitted Intangible Assets were either developed by the
Special Preferred Enterprise or acquired from a foreign company on or after January 1, 2017, and the sale received prior approval
from the IIA. A Special Preferred Technological Enterprise that acquires Benefitted Intangible Assets from a foreign company for
more than NIS 500 million will be eligible for these benefits for at least ten years, subject to certain approvals as specified
in the Investment Law.
Dividends distributed by a
Preferred Technological Enterprise or a Special Preferred Technological Enterprise, paid out of Preferred Technological Income, are generally
subject to tax at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. The withholding tax rate applicable
to distribution of dividend from such income to non-Israeli residents is 25% (or 30% if distributed to a “substantial shareholder”
at the time of the sale or at any time during the preceding twelve months period), which may be reduced under an applicable tax treaty
by applying in advance for a withholding certificate from the ITA. In addition, if such dividends are distributed to a foreign company
that holds solely or together with other foreign companies 90% or more in the Israeli company and other conditions are met, the withholding
tax rate will be 4% (subject to the receipt in advance of a valid certificate from the ITA allowing for a reduced tax rate). However,
if such dividends are paid to an Israeli company, no tax is required to be withheld.
Taxation of Our Securityholders
Capital gains Tax on Sales of the ordinary
shares
Israeli law generally imposes
a capital gains tax on the sale of any capital assets by Israeli residents, as defined for Israeli tax purposes, and on the sale of capital
assets located in Israel, including shares of Israeli companies, by both Israeli residents and non-Israeli residents, unless a specific
exemption is available or unless a tax treaty between Israel and the shareholder’s country of residence provides otherwise. The
Ordinance distinguishes between real gain and inflationary surplus. The inflationary surplus is a portion of the total capital gain equivalent
to the increase of the relevant asset’s purchase price attributable to an increase in the Israeli consumer price index, or, in certain
circumstances, a foreign currency exchange rate, between the date of purchase and the date of sale. Inflationary surplus is currently
not subject to tax in Israel. The real gain is the excess of the total capital gain over the inflationary surplus.
Capital Gains Taxes Applicable to Non-Israeli
Resident Securityholders
A non-Israeli resident who
derives capital gains from the sale of shares or warrants in an Israeli resident company that were purchased after the company was listed
for trading on a stock exchange outside of Israel, will be exempt from Israeli tax as long as (among other conditions) the securities
were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not
be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of more than 25% in such non-Israeli
corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation,
whether directly or indirectly. In addition, such exemption is not applicable to a person whose gains from selling or otherwise disposing
of the securities are deemed to be business income.
Additionally, a sale of securities
by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example,
under the Convention Between the Government of the United States of America and the Government of the State of Israel with respect
to Taxes on Income, as amended, or the U.S. Israel Tax Treaty, the sale, exchange or other disposition of shares by a shareholder
who is a United States resident (for purposes of the treaty) holding the shares as a capital asset and is entitled to claim the benefits
afforded to such a resident by the U.S. Israel Tax Treaty (a “U.S. Resident”) is generally exempt from Israeli capital
gains tax unless: (i) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in Israel;
(ii) the capital gain arising from such sale, exchange or disposition is attributed to royalties; (iii) the capital gain arising
from the such sale, exchange or disposition is attributed to a permanent establishment in Israel, under certain terms; (iv) such
U.S. Resident holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12 month
period preceding the disposition, subject to certain conditions; or (v) such U.S. Resident is an individual and was present
in Israel for 183 days or more during the relevant taxable year. In any such case, the sale, exchange or disposition of such shares
by the U.S. Resident would be subject to Israeli tax (unless exempt under the Israeli domestic law as described above). Under the
U.S. Israel Tax Treaty, the gain may be treated as foreign source income for United States foreign tax credit purposes, upon
an election by the U.S. Resident, and such U.S. Resident may be permitted to claim a credit for such taxes against the United States
federal income tax imposed on such sale, subject to the limitations under the United States federal income tax laws applicable to
foreign tax credits. The U.S. Israel Tax Treaty does not provide such credit against any United States state or local taxes.
Regardless of whether securityholders
may be liable for Israeli tax on the sale of our securities, the payment of the consideration may be subject to the withholding of Israeli
tax at source. Securityholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding
at source at the time of sale (i.e., provide a non-Israeli resident certificate and other documentation).
Capital Gains Taxes Applicable to Israeli
Resident Securityholders
An Israeli resident corporation
who derives capital gains from the sale of shares or warrants in an Israeli resident company will generally be subject to tax on the real
capital gains generated on such sale at the corporate tax rate (currently of 23%). An Israeli resident individual will generally be subject
to capital gain tax at the rate of 25%. However, if the individual securityholder is claiming deduction of interest expenditures or is
a “substantial shareholder” at the time of the sale or at any time during the preceding twelve months period, such gain
will be taxed at the rate of 30%. Individual holders dealing in securities in Israel for whom the income from the sale of securities is
considered “business income” as defined in section 2(1) of the Ordinance are taxed at the marginal tax rates applicable
to business income (up to 47% in 2023 plus 3% Surtax). Certain Israeli institutions who are exempt from tax under section 9(2) or
section 129(C)(a)(1) of the Ordinance (such as exempt trust funds and pension funds) may be exempt from capital gains tax from the
sale of the securities.
Taxation of Israeli Shareholders on Receipt
of Dividends
An Israeli resident individual
is generally subject to Israeli income tax on the receipt of dividends paid on the ordinary shares at the rate of 25%. With respect to
a person who is a “substantial shareholder” at the time of receiving the dividend or on any time during the preceding twelve months,
the applicable tax rate is 30%. Such dividends are generally subject to Israeli withholding tax at a rate of 25% if the shares are registered
with a nominee company (whether the recipient is a substantial shareholder or not). If the recipient of the dividend is an Israeli resident
corporation such dividend income will be exempt from tax provided the income from which such dividend is distributed was derived or accrued
within Israel and was received directly or indirectly from another corporation that is liable to Israeli corporate tax. An exempt trust
fund, pension fund or other entity that is exempt from tax under section 9(2) or section 129C(a)(1) of the Ordinance is exempt
from tax on a dividend.
Dividend distribution by a
Preferred Technology Enterprise or a Special Preferred Technology Enterprise is subject to beneficial withholding tax rates. For a further
discussion, see “Taxation and Government Programs — Israeli Tax Considerations — Law for the Encouragement
of Capital Investments, 5719-1959 — New Tax Benefits Under the 2017 Amendment that Became Effective on January 1,
2017.”
Taxation of Non-Israeli Shareholders on
Receipt of Dividends
Non-Israeli residents (either
individuals or corporations) are generally subject to Israeli income tax on the receipt of dividends paid on the ordinary shares at the
rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty between Israel and the shareholder’s country
of residence. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any
time during the preceding twelve months, the applicable tax rate is 30%. Such dividends are generally subject to Israeli withholding
tax at a rate of 25% if the shares are registered with a nominee company (whether the recipient is a substantial shareholder or not),
unless a reduced rate is provided under an applicable tax treaty (subject to the receipt in advance of a valid certificate from the ITA
allowing for a reduced tax rate). For example, under the U.S. Israel Tax Treaty, the maximum rate of tax withheld at source in Israel
on dividends paid to a holder of the ordinary shares who is a U.S. Resident is 25%. However, generally, the maximum rate of withholding
tax on dividends, not generated by a Preferred Technological Enterprise, Preferred Enterprise, Approved Enterprise or Beneficial Enterprise,
that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout the tax year in which
the dividend is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25% of the gross income for
such preceding year consists of certain types of dividends and interest. Notwithstanding the foregoing, dividends distributed from income
attributed to an Approved Enterprise, Preferred Technological Enterprise, Benefited Enterprise or Preferred Enterprise are not entitled
to such reduction under the tax treaty but are subject to a withholding tax rate of 15% for a shareholder that is a U.S. corporation,
provided that the conditions related to the outstanding voting rights and the gross income for the previous year (as set forth in the
previous sentences) are met. If the dividend is attributable partly to income derived from an Approved Enterprise, Preferred Technological
Enterprise, Benefited Enterprise or Preferred Enterprise, and partly to other sources of income, the withholding rate may be a blended
rate reflecting the relative portions of the two types of income. We cannot assure you that we will designate the profits that we may
distribute in a way that will reduce shareholders’ tax liability.
Application for the reduced
tax rate requires appropriate documentation presented and specific instruction received from the ITA. To the extent tax is withheld
at source at the maximum rates (see above), a qualified tax treaty recipient will have to comply with some administrative procedures with
the ITA in order to receive a refund of any excess tax withheld.
A foreign resident who had
income from a dividend that was accrued from Israeli source, from which, among other conditions, the full tax was deducted, will be exempt
from filing a tax return in Israel, unless (i) such income was generated from a business conducted in Israel by such foreign resident,
(ii) such foreign resident has other taxable sources of income in Israel with respect to which a tax return is required to be filed, or
(iii) such foreign resident is liable to Surtax (see below) in accordance with section 121B of the Ordinance.
Dividend distribution by a
Preferred Technology Enterprise or a Special Preferred Technology Enterprise is subject to beneficial withholding tax rates. For a further
discussion, see “Taxation and Government Programs — Israeli Tax Considerations — Law for the Encouragement
of Capital Investments, 5719-1959 — New Tax benefits Under the 2017 Amendment that Became Effective on January 1,
2017.”
Surtax
Subject to the provisions of
an applicable tax treaty, individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 3% on annual
income (including, but not limited to, dividends, interest and capital gain) exceeding NIS 698,280 for 2023, which amount is linked to
the annual change in the Israeli consumer price index.
Estate and Gift Tax
Israeli law presently does
not impose estate or gift taxes.
DESCRIPTION OF SECURITIES
The
securities to be registered on this registration statement on Form F-1 of which this prospectus forms a part include up to an aggregate
amount of 1,655,000 ordinary shares issuable upon exercise of the Public
Warrants at the exercise price of $6.00 per ordinary share and 100,000 ordinary shares issuable upon exercise of the Underwriter Warrants
at an exercise price of $7.50 per ordinary share issued as part of our initial public offering.
Ordinary shares
The Company’s authorized
share capital consists of 60,000,000 ordinary shares, no par value per share, of which 13,666,042 ordinary shares were issued
and outstanding as of September 30, 2023.
All of the outstanding ordinary
shares are validly issued, fully paid and non-assessable. The ordinary shares are not redeemable and do not have any pre-emptive rights.
Voting Rights and Conversion
All ordinary shares will have
identical voting and other rights in all respects. None of our major shareholders have different voting rights than our other shareholders.
Transfer of Shares
Fully paid ordinary shares
are issued in registered form and may be freely transferred under the Company’s amended and restated articles of association, unless
the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are
listed for trading. The ownership or voting of the ordinary shares by non-residents of Israel is not restricted in any way by the amended
and restated articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are,
or have been, in a state of war with Israel.
Liability to Further Capital Calls
Our board of directors may
make, from time to time, such calls as it may deem fit upon shareholders with respect to any sum unpaid with respect to ordinary shares
held by such shareholders, which is not payable at a fixed time. Such shareholder shall pay the amount of every call so made upon him.
Unless otherwise stipulated by our board of directors, each payment in response to a call shall be deemed to constitute a pro rata payment
on account of all shares with respect to which such call was made. A shareholder shall not be entitled to his rights as shareholder, including
the right to dividends, unless such shareholder has fully paid all the notices of call delivered to him, or which according to the Company’s
amended and restated articles of association are deemed to have been delivered to him, together with interest, linkage and expenses, if
any, unless otherwise determined by our board of directors.
Business Combinations
Under the Company’s amended
and restated articles of association, we may not engage in any “business combinations” with any “interested shareholder”
for a three-year period following the time that such shareholder became an interested shareholder, unless:
| ● | prior to the time that such shareholder became an interested
shareholder, our board of directors approved either the business combination or the transaction which resulted in the shareholder becoming
an interested shareholder; |
| ● | upon consummation of the transaction which resulted in the
shareholder becoming an interested shareholder, the interested shareholder owned at least 85% of our voting shares outstanding at the
time the transaction commenced, excluding for purposes of determining our voting shares outstanding (but not the outstanding voting
shares owned by the interested shareholder) those shares owned by persons who are directors and also officers; or |
| ● | at the time that such shareholder became an interested shareholder,
or subsequent to such time, the business combination is approved by our board of directors and authorized at a general meeting of shareholders
by the affirmative vote of at least 662/3% of our voting shares outstanding that are not owned by the interested shareholder. |
Generally, a “business
combination” includes any merger, consolidation, sale or other transaction resulting in a financial benefit to the interested shareholder.
Subject to certain exceptions, an “interested shareholder” is any person (other than the Company and any of the Company’s
direct or indirect majority-owned subsidiaries) who, together with such person’s affiliates and associates, owns, or within the
previous three years owned, 15% or more of our voting shares.
Under certain circumstances,
this provision will make it more difficult for a person who would be an “interested shareholder” to effect various business
combinations with a corporation for a three-year period. This provision may encourage companies interested in acquiring us to negotiate
in advance with our board of directors because the shareholder approval requirement would be avoided if our board of directors approves
either the business combination or the transaction which results in the shareholder becoming an interested shareholder. These provisions
also may have the effect of preventing changes in our board of directors and may make it more difficult to accomplish transactions which
shareholders may otherwise deem to be in their best interests.
Election of Directors
The ordinary shares do not
have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at
a shareholders meeting have the power to elect our directors, subject to the special approval requirements for external directors under
the Companies Law. Under the Company’s amended and restated articles of association, the number of directors on our board of directors
must be no less than three and no more than nine, including any external directors required to be appointed under the Companies Law. The
minimum and maximum number of directors may be changed, at any time and from time to time, by a special vote of the holders of at least
662/3% of the outstanding shares.
Other than external directors,
for whom special election requirements apply under the Companies Law, the vote required to appoint a director is a simple majority vote.
In addition, under the Company’s amended and restated articles of association, our board of directors may elect new directors to
fill vacancies (whether such vacancy is due to a director no longer serving or due to the number of directors serving being less than
the maximum required in our amended and restated articles of association), provided that the total number of directors shall not, at any
time, exceed nine directors and provided that our board of directors may not elect external directors. The Company’s amended and
restated articles of association provide that the term of a director appointed by our board of directors to fill any vacancy will be for
the remaining term of office of the director(s) whose office(s) have been vacated. Furthermore, under the Company’s amended
and restated articles of association, our directors, other than external directors, are divided into three classes with staggered three-year
terms. Each class of directors consists, as nearly as possible, of 1/3 of the total number of directors constituting the entire board
of directors (other than the external directors).
External directors are elected
for an initial term of three years, may be elected for additional three-year terms, and may be removed from office pursuant to the
terms of the Companies Law.
Dividend and Liquidation Rights
We may declare a dividend to
be paid to the holders of the ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions
are determined by our board of directors and do not require the approval of the shareholders of a company unless the Company’s articles
of association provide otherwise. The Company’s amended and restated articles of association to be effective immediately prior to
the closing of this offering do not require shareholder approval of a dividend distribution and provide that dividend distributions may
be determined by our board of directors.
Pursuant to the Companies Law,
the distribution amount is limited to the greater of retained earnings or earnings generated over the previous two years, according
to our then last reviewed or audited financial statements (less the amount of previously distributed dividends, if not reduced from the
earnings), provided that the end of the period to which the financial statements relate is not more than six months prior to the
date of the distribution. If the Company does not meet such criteria, then the Company may distribute dividends only with court approval.
In each case, the Company is only permitted to distribute a dividend if our board of directors and, if applicable, the court determines
that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations
as they become due. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to
the holders of the ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be
affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that
may be authorized in the future.
Exchange Controls
There are currently no Israeli
currency control restrictions on remittances of dividends on the ordinary shares, proceeds from the sale of ordinary shares or interest
or other payments to non-residents of Israel, except for shareholders who are subjects of certain countries that are, or have been, in
a state of war with Israel at such time.
Shareholder Meetings
Under Israeli law, the Company
is required to hold an annual general meeting of our shareholders once every calendar year that must be held no later than 15 months
after the date of the previous annual general meeting. All general meetings other than the annual meeting of shareholders are referred
to in our amended and restated articles of association as special meetings. Our board of directors may call special meetings whenever
it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our
board of directors is required to convene a special general meeting upon the written request of (i) any two of our directors or one-quarter
of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of
our outstanding issued shares and 1% or more of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Under Israeli law, one or more
shareholders holding at least 1% of the voting rights at the general meeting may request that the board of directors include a matter
in the agenda of a general meeting to be convened in the future, such as nominating a director candidate, provided that it is appropriate
to discuss such a matter at the general meeting. The Company’s amended and restated articles of association contain procedural guidelines
and disclosure items with respect to the submission of shareholder proposals for shareholders meetings.
Subject to the provisions of
the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the
shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date
of the meeting. Furthermore, the Companies Law requires that resolutions regarding, among other things, the following matters must be
passed at a general meeting of our shareholders:
| ● | amendments to our amended and restated articles of association; |
| ● | appointment or termination of our auditors; |
| ● | election of directors, including external directors (unless
otherwise determined in our amended and restated articles of association); |
| ● | approval of certain related party transactions; |
| ● | increases or reductions of our authorized share capital; |
| ● | the exercise of our board of directors’ powers by a
general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our
proper management. |
Under our amended and restated
articles of association, we are not required to give notice to our registered shareholders pursuant to the Companies Law, unless otherwise
required by law. The Companies Law requires that a notice of any annual general meeting or special general meeting be provided to shareholders
at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of directors, the approval
of transactions with office holders or interested or related parties, or an approval of a merger, or as otherwise required under applicable
law, notice must be provided at least 35 days prior to the meeting. Under the Companies Law, shareholders of a public company are
not permitted to take action by written consent in lieu of a meeting.
Voting Rights
Quorum Requirements
Pursuant to the Company’s
amended and restated articles of association, holders of the ordinary shares have one vote for each Ordinary Share held on all matters
submitted to a vote before the shareholders at a general meeting. Under the Company’s amended and restated articles of association,
the quorum required for general meetings of shareholders must consist of at least two shareholders present in person or by proxy (including
by voting deed) holding 25% or more of our voting rights. A meeting adjourned for lack of a quorum will generally be adjourned to the
same day of the following week at the same time and place, or to such other day, time or place as indicated by our board of
directors if so specified in the notice of the meeting. At the reconvened meeting, any number of shareholders present in person or by
proxy shall constitute a lawful quorum.
Vote Requirements
The Company’s amended
and restated articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise
required by the Companies Law or by our amended and restated articles of association. The Company’s amended and restated articles
of association provide that all resolutions of our board of directors require a simple majority vote of the directors present and voting
at such meeting, unless otherwise required by the Companies Law or by the Company’s amended and restated articles of association.
Pursuant to the Company’s amended and restated articles of association, in the event the vote is tied, the chair of the board of
directors will have a casting vote.
Pursuant to the Company’s
amended and restated articles of association, an amendment to the Company’s amended and restated articles of association regarding
any change of the composition or election procedures of our directors will require a special majority vote (66⅔%). In addition,
any change to the rights and privileges of the holders of any class of our shares requires a simple majority of the class so affected
(or otherwise in accordance with the terms of such class), in addition to the simple majority vote of all classes of shares voting together
as a single class at a shareholder meeting.
Under the Companies Law, each
of (i) the approval of an extraordinary transaction with a controlling shareholder and (ii) the terms of employment or other
engagement of the controlling shareholder of the company or such controlling shareholder’s relative (even if not extraordinary)
requires the approval based on the codified fiduciary duties that the office holders owe to a company in the Companies Law. Certain transactions
with respect to remuneration of our office holders and directors require further approvals the approval based on the codified fiduciary
duties that the office holders owe to a company in the Companies Law. Another exception to the simple majority vote requirement is a resolution
for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350
of the Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting, in person or by proxy
and voting on the resolution.
Access to Corporate Records
Under the Companies Law, shareholders
are provided access to: minutes of our general meetings; our shareholders register and material shareholders register; the Company’s
amended and restated articles of association; our financial statements; and any document that we are required by law to file publicly
with the Israeli Companies Registrar (the “ISA”). In addition, shareholders may request to be provided with any document related
to an action or transaction requiring shareholder approval under the related party transaction provisions of the Companies Law. We may
deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a
trade secret or patent.
Modification of Class Rights
Under the Companies Law and
the Company’s amended and restated articles of association, the rights attached to any class of share, such as voting, liquidation
and dividend rights, may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a
separate class meeting, or otherwise in accordance with the terms of such class of shares, in addition to the simple majority vote of
all classes of shares voting together as a single class at a shareholder meeting, as set forth in our amended and restated articles of
association.
Acquisitions Under Israeli Law
Full Tender Offer. A
person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the target company’s issued
and outstanding share capital is required by the Companies Law to make a tender offer to all of the company’s shareholders for the
purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of an Israeli public company and
who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender
offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of
that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company
or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer,
all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer
will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of
the company or of the applicable class of shares.
Upon a successful completion
of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer
or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the
tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions,
the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli
court as described above.
If (a) the shareholders
who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company or of the applicable
class or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in
the acceptance of the tender offer or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and
outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase
its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from shareholders
who accepted the tender offer.
Special Tender Offer. The
Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as
a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. Similarly, the Companies
Law provides that an acquisition of shares in a public company must be made by means of a special tender offer if as a result of the acquisition
the purchaser would become a holder of more than 45% of the voting rights in the company. These requirements do not apply if, in general,
the acquisition (1) was made in a private placement that received shareholder approval, (2) was from a 25% or greater shareholder
of the company which resulted in the acquirer becoming a 25% or greater shareholder of the company, or (3) was from a 45% or greater
shareholder of the company which resulted in the acquirer becoming a 45% or greater shareholder of the company.
A special tender offer must
be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting
power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders. A special tender
offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired
by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer
(excluding the purchaser and its controlling shareholders, holders of 25% or more of the voting rights in the company or any person having
a personal interest in the acceptance of the tender offer or any other person acting on their behalf, including the relatives and entities
under such person’s control). If a special tender offer is accepted, then the purchaser or any person or entity controlling it or
under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of
shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer,
unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger. The
Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described
under the Companies Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority vote
of each class of its shares, voted on the proposed merger at a shareholders meeting.
For purposes of the shareholder
vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares represented at the shareholders
meeting that are held by parties other than the other party to the merger, or by any person (or group of persons acting in concert) who
holds (or hold, as the case may be) 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other
party, vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the
controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same special majority approval
that governs all extraordinary transactions with controlling shareholders. See “Management — Approval of Related
Party Transactions under Israeli Law — Fiduciary Duties of Directors and Officers — Disclosure of Personal
Interests of an Office Holder and Approval of Certain Transactions.”
If the transaction would have
been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain
shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights
of a company, if the court holds that the merger is fair and reasonable, taking into account the value to the parties to the merger and
the consideration offered to the shareholders of the company.
Upon the request of a creditor
of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern
that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further
give instructions to secure the rights of creditors.
In addition, a merger may not
be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each
party with the IRC and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Israeli tax law treats some
acquisitions, such as share-for-share exchanges between an Israeli company and a foreign company, less favorably than U.S. tax laws.
For example, Israeli tax law may, under certain circumstances, subject a shareholder who exchanges his ordinary shares for shares in another
corporation to taxation prior to the sale of the shares received in such share-for-share swap.
Anti-Takeover Measures Under Israeli Law
The Companies Law allows us
to create and issue shares having rights different from those attached to the ordinary shares, including shares providing certain preferred
rights with respect to voting, distributions or other matters and shares having pre-emptive rights. As of the date of this prospectus,
no preferred shares were authorized under the Company’s amended and restated articles of association. In the future, if we do authorize,
create and issue a specific class of preferred shares, such class of shares, depending on the specific rights that may be attached to
it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over
the market value of their ordinary shares. The authorization and designation of a class of preferred shares will require an amendment
to the Company’s amended and restated articles of association, which requires the prior approval of the holders of a majority of
the voting power attaching to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled
to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Companies
Law as described above in “— Voting Rights.”
Borrowing Powers
Pursuant to the Companies Law
and the Company’s amended and restated articles of association, our board of directors may exercise all powers and take all actions
that are not required under law or under our amended and restated articles of association to be exercised or taken by our shareholders,
including the power to borrow money for company purposes.
Changes in Capital
The Company’s amended
and restated articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions
of the Companies Law and must be approved by a resolution duly adopted by our shareholders at a general meeting. In addition, transactions
that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings
or profits, require the approval of both our board of directors and an Israeli court.
Establishment
We were incorporated under
the laws of the State of Israel on February 13, 2017. We are registered with the IRC.
Exclusive Forum
The Company’s amended
and restated articles of association to be effective immediately prior to the closing of this offering provide that unless we consent
in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum
for the resolution of any complaint asserting a cause of action arising under the Securities Act. This choice of forum provision may limit
a shareholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers
or other employees and may increase the costs associated with such lawsuits, which may discourage such lawsuits against us and our directors,
officers and employees.
This exclusive forum provision
would not apply to claims brought pursuant to the Securities Act or the Exchange Act or any other claim for which U.S. federal
courts would have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of
the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act
or the rules and regulations thereunder.
Alternatively, if a court were
to find these provisions of the Company’s amended and restated articles of association to be effective immediately prior to the
closing of this offering inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings,
we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business
and financial condition.
The Company’s amended
and restated articles of association to be effective immediately prior to the closing of this offering also provide that unless we consent
in writing to the selection of an alternative forum, the competent courts in Tel Aviv, Israel shall be the exclusive forum for any derivative
action or proceeding brought on behalf of the Company, any action asserting a breach of a fiduciary duty owed by any of our directors,
officers or other employees to the Company or our shareholders or any action asserting a claim arising pursuant to any provision of the
Companies Law or the Israeli Securities Law.
Any person or entity purchasing
or otherwise acquiring any interest in our share capital shall be deemed to have notice of and to have consented to these choice of forum
provisions.
History of Share Capital
Since January 1, 2020,
our issued share capital has changed as provided below.
On February 18, 2020,
we entered into a crowd-funding transaction in which we issued 370,356 ordinary shares to 536 different investors for total net consideration
of $447 thousand (net of $52 thousand issuance cost).
On April 26 2020, we entered
into another crowd-funding transaction in which we issued 47,688 shares to 161 different investors for total net consideration of $61
thousand (net of $8 thousand issuance cost).
In February, May, June and
July 2021, we entered into Simple Agreements for Future Equity (each, a “SAFE”) with four separate investors for aggregate
proceeds of $800 thousand. Pursuant to the terms of each SAFE, upon consummation of an equity financing, we were required to issue to
each investor the number of ordinary shares equal to the purchase amount divided by the SAFE price, which was defined as the price per
share equal to 80% of the equity financing valuation (to be no less than $25,000 thousand). The SAFE Agreements also provide the investor
the right to automatically receive ordinary shares in the case of a liquidity event, defined as a change of control event or an initial
public offering. In the case of a liquidity event the investors are entitled to the number of ordinary shares equal to the investment
amount divided by the liquidity price. The liquidity price is defined as the price per share equal to the Company’s valuation at
the time of the liquidity event, multiplied by 80%, and divided by the Company’s capitalization (to be no less than $25,000 thousand).
As part of our initial public offering, the SAFEs were converted at a price per share of $2.892 into 276,672 ordinary shares.
In May and October 2021,
we issued 240,000 and 45,000 ordinary shares, respectively, pursuant to the exercise of options at an exercise price of $0.033 per
share.
In August and September 2021,
we issued 1,837,500 ordinary shares pursuant to the exercise of warrants at a weighted average price of $0.67 per share.
On November 9, 2021, we
implemented a 1-for-3 split of our share capital, resulting in an increase of the issued and outstanding ordinary shares.
On December 13, 2021,
we completed our initial public offering and issued 2,000,000 units, each consisting of one ordinary share and a warrant representing
the right to purchase one ordinary share with an exercise price of $6.00 per share, at an initial public offering price of $6.00 per unit.
We also issued Warrants to purchase an additional 300,000 ordinary shares at an exercise price of $6.00 per share upon exercise by the
underwriter of their overallotment option.
In connection with the closing
of our initial public offering, on December 13, 2021, we issued 961,440 ordinary shares pursuant to the exercise of options
at a weighted average exercise price of $0.079 per share.
In March 2022, we issued
645,000 ordinary shares upon exercise of warrants issued in our initial public offering at a price per share of $6.00.
In October 2022, we issued
35,980 ordinary shares pursuant to vesting of restricted stock units (“RSUs”) that were granted to one of our officers
in October 2021.
In December 2022, we issued
72,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in November 2021.
In 2022, we issued 85,449 ordinary
shares to certain consultants in exchange for their services. One of the consultants received 44,000 shares of restricted stock which
were issued in 4 equal installments and subject to 2 year lock-up period. The other consultant’s received ordinary shares subject
to a 6 month lock-up.
In March, June and September 2023,
we issued 54,000 ordinary shares pursuant to vesting of RSUs that were granted to our officers in November 2021.
In May 2023, we issued
3,600 ordinary shares for gross proceeds of $7,200 in connection with our at-the-market offering program. The ordinary Shares were
sold at market prices at the time of sale.
In May and June we issued
320,479 ordinary shares pursuant to vesting of RSUs that were granted to our officers in March 2023.
In June and September2023,
we issued 50,000 ordinary shares pursuant to vesting of RSUs that were granted to our employees in March 2023.
In June 2023, we issued
126,000 ordinary shares pursuant to the exercise of options at an exercise price of $0.033 per share.
In June 2023, we issued
1,330,000 ordinary shares pursuant to a registered direct offering at a price per share of $1.50.
Transfer Agent and Registrar of ordinary shares
The transfer agent and registrar
for the ordinary shares American Stock Transfer & Trust Company, LLC. Its address is 6201 15th Avenue, Brooklyn,
New York 11219, and its telephone number is (800) 937-5449.
PLAN OF DISTRIBUTION
The
ongoing offer and sale by us of the ordinary shares issuable upon exercise of the Warrants is being made pursuant to this prospectus.
We
will deliver ordinary shares upon exercise of the Warrants, in whole or in part. We will not issue fractional ordinary shares. Each Warrant
contains instructions for the exercise. In order to exercise a Warrant, the holder must deliver the information required by the applicable
warrant agreement, along with payment of the exercise price, if the exercise price is being paid in cash, for the ordinary shares to be
purchased. We will then deliver our ordinary shares in the manner described in the applicable warrant agreement.
Offer Restrictions Outside the United States
Other than in the United States,
no action has been taken by us that would permit a public offering of the securities offered by this prospectus in any jurisdiction where
action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor
may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed
or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations
of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions
relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation
of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
LEGAL MATTERS
The validity of our ordinary shares offered by this
prospectus relating to Israeli law will be passed upon for us by Goldfarb Gross Seligman & Co., Israel. Certain members of Goldfarb
Gross Seligman & Co. are the beneficial owners of an aggregate of less than 1.0% of our ordinary shares.
EXPERTS
The consolidated financial
statements of NeuroSense Therapeutics Ltd. as of December 31, 2022 and 2021 and for each of the years in the three-year period ended December
31, 2022 have been incorporated by reference herein in reliance upon the report of Somekh Chaikin, a member firm of KPMG International,
independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting
and auditing. The audit report covering the December 31, 2022 consolidated financial statements contains an explanatory paragraph that
states that the Company’s recurring losses and its expectation to incur significant additional losses raise substantial doubt about
the entity’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might
result from the outcome of that uncertainty.
WHERE YOU CAN FIND MORE INFORMATION
This
prospectus is part of the registration statement on Form F-1 we filed with the SEC under the Securities Act, with respect to the securities
offered by this prospectus. However, as is permitted by the rules and regulations of the SEC, this prospectus, which is part of our registration
statement on Form F-1, omits certain non-material information, exhibits, schedules and undertakings set forth in the registration statement.
For further information about us, and the securities offered by this prospectus, please refer to the registration statement and the exhibits
and schedules filed as a part of the registration statement. You should rely only on the information contained in this prospectus or incorporated
by reference. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities
in any state where the offer is not permitted.
We are subject to the reporting
requirements of the Exchange Act that are applicable to a foreign private issuer. In accordance with the Exchange Act, we file reports,
including annual reports on Form 20-F by April 30 of each year. We also furnish to the SEC under cover of Form 6-K material information
required to be made public in Israel, filed with and made public by any stock exchange or distributed by us to our shareholders.
The SEC maintains an Internet
site that contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically
with the SEC (http://www.sec.gov).
As a foreign private issuer,
we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders and our
officers, directors and principal shareholders are exempt from the “short-swing profits” reporting and liability provisions
contained in Section 16 of the Exchange Act and related Exchange Act rules.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
We file annual and special
reports and other information with the SEC (File Number 001-41084). These filings contain important information which does not appear
in this prospectus. The SEC allows us to “incorporate by reference” information into this prospectus, which means that we
can disclose important information to you by referring you to other documents which we have filed with the SEC. We are incorporating by
reference in this prospectus the documents listed below:
| |
● |
our Annual Report on Form 20-F for the fiscal year ended on December 31, 2022, filed with the SEC on March 22, 2023; and |
| |
● |
our reports on Form 6-K furnished to the SEC on April
14, 2023, April 17, 2023
(with respect to Exhibit
99.1, all of the text other than the paragraph immediately preceding the heading “About NeuroSense”), May
2, 2023 (with respect to Exhibit
99.1, all of the text other than the three paragraphs immediately preceding the heading “About Parkinson’s Disease”),
May 15, 2023 (with respect
to Exhibit 99.1, all
of the text other than the three paragraphs immediately preceding the heading “About ALS”), May
25, 2023, May 31, 2023,
June 1, 2023 (with respect
to Exhibit 99.1, all
of the text other than the paragraph immediately preceding the heading “Business Update”), June
13, 2023, June 23, 2023,
July 6, 2023 (with respect
to Exhibit 99.1, all
of the text other than the paragraph immediately preceding the heading “About NeuroSense”), August
16, 2023 (with respect to Exhibit
99.1, only the text under the headings “Financial Summary” and “Forward-Looking Statements”), September
19, 2023 (other than the paragraph immediately preceding the heading “About NeuroSense” in Exhibit
99.1), September 20, 2023
(with respect to Exhibit
99.1, all of the text other than the paragraph immediately preceding the heading “About NeuroSense”), September
29, 2023, October 2, 2023
(with respect to Exhibit
99.1, only the first paragraph), October
4, 2023 (with respect to Exhibit
99.1, only the first three paragraphs), October
17, 2023 (with respect to Exhibit
99.1, all of the text other than the paragraph immediately preceding the heading “About NeuroSense”), November
6, 2023 (with respect to Exhibit
99.1, all of the text other than for the two paragraphs immediately preceding the heading “About ALS”), November
13, 2023 (with respect to Exhibit
99.1, all of the text other than for the second paragraph), November 28, 2023 (with respect to Exhibit 99.1, all of the text
other than the second paragraph) and our report on Form 6-K/A furnished to the SEC on
January 19, 2023 (with
respect to Exhibit 99.1,
only the text under the headings “Business Update” (first two paragraphs), “Financial Summary”, “Forward-Looking
Statements” and financial tables); and |
| |
|
|
| |
● |
the description of the ordinary shares contained under the heading
“Item 1. Description of Registrant’s Securities to be Registered” in our registration statement on Form
8-A, as filed with the SEC on November 18, 2021, including any subsequent amendment or any report filed for the purpose of updating
such description. |
Certain statements in and portions of this prospectus
update and replace information in the above listed documents incorporated by reference.
We will provide each person,
including any beneficial owner to whom a prospectus is delivered, without charge, upon a written or oral request, a copy of any of the
documents incorporated by reference in this prospectus, other than exhibits to such documents which are not specifically incorporated
by reference into such documents. Please direct your written or telephone requests to NeuroSense Therapeutics Ltd., 11 HaMenofim Street,
Building B, Herzliya, 4672562, Israel, Attn: Or Eisenberg, telephone number +972587531153. You may also obtain information about us by
visiting our website at www.neurosense-tx.com. Information contained in our website is not part of this prospectus.
SERVICE OF PROCESS AND ENFORCEMENT OF CIVIL
LIABILITIES
We are incorporated under
the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this
prospectus, substantially all of whom reside outside the United States, may be difficult to obtain within the United States. Furthermore,
because substantially all of our assets and substantially all of our directors and officers are located outside the United States, any
judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
We have irrevocably appointed
Cogency Global Inc. as our agent to receive service of process in any action against us in any U.S. federal or state court arising out
of this offering or any purchase or sale of securities in connection with this offering. The address of our agent is 122 East 42nd Street,
18th Floor, New York, NY 10168.
We have been informed by
our legal counsel in Israel, Goldfarb Gross Seligman & Co., that it may be difficult to initiate an action with respect to U.S. securities
law in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is
not the most appropriate forum to hear such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that
Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must
be proved as a fact by expert witnesses which can be a time-consuming and costly process. Certain matters of procedure may also be governed
by Israeli law.
Subject to certain time
limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is
non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including
a monetary or compensatory judgment in a non-civil matter, provided that:
| |
● |
the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment; |
| |
● |
the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and |
| |
● |
the judgment is executory in the state in which it was given. |
Even if these conditions are
met, an Israeli court will not declare a foreign civil judgment enforceable if:
| |
● |
the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases); |
| |
● |
the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel; |
| |
● |
the judgment was obtained by fraud; |
| |
● |
the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the Israeli court; |
| |
● |
the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in Israel; |
| |
● |
the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid; or |
| |
● |
at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in Israel. |
If a foreign judgment is
enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency
and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency
is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date
of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli
court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate
set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
Up to 1,755,000 Ordinary Shares Underlying Warrants

Prospectus
, 2023
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 6. Indemnification of Directors and Officers
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Companies Law, a company may not exculpate
an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from
liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a
provision authorizing such exculpation is included in its articles of association. Our amended and restated articles of association, include
such a provision. A company may not exculpate in advance a director from liability arising from a breach of his or her duty of care in
connection with a prohibited dividend or distribution to shareholders.
As permitted under the Companies Law, our amended
and restated articles of association provide that we may indemnify an office holder in respect of the following liabilities, payments
and expenses incurred for acts performed by him or her as an office holder, either in advance of an event or following an event:
| ● | a monetary liability incurred by or imposed on the office
holder in favor of another person pursuant to a court judgment, including pursuant to a settlement confirmed as judgment or arbitrator’s
decision approved by a competent court. However, if an undertaking to indemnify an office holder with respect to such liability is provided
in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based
on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by
the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and
amount or criteria; |
| ● | reasonable litigation expenses, including reasonable attorneys’
fees, which were incurred by the office holder as a result of an investigation or proceeding filed against the office holder by an authority
authorized to conduct such investigation or proceeding, provided that such investigation or proceeding was either (i) concluded without
the filing of an indictment against such office holder and without the imposition on him of any monetary obligation in lieu of a criminal
proceeding, (ii) concluded without the filing of an indictment against the office holder but with the imposition of a monetary obligation
on the office holder in lieu of criminal proceedings for an offense that does not require proof of criminal intent or (iii) in connection
with a monetary sanction; |
| ● | reasonable litigation expenses, including attorneys’
fees, incurred by the office holder or which were imposed on the office holder by a court (i) in a proceeding instituted against him
or her by the company, on its behalf, or by a third party, (ii) in connection with criminal indictment of which the office holder was
acquitted or (iii) in a criminal indictment for which the office holder was convicted of an offense that does not require proof of criminal
intent; |
| ● | expenses he or she incurs as a result of administrative proceedings
that may be instituted against him or her under Israeli securities laws, if applicable, and payments made to injured persons under specific
circumstances thereunder; and |
| ● | any other matter in respect of which it is permitted or will
be permitted under applicable law to indemnify an office holder in the company. |
As permitted under the Companies Law, our amended and restated articles of association provide that we may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder:
| ● | a breach of the duty of loyalty to the company, provided
that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company; |
| ● | a breach of duty of care to the company or to another person,
to the extent such a breach arises out of the negligent conduct of the office holder; |
| ● | a monetary liability imposed on the office holder in favor
of a third party; |
| ● | expenses he or she incurs as a result of administrative proceedings
that may be instituted against him or her under the Israeli securities laws if applicable, and payments made to injured persons under
specific circumstances thereunder; and |
| ● | any other matter in respect of which it is permitted or will
be permitted under applicable law to insure the liability of an office holder in the company. |
Under the Companies Law, a company may not indemnify,
exculpate or insure an office holder against any of the following:
| ● | a breach of the duty of loyalty, except for indemnification
and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable
basis to believe that the act would not prejudice the company; |
| ● | a breach of duty of care committed intentionally or recklessly,
excluding a breach arising out of the negligent conduct of the office holder; |
| ● | an act or omission committed with intent to derive illegal
personal benefit; or |
| ● | a fine or forfeit levied against the office holder. |
Under the Companies Law, exculpation, indemnification
and insurance of office holders must be approved by the compensation committee and the board of directors and, with respect to directors
or controlling shareholders, their relatives and third parties in which controlling shareholders have a personal interest, also by the
shareholders.
Our amended and restated articles of association
permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted or to be permitted by law. Our office
holders are currently covered by a directors’ and officers’ liability insurance policy. As of the date of this prospectus,
no claims for directors’ and officers’ liability insurance have been filed under this policy and we are not aware of any pending
or threatened litigation or proceeding involving any of our office holders, including our directors, in which indemnification is sought.
We have entered into agreements with each of our
current office holders exculpating them from a breach of their duty of care to us to the fullest extent permitted by law, subject to limited
exceptions, and undertaking to indemnify them to the fullest extent permitted by law, subject to limited exceptions, including, with respect
to liabilities resulting from this offering, to the extent that these liabilities are not covered by insurance. This indemnification is
limited, with respect to any monetary liability imposed in favor of a third party, to events determined as foreseeable by the board of
directors based on our activities. The maximum aggregate amount of indemnification that we may pay to our office holders based on such
indemnification agreement is the greater of (i) an amount equal to 25% of our shareholders’ equity on a consolidated basis, based
on our most recent financial statements made publicly available before the date on which the indemnity payment is made, and (ii) $25 million.
Such indemnification amounts are in addition to any insurance amounts. These indemnification agreements will supersede all previous letters
of indemnification that we have provided to him or her in the past, if any. However, in the opinion of the SEC, indemnification of office
holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
Item 7. Recent Sales of Unregistered Securities
The following sets forth information regarding all
unregistered securities sold since January 2020. These issuances did not result in changes to the voting rights attached to our ordinary
shares.
Issuance of Capital Stock
| ● | In February and April 2020, we issued an aggregate of 418,044 ordinary shares, at a weighted average price
per share of $1.43. |
| ● | In June 2023, as part of a registered direct offering of 1,330,000 ordinary
shares and pre-funded warrants to purchase up to 1,670,000 ordinary shares at an exercise price of $0.0001, we issued 3,000,000
warrants to purchase ordinary shares at an exercise price of $1.50 per
ordinary share in a private placement. |
The offers, sales and issuances of the securities
described in the preceding paragraphs were exempt from registration either (a) under Section 4(a)(2) of the Securities Act and the rules
and regulations promulgated thereunder (including Regulation D and Rule 506), in that the transactions were between an issuer and sophisticated
investors or members of its senior executive management and did not involve any public offering within the meaning of Section 4(a)(2),
(b) under Regulation S promulgated under the Securities Act in that offers, sales and issuances were not made to persons in the United
States and no directed selling efforts were made in the United States, (c) under Rule 144A under the Securities Act in that the shares
were offered and sold by the initial purchasers to qualified institutional buyers or (d) under Rule 701 promulgated under the Securities
Act in that the transactions were under compensatory benefit plans and contracts relating to compensation.
Item 8. Exhibits and Financial Statement Schedules
(a) The following
documents are filed as part of this registration statement:
| * | Previously filed. |
| ! | Annexes, schedules and exhibits have been omitted pursuant
to Item 601(b)(2) of Regulation S-K. The registrant agrees to furnish supplementally a copy of any omitted attachment to the Securities
and Exchange Commission on a confidential basis upon request. |
| + | Management contract or compensatory plan or arrangement. |
| # | English translation of original Hebrew document. |
Item 9. Undertakings
| a. | The undersigned registrant hereby undertakes: |
| 1. | To file, during any period in which offers or sales are being
made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by section 10(a)(3) of
the Securities Act of 1933 (the “Securities Act”); |
| ii. | To reflect in the prospectus any facts or events arising
after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in
the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing,
any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which
was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus
filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in
the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration
statement; and |
| iii. | To include any material information with respect to the plan
of distribution not previously disclosed in the registration statement or any material change to such information in the registration
statement. |
| 2. | That, for the purpose of determining any liability under the
Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered
therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 3. | To remove from registration by means of a post-effective amendment
any of the securities being registered which remain unsold at the termination of the offering. |
| 4. | To file a post-effective amendment to the registration statement
to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous
offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that
the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph
(a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those
financial statements. |
| 5. | That, for the purpose of determining liability under the Securities
Act to any purchaser, each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration
statement as of the date the filed prospectus was deemed part of and included in the registration statement; and each prospectus required
to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an
offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of
the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of
prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the
prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date
shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to
which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement
or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of
the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify
any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such
document immediately prior to such effective date. |
| 6. | That, for the purpose of determining liability of the registrant
under the Securities Act to any purchaser in the initial distribution of the securities:, the undersigned registrant undertakes that
in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting
method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following
communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities
to such purchaser: |
| i. | Any preliminary prospectus or prospectus of the undersigned
registrant relating to the offering required to be filed pursuant to Rule 424; |
| ii. | Any free writing prospectus relating to the offering prepared
by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| iii. | The portion of any other free writing prospectus relating
to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned
registrant; and |
| iv. | Any other communication that is an offer in the offering
made by the undersigned registrant to the purchaser. |
| 8. | Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions,
or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed
in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being
registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to
a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act
and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements
for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly
authorized, in Herzliya, Israel on December 4, 2023.
| |
NEUROSENSE THERAPEUTICS LTD. |
| |
|
| |
By: |
/s/ Alon Ben-Noon |
| |
Name: |
Alon Ben-Noon |
| |
Title: |
Chief Executive Officer |
Pursuant to the requirements
of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons on December 4, 2023
and in the capacities indicated.
| Name |
|
Title |
| |
|
|
* |
|
Chair of the Board of Directors |
| Mark Leuchtenberger |
|
|
| |
|
|
| /s/ Alon Ben-Noon |
|
Chief Executive Officer and Director |
| Alon Ben-Noon |
|
(principal executive officer
and director) |
| |
|
|
| * |
|
Chief Financial Officer |
| Or Eisenberg |
|
(principal financial officer and principal accounting officer) |
| |
|
|
* |
|
External Director |
| Cary Claiborne |
|
|
| |
|
|
* |
|
External Director |
| Christine Pellizzari |
|
|
| |
|
|
* |
|
Director |
| Caren Deardorf |
|
|
| |
|
|
* |
|
Director |
| Dr. Revital Mandil-Levin |
|
|
| *By: |
/s/ Alon Ben-Noon |
|
| Alon Ben-Noon |
|
| Attorney-in-fact |
|
SIGNATURE OF AUTHORIZED U.S. REPRESENTATIVE
OF REGISTRANT
Pursuant to the requirements
of the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of NeuroSense Therapeutics
Ltd. has signed this Post-effective Amendment to Registration Statement on Form F-1 on December 4, 2023.
| |
By: |
/s/ Colleen A. De Vries |
| |
Name: |
Colleen A. De Vries |
| |
Title: |
Sr. Vice -President, on behalf of Cogency Global |
II-6
Exhibit 23.1
Consent of Independent Registered
Public Accounting Firm
We consent to the use of our report dated March 20, 2023, with respect to the consolidated financial statements of NeuroSense
Therapeutics Ltd., incorporated herein by reference and to the reference to our firm under the heading “Experts” in the prospectus.
| /s/ Somekh Chaikin |
|
| Somekh Chaikin |
|
| Member Firm of KPMG International |
|
| Tel-Aviv, Israel |
|
| December 4, 2023 |
|
NeuroSense Therapeutics (NASDAQ:NRSN)
過去 株価チャート
から 12 2024 まで 1 2025
NeuroSense Therapeutics (NASDAQ:NRSN)
過去 株価チャート
から 1 2024 まで 1 2025