0001620463false00016204632024-09-032024-09-03
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 3, 2024
Athira Pharma, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
001-39503 |
45-3368487 |
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
18706 North Creek Parkway, Suite 104
Bothell, WA 98011
(Address of principal executive offices, including zip code)
(425) 620-8501
(Registrant’s telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, $0.0001 par value per share |
ATHA |
The Nasdaq Stock Market LLC
(The Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
|
|
Emerging Growth Company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act). ☐
Item 8.01 Other Events.
On September 3, 2024, Athira Pharma, Inc. (the “Company”) issued a press release announcing the topline results from the Company’s Phase 2/3 LIFT-AD clinical trial.
A copy of the Company’s press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
Item 7.01 Regulation FD Disclosure.
The Company will host a live webcast to discuss the LIFT-AD topline results at 4:30 PM Eastern time today, September 3, 2024. To access the live webcast, please visit the “Events and Presentations” page within the Investors section of the Athira website https://investors.athira.com/news-and-events/events-and-presentations-investor. The call can also be accessed by phone at 800-715-9871, conference ID: 4911242. As part of the webcast, the Company will present certain slides relating to the LIFT-AD topline results, which slides are attached as Exhibit 99.2 hereto.
On September 3, 2024, the Company announced certain information relating to the Company’s financial condition as of August 31, 2024, including its preliminary and unaudited estimate of cash resources, which consist of cash, cash equivalents and investments, of approximately $75.1 million. The preliminary and unaudited estimate of cash resources is based on management’s initial analysis of operations as of August 31, 2024, and is subject to further internal review.
The information in Item 7.01 of this Current Report on Form 8-K, including the slides to be used during the webcast and attached as Exhibit 99.2 hereto, are being furnished and not filed pursuant to Item 7.01 of Form 8-K. Such information shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any of the Company’s filings under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act whether made before or after the date hereof and regardless of any general incorporation language in such filings, except to the extent expressly set forth by specific reference in such a filing.
Forward-Looking Statements
This Current Report on Form 8-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. Forward-looking statements are identified by such words as “believe,” “expect,” “anticipate” and words of similar import and are based on current expectations that involve risks and uncertainties, such as the Company’s plans, projections, objectives, expectations and intentions. All statements other than historical or current facts are forward-looking statements, including, without limitation, statements about the Company’s preliminary and unaudited estimate of cash resources, which consist of cash, cash equivalents and investments. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. These statements, like all statements in this report, speak only as of their date. The preliminary expectations regarding cash, cash equivalents, and investments as of August 31, 2024 are the responsibility of management, are subject to management’s review and actual results could differ from management’s expectations. No assurance is given by the Company’s independent registered public accounting firm on such preliminary expectations. You should not draw any conclusions as to any other financial results as of August 31, 2024, based on the foregoing estimates.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Athira Pharma, Inc. |
|
|
|
|
Date: |
September 3, 2024 |
By: |
/s/ Mark Litton |
|
|
|
Mark Litton |
|
|
|
President and Chief Executive Officer |
Exhibit 99.1

Athira Pharma Announces Topline Results from Phase 2/3 LIFT-AD Clinical Trial of Fosgonimeton for Mild-to-Moderate Alzheimer’s Disease
LIFT-AD trial did not meet primary endpoint of GST and key secondary endpoints of cognition (ADAS-Cog11) and function (ADCS-ADL23)
In pre-specified subgroups of patients with moderate Alzheimer’s disease or who are APOE4 carriers, fosgonimeton showed a numerically greater treatment effect
All biomarkers associated with Alzheimer’s disease pathology showed changes with fosgonimeton treatment consistent with the broad neuroprotective mechanism of HGF modulation
Athira to host live webcast today at 4:30 PM Eastern time
BOTHELL, Wash., September 3, 2024 – Athira Pharma, Inc. (NASDAQ: ATHA), a late clinical-stage biopharmaceutical company focused on developing small molecules to restore neuronal health and slow neurodegeneration, today announced topline results from its Phase 2/3 LIFT-AD clinical trial of fosgonimeton, a hepatocyte growth factor (HGF) positive modulator, in patients with mild-to-moderate Alzheimer’s disease (AD).
The topline results showed that neither the trial's primary endpoint (the Global Statistical Test (GST), a combination of results from measures of cognition (ADAS-Cog11) and function (ADCS-ADL23)) nor its key secondary endpoints of ADAS-Cog11 and ADCS-ADL23 reached statistical significance compared with placebo at 26 weeks. However, both components of GST, cognition (ADAS-Cog11) and function (ADCS-ADL23), directionally favored fosgonimeton treatment, and in pre-specified subgroups characterized by more rapid disease progression (moderate AD and APOE4 carriers), cognition and function improved or stabilized in the fosgonimeton treated group. In addition, data across biomarkers of protein pathology (Aβ42/40, p-Tau181, and p-Tau217), inflammation (GFAP) and neurodegeneration (NfL) showed directional improvements with fosgonimeton treatment that are consistent with the broad neuroprotective mechanism of HGF modulation.
“These are not the results we hoped for, as the lack of clinical decline in the placebo group, combined with the short duration of the study, may have impacted the trial’s ability to translate the effect of fosgonimeton treatment into meaningful clinical benefit,” said Javier San Martin, M.D., Chief Medical Officer of Athira. “However, we believe the totality of the data continues to suggest that positive modulation of the HGF pathway has the potential to translate into improvement in parameters of neuronal health and may mitigate disease progression.”
“While the trial did not meet its primary endpoint, the biomarker and subgroup data are intriguing and remarkably consistent not only across endpoints but also with our understanding of fosgonimeton’s neuroprotective mechanism of action,” added Anton P. Porsteinsson, M.D., Director of the University of Rochester Alzheimer’s Disease Care, Research, and Education Program (AD-CARE) and a LIFT-AD investigator.
Phase 2/3 LIFT-AD Clinical Trial Design and Topline Results
LIFT-AD (NCT04488419) was a randomized, placebo-controlled, double-blind study that evaluated the efficacy and safety of once-daily subcutaneous injections of fosgonimeton (40 mg) in 312 mild-to-moderate AD patients not on acetylcholinesterase inhibitors (AChEIs) compared to placebo over a 26-week treatment period. The primary endpoint of LIFT-AD was the change from baseline after 26 weeks of treatment using the Global Statistical Test (GST), a combination of results from measures of cognition
(ADAS-Cog11) and function (ADCS-ADL23). Secondary endpoints included cognition (ADAS-Cog11), function (ADCS-ADL23), and a plasma biomarker of neurodegeneration, neurofilament light chain (NfL). The trial explored additional plasma biomarkers including glial fibrillary acidic protein (GFAP), a marker of neuroinflammation, and both amyloid beta and phosphorylated tau (pTau), hallmark measures of AD pathology.
Primary Analysis Population
Topline results from the LIFT-AD study of fosgonimeton in mild-to-moderate AD patients after 26 weeks showed:
•A -0.08 change in GST favoring fosgonimeton that did not reach statistical significance (p=0.70)
•The change in cognition from baseline as assessed by ADAS-Cog11, for which a decrease from baseline represents improvement, was -0.39 for the placebo group and -1.09 for the fosgonimeton treated group, a difference of -0.70 (p=0.35) favoring fosgonimeton
•In the fosgonimeton treated group, there was an increase (improvement) of 0.65 in function as measured by ADCS-ADL23 versus a decline of -0.02 in placebo, although this difference did not meet statistical significance (p=0.61)
Prespecified Biomarker Analyses
Data from the plasma biomarkers of neurodegeneration (NfL), inflammation (GFAP), and protein pathology (p-Tau181, p-Tau217, and amyloid beta 42/40 ratio) showed consistent directional improvements favoring fosgonimeton versus placebo after 26 weeks. Notably, fosgonimeton treatment reduced plasma levels of pTau217, a hallmark of AD, by -0.12 pg/mL compared to placebo (p<0.01).
Prespecified Subgroup Analyses
In a prespecified subgroup analyses of more advanced AD patients, a greater numerical treatment effect in clinical outcomes was observed in the fosgonimeton treatment group compared to placebo after 26 weeks:
•The change in cognition from baseline as assessed by ADAS-Cog11 in moderate AD patients, for which a decrease from baseline represents improvement, showed the fosgonimeton treatment group (n=61) improved compared to placebo (n=70), with a delta of -1.16 (p=0.39)
•For AD patients who are carriers of the APOE4 gene, the placebo group (n=74) declined in cognition as assessed by ADAS-Cog11 over the 26-week period as expected, whereas the fosgonimeton treatment group (n=74) remained stable, with a delta of -1.07 (p=0.33)
Post Hoc Subgroup Analyses
In a post hoc analyses by disease severity as defined by baseline ADAS-Cog11 (>30) and Clinical Dementia Rating (CDR) 2, fosgonimeton showed a larger effect size mainly driven by an improvement in cognition at week 26.
•Patients with the highest baseline ADAS-Cog11 (>30) who were treated with fosgonimeton (n=42) compared to placebo (n=52) showed a -2.51 improvement in cognition as assessed by ADAS-Cog11 (p=0.16), for which a lower number represents improvement
•A small subset of patients with a CDR 2 (moderate dementia) who were treated with fosgonimeton (n=20) compared to placebo (n=19) showed a -3.74 improvement in cognition as assessed by ADAS-Cog11 (p=0.21)
Safety and Tolerability
Fosgonimeton was generally well tolerated, with a favorable safety profile. Participants treated with fosgonimeton (40 mg) for 26 weeks showed a higher incidence of treatment emergent adverse events compared to placebo, mainly driven by injection site reactions. The incidence of serious adverse events (SAEs) was similar between treatment groups, with few treatment-related SAEs and no deaths observed in the study. The study had a 22 percent early termination rate.
“In addition to fosgonimeton, we have a pipeline of next-generation, orally delivered HGF modulators, with improved pharmacological properties, that we are currently evaluating in neurodegenerative diseases,” stated Mark Litton, Ph.D., President and Chief Executive Officer of Athira. “We want to express our sincere appreciation for the patients, caregivers, their families, and healthcare professionals who participated in the LIFT-AD trial. I also want to thank all Athira employees for their dedication and tireless contributions to advancing the science to benefit patients battling neurodegenerative diseases.”
Full analysis of these results is scheduled to be reviewed at the 17th Annual Clinical Trials on Alzheimer’s Disease (CTAD) taking place October 29 - November 1, 2024, in Madrid, Spain.
Athira continues to evaluate ATH-1105, a next-generation, orally administered, small molecule drug candidate in development for the potential treatment of amyotrophic lateral sclerosis (ALS), AD, and other neurodegenerative diseases. Athira is currently conducting a first-in-human, dose escalation Phase 1 clinical trial evaluating safety, tolerability and pharmacokinetics of ATH-1105 in up to 80 healthy volunteers. Athira completed the first cohort of healthy volunteers in June 2024 and expects trial completion by year-end 2024, with a goal to be in ALS patients in 2025. ATH-1105’s potential is supported by a growing body of preclinical evidence demonstrating improvements in nerve and motor function, biomarkers of inflammation and neurodegeneration, and survival in various models of ALS.
Live Webcast
Athira management will host a live webcast to discuss the LIFT-AD topline results at 4:30 PM Eastern time today, Tuesday, September 3, 2024. The live webcast can be accessed at Events and Presentations | Athira Pharma. The call can also be accessed by phone at 800-715-9871, conference ID: 4911242. A replay of the webcast will be available two hours after the call and will be archived on the Events for approximately 90 days.
About Athira Pharma, Inc.
Athira Pharma, Inc., headquartered in the Seattle, Washington area, is a late clinical-stage biopharmaceutical company focused on developing small molecules to restore neuronal health and slow neurodegeneration. Athira aims to alter the course of neurological diseases by advancing its pipeline of drug candidates that modulate the neurotrophic HGF system. For more information, visit www.athira.com. You can also follow Athira on Facebook, LinkedIn, X (formerly known as Twitter) and Instagram.
Forward-Looking Statements
This communication contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are not based on historical fact and include statements regarding: Athira’s drug candidates as potential treatments for Alzheimer’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases; future development plans; the anticipated reporting of data; the potential learnings from preclinical studies and other nonclinical data, the LIFT-AD trial and the ongoing Phase 1 trial of ATH-1105 and their ability to inform and improve future clinical development plans; expectations regarding the potential efficacy and commercial potential of Athira’s drug candidates; and Athira’s ability to advance its drug candidates into later stages of development. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “on track,” “would,” “expect,” “plan,” “believe,” “intend,” “pursue,” “continue,” “suggest,” “potential, target” and similar expressions. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the data from preclinical and clinical trials may not support the safety, efficacy and tolerability of Athira’s drug candidates; development of drug candidates may cease or be delayed; regulatory authorities could object to protocols, amendments and other submissions; future potential regulatory milestones for drug candidates, including those related to current and planned clinical studies, may be insufficient to support regulatory submissions or approval; Athira may not be able to recruit sufficient patients for its clinical trials; the outcome of legal proceedings that have been or may in the future be instituted against Athira, its directors and officers; possible negative interactions of Athira's drug candidates with other treatments; Athira’s assumptions regarding its financial condition and the sufficiency of its cash, cash equivalents and investments to fund its planned operations may be incorrect; adverse conditions in the general domestic and global economic markets; the impact of competition; the impact of expanded drug candidate development and clinical activities on operating expenses; the impact of new or changing laws and regulations; as well as the other risks detailed in Athira’s filings with the Securities and Exchange Commission from time to time. These forward-looking statements speak only as of the date hereof and Athira undertakes no obligation to update forward-looking statements. Athira may not actually achieve the plans, intentions, or expectations disclosed in its forward-looking statements, and you should not place undue reliance on the forward-looking statements.
Investor & Media Contact:
Julie Rathbun
Athira Pharma
Julie.rathbun@athira.com
206-769-9219

LIFT-AD Topline Readout September 3, 2024 Exhibit 99.2

Disclaimer This presentation and the accompanying oral commentary contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, timing and success of our planned development activities, our ability to obtain regulatory approval, the potential therapeutic benefits and economic value of our product candidates as a potential treatment for Alzheimer’s disease, amyotrophic lateral sclerosis, and other neurodegenerative diseases, the anticipated reporting of data, and the potential learnings from preclinical studies and other nonclinical data, the LIFT-AD trial and their ability to inform and improve future clinical development plans. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described in greater detail in our filings with the Securities and Exchange Commission (“SEC”) may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and readers are cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. This presentation contains estimates, projections and other information concerning market, industry and other data. We obtained this data from our own internal estimates and research and from academic and industry research, publications, surveys, and studies conducted by third parties, including governmental agencies. These data involve a number of assumptions and limitations, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed in our filings with the SEC. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. While we believe such information is generally reliable, we have not independently verified any third-party information. This presentation concerns drug candidates that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. The drug candidates are currently limited by federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. We announce material information to the public through a variety of means, including filings with the SEC, press releases, public conference calls, our website (www.athira.com), our investor relations website (investors.athira.com), and our news site (investors.athira.com/news-and-events/press-releases). We use these channels, as well as social media, including our LinkedIn (https://www.linkedin.com/company/athirapharma), Facebook (https://www.facebook.com/athirapharmainc) and @athirapharma on X (formerly known as Twitter) and Instagram to communicate with investors and the public about Athira, our products, and other matters. Therefore, we encourage investors, the media, and others interested in Athira to review the information we make public in these locations, as such information could be deemed to be material information. © Athira Pharma, Inc.
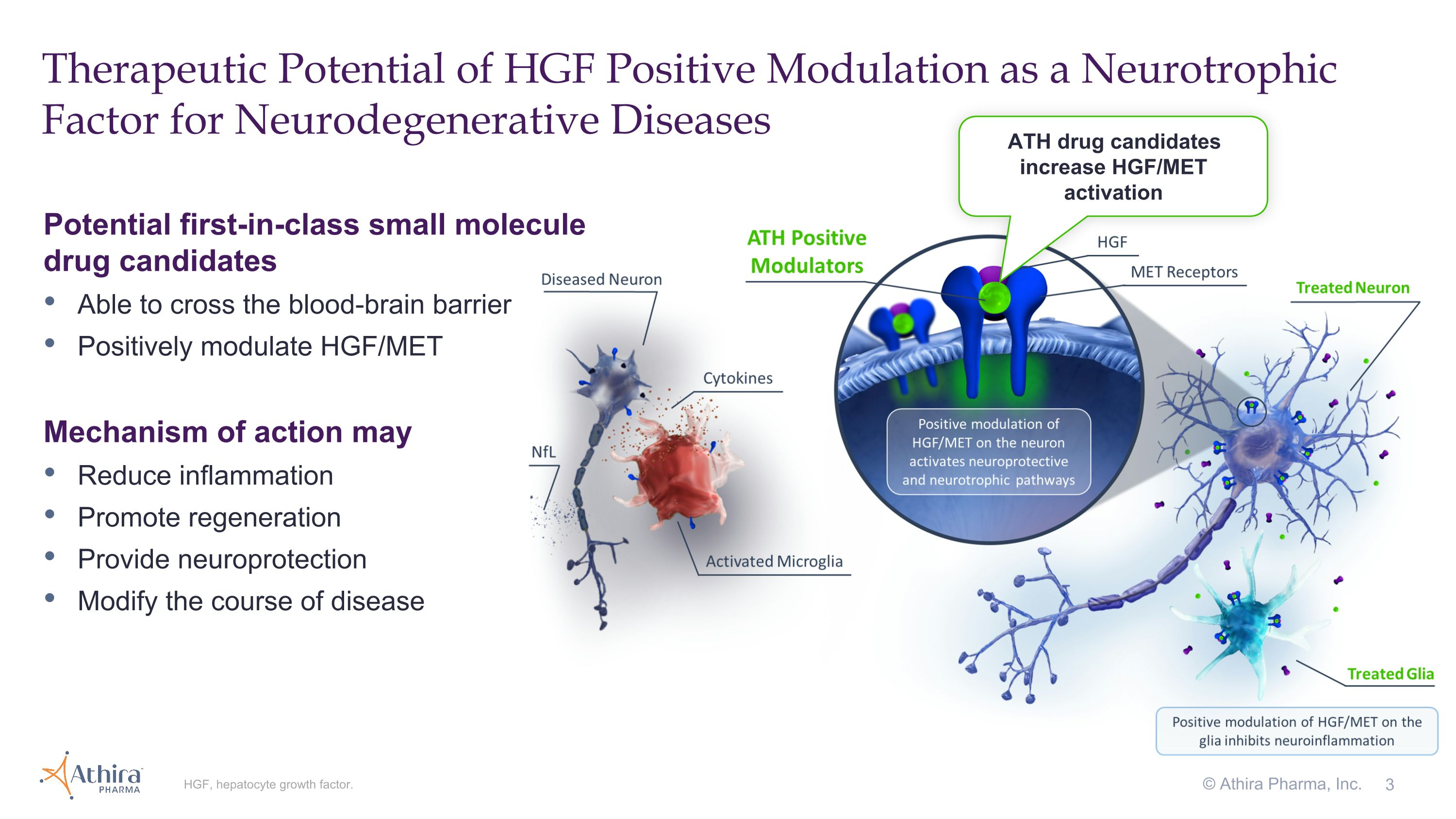
3 Therapeutic Potential of HGF Positive Modulation as a Neurotrophic Factor for Neurodegenerative Diseases HGF, hepatocyte growth factor. Potential first-in-class small molecule drug candidates Able to cross the blood-brain barrier Positively modulate HGF/MET Mechanism of action may Reduce inflammation Promote regeneration Provide neuroprotection Modify the course of disease ATH drug candidates increase HGF/MET activation © Athira Pharma, Inc.
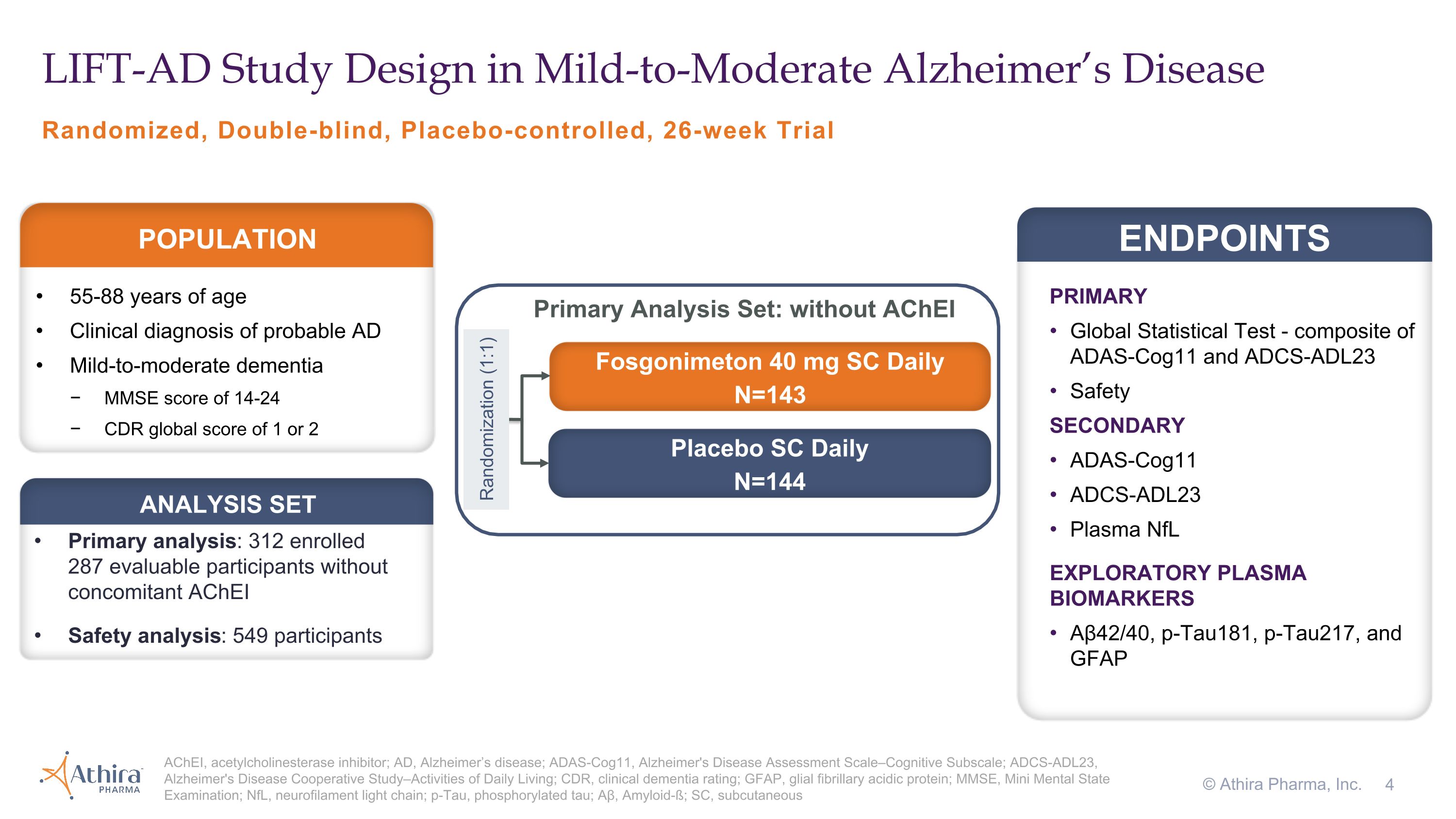
Randomized, Double-blind, Placebo-controlled, 26-week Trial LIFT-AD Study Design in Mild-to-Moderate Alzheimer’s Disease AChEI, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL23, Alzheimer's Disease Cooperative Study–Activities of Daily Living; CDR, clinical dementia rating; GFAP, glial fibrillary acidic protein; MMSE, Mini Mental State Examination; NfL, neurofilament light chain; p-Tau, phosphorylated tau; Aβ, Amyloid-ß; SC, subcutaneous © Athira Pharma, Inc. PRIMARY Global Statistical Test - composite of ADAS-Cog11 and ADCS-ADL23 Safety SECONDARY ADAS-Cog11 ADCS-ADL23 Plasma NfL EXPLORATORY PLASMA BIOMARKERS Aβ42/40, p-Tau181, p-Tau217, and GFAP ENDPOINTS 55-88 years of age Clinical diagnosis of probable AD Mild-to-moderate dementia MMSE score of 14-24 CDR global score of 1 or 2 POPULATION Fosgonimeton 40 mg SC Daily N=143 Placebo SC Daily N=144 Randomization (1:1) Primary analysis: 312 enrolled �287 evaluable participants without concomitant AChEI Safety analysis: 549 participants ANALYSIS SET Primary Analysis Set: without AChEI
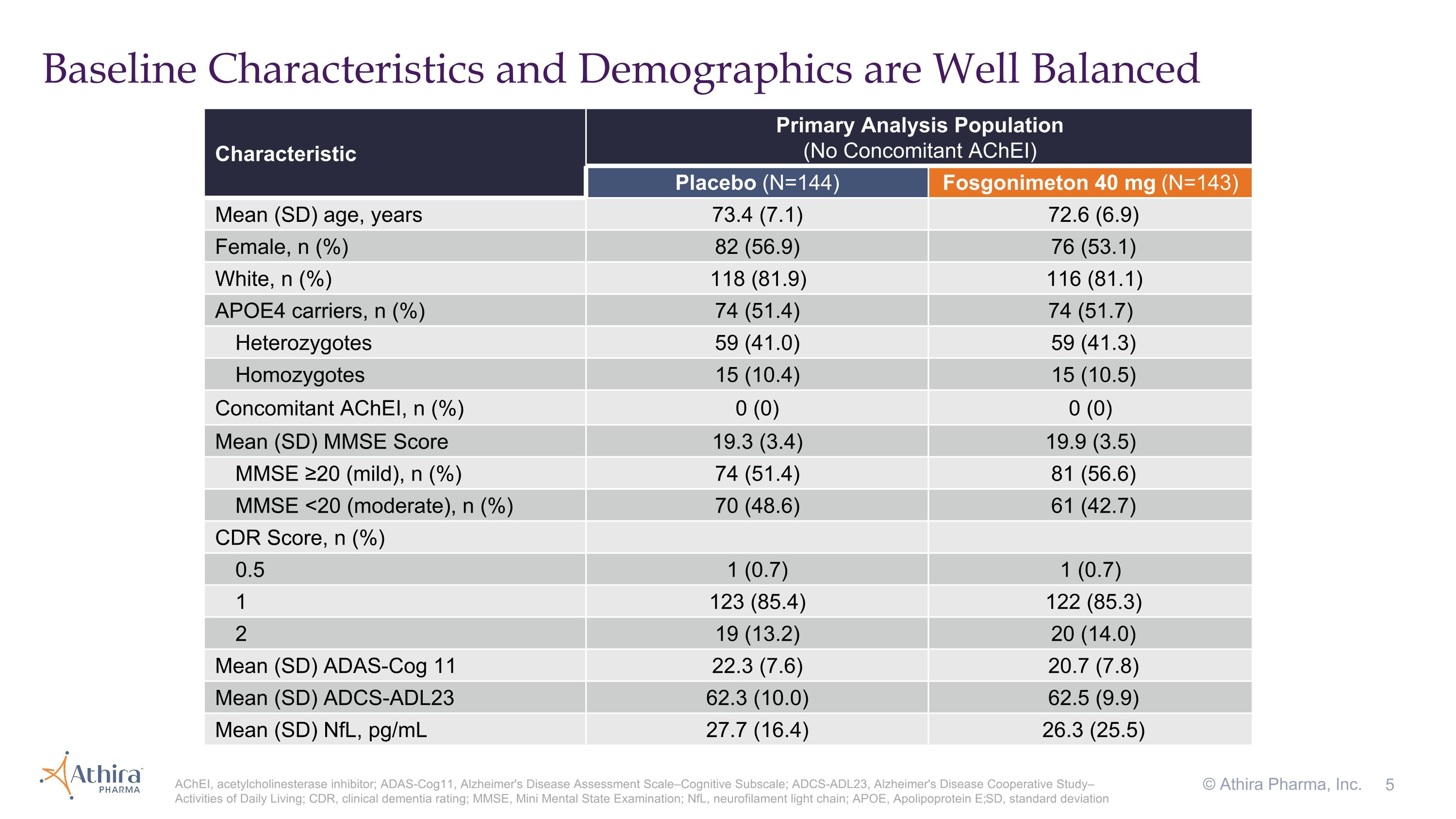
Baseline Characteristics and Demographics are Well Balanced © Athira Pharma, Inc. AChEI, acetylcholinesterase inhibitor; ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL23, Alzheimer's Disease Cooperative Study–Activities of Daily Living; CDR, clinical dementia rating; MMSE, Mini Mental State Examination; NfL, neurofilament light chain; APOE, Apolipoprotein E; SD, standard deviation Characteristic Primary Analysis Population (No Concomitant AChEI) Placebo (N=144) Fosgonimeton 40 mg (N=143) Mean (SD) age, years 73.4 (7.1) 72.6 (6.9) Female, n (%) 82 (56.9) 76 (53.1) White, n (%) 118 (81.9) 116 (81.1) APOE4 carriers, n (%) 74 (51.4) 74 (51.7) Heterozygotes 59 (41.0) 59 (41.3) Homozygotes 15 (10.4) 15 (10.5) Concomitant AChEI, n (%) 0 (0) 0 (0) Mean (SD) MMSE Score 19.3 (3.4) 19.9 (3.5) MMSE ≥20 (mild), n (%) 74 (51.4) 81 (56.6) MMSE <20 (moderate), n (%) 70 (48.6) 61 (42.7) CDR Score, n (%) 0.5 1 (0.7) 1 (0.7) 1 123 (85.4) 122 (85.3) 2 19 (13.2) 20 (14.0) Mean (SD) ADAS-Cog 11 22.3 (7.6) 20.7 (7.8) Mean (SD) ADCS-ADL23 62.3 (10.0) 62.5 (9.9) Mean (SD) NfL, pg/mL 27.7 (16.4) 26.3 (25.5)
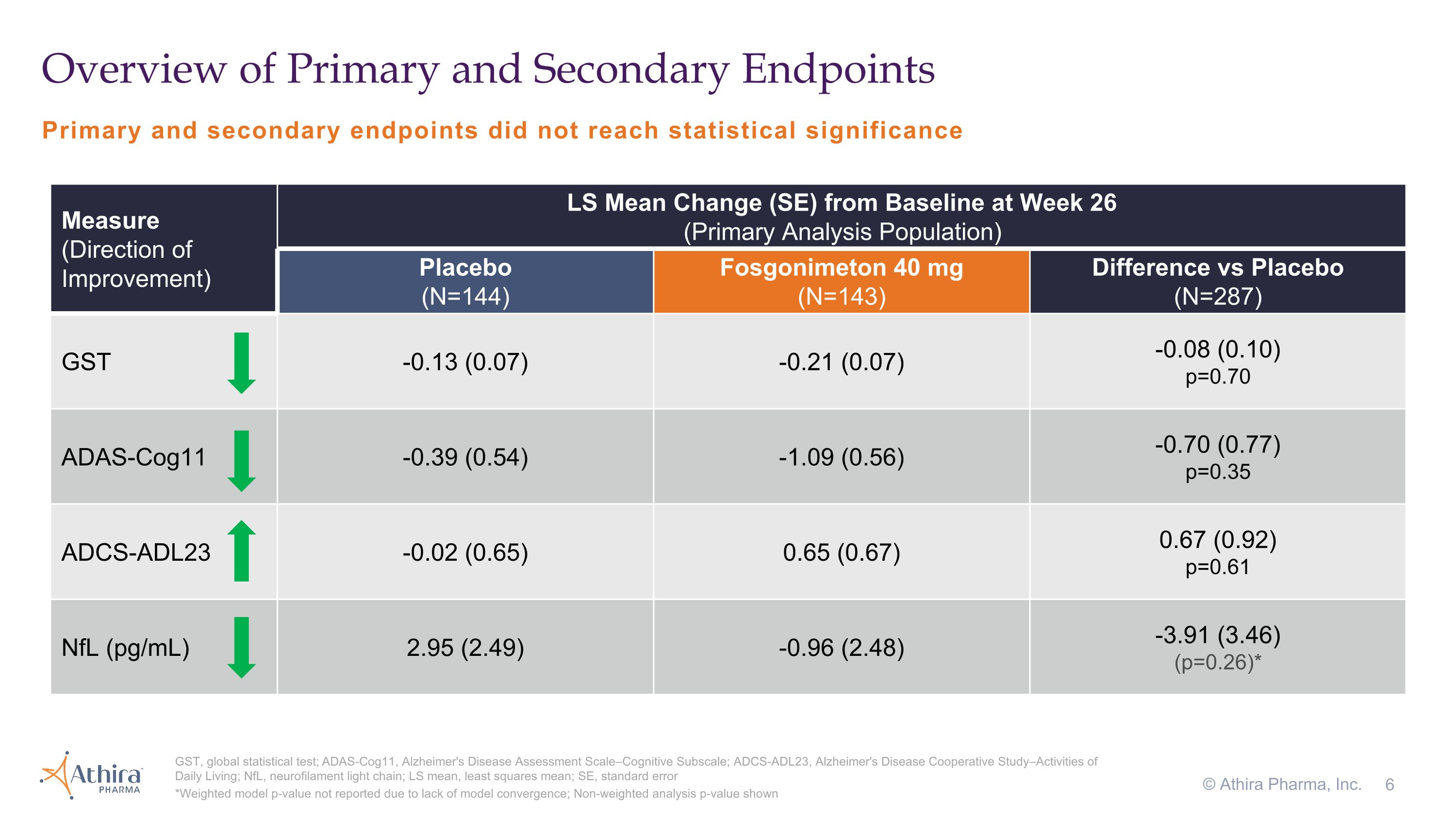
Primary and secondary endpoints did not reach statistical significance Overview of Primary and Secondary Endpoints GST, global statistical test; ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL23, Alzheimer's Disease Cooperative Study–Activities of Daily Living; NfL, neurofilament light chain; LS mean, least squares mean; SE, standard error *Weighted model p-value not reported due to lack of model convergence; Non-weighted analysis p-value shown © Athira Pharma, Inc. Measure �(Direction of Improvement) LS Mean Change (SE) from Baseline at Week 26�(Primary Analysis Population) Placebo�(N=144) Fosgonimeton 40 mg (N=143) Difference vs Placebo�(N=287) GST -0.13 (0.07) -0.21 (0.07) -0.08 (0.10) p=0.70 ADAS-Cog11 -0.39 (0.54) -1.09 (0.56) -0.70 (0.77) p=0.35 ADCS-ADL23 -0.02 (0.65) 0.65 (0.67) 0.67 (0.92) p=0.61 NfL (pg/mL) 2.95 (2.49) -0.96 (2.48) -3.91 (3.46) (p=0.26)*
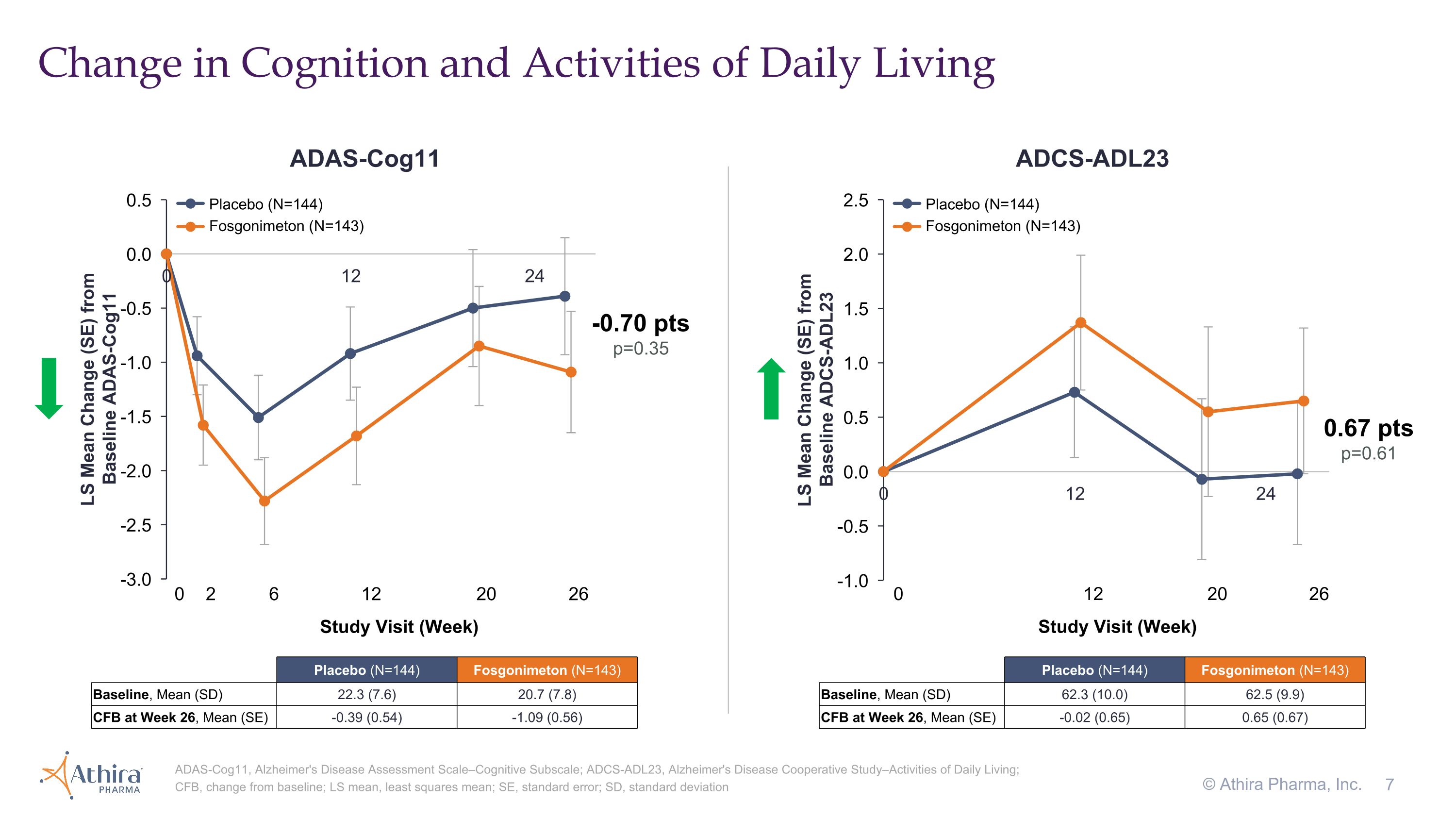
Change in Cognition and Activities of Daily Living ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ADCS-ADL23, Alzheimer's Disease Cooperative Study–Activities of Daily Living; CFB, change from baseline; LS mean, least squares mean; SE, standard error; SD, standard deviation © Athira Pharma, Inc. 0.67 pts p=0.61 0 12 20 26 Study Visit (Week) -0.70 pts p=0.35 0 2 6 12 20 26 Study Visit (Week) ADAS-Cog11 ADCS-ADL23 Placebo (N=144) Fosgonimeton (N=143) Placebo (N=144) Fosgonimeton (N=143) Placebo (N=144) Fosgonimeton (N=143) Baseline, Mean (SD) 22.3 (7.6) 20.7 (7.8) CFB at Week 26, Mean (SE) -0.39 (0.54) -1.09 (0.56) Placebo (N=144) Fosgonimeton (N=143) Baseline, Mean (SD) 62.3 (10.0) 62.5 (9.9) CFB at Week 26, Mean (SE) -0.02 (0.65) 0.65 (0.67)
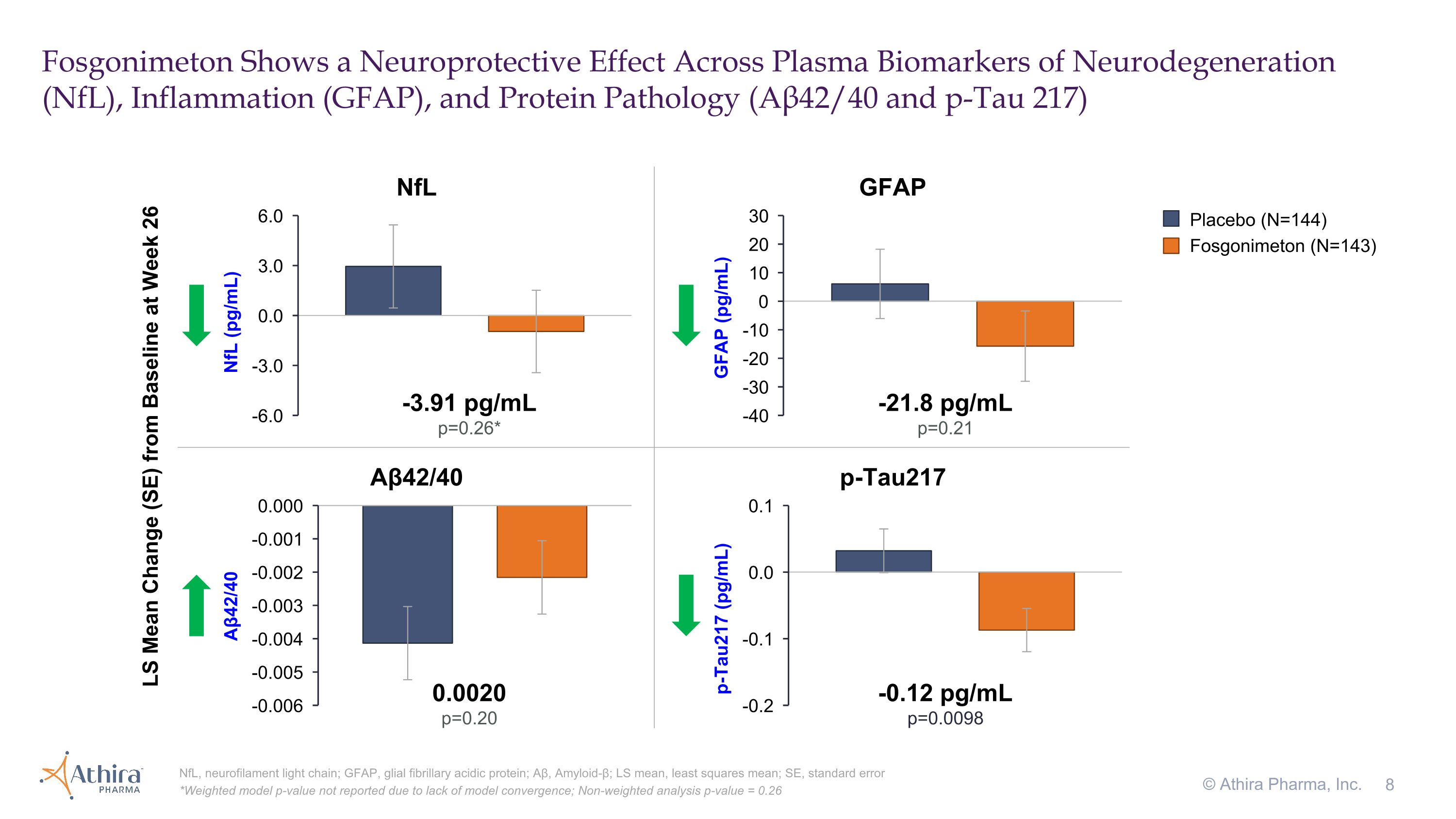
Fosgonimeton Shows a Neuroprotective Effect Across Plasma Biomarkers of Neurodegeneration (NfL), Inflammation (GFAP), and Protein Pathology (Aβ42/40 and p-Tau 217) NfL, neurofilament light chain; GFAP, glial fibrillary acidic protein; Aβ, Amyloid-β; LS mean, least squares mean; SE, standard error *Weighted model p-value not reported due to lack of model convergence; Non-weighted analysis p-value = 0.26 © Athira Pharma, Inc. -3.91 pg/mL p=0.26* NfL LS Mean Change (SE) from Baseline at Week 26 Aβ42/40 p-Tau217 GFAP -21.8 pg/mL p=0.21 0.0020 p=0.20 -0.12 pg/mL p=0.0098 Placebo (N=144) Fosgonimeton (N=143)
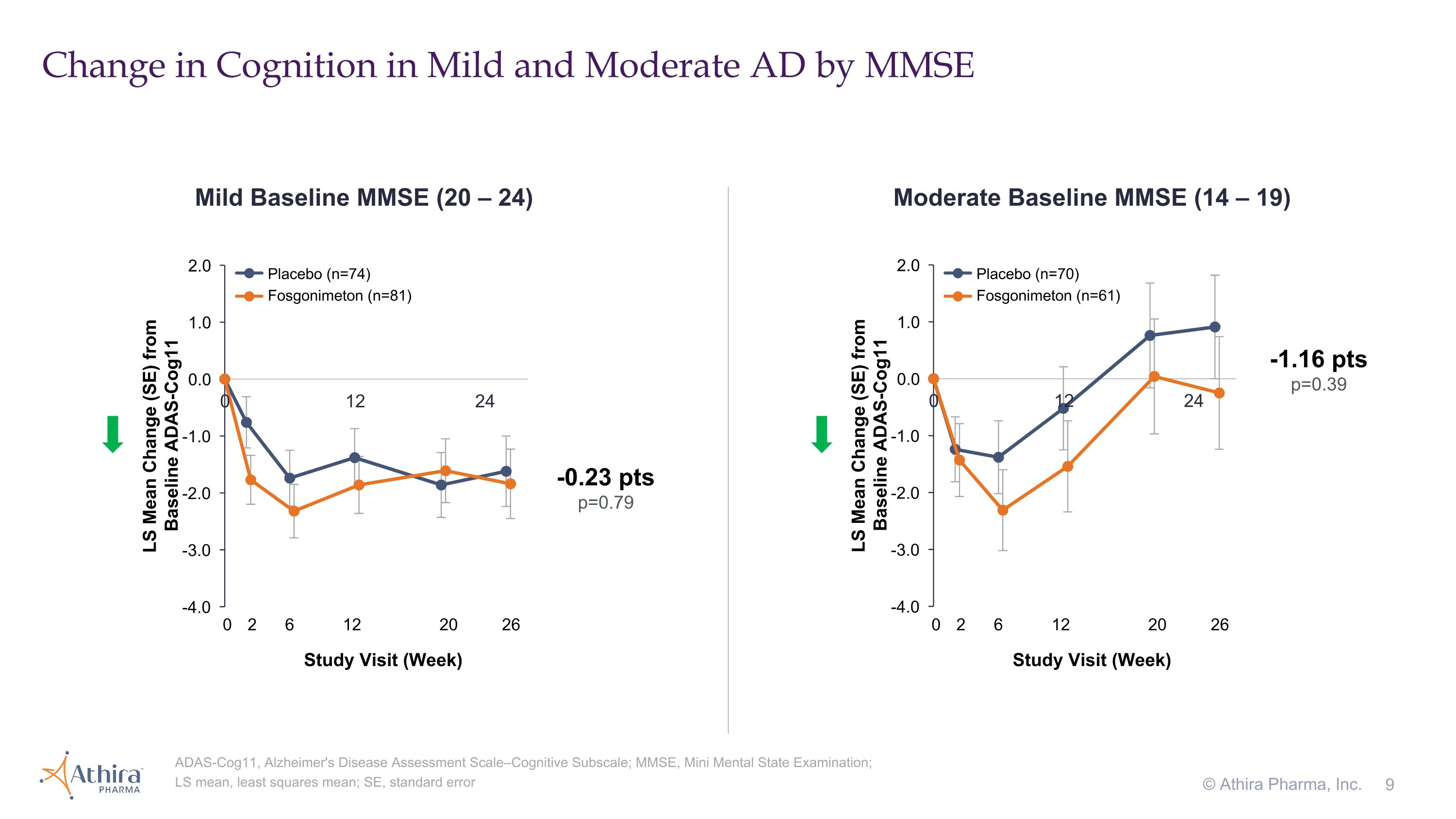
Change in Cognition in Mild and Moderate AD by MMSE © Athira Pharma, Inc. ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; MMSE, Mini Mental State Examination; LS mean, least squares mean; SE, standard error Mild Baseline MMSE (20 – 24) Moderate Baseline MMSE (14 – 19) 0 2 6 12 20 26 Study Visit (Week) -1.16 pts p=0.39 -0.23 pts p=0.79 0 2 6 12 20 26 Study Visit (Week) Placebo (n=74) Fosgonimeton (n=81) Placebo (n=70) Fosgonimeton (n=61)
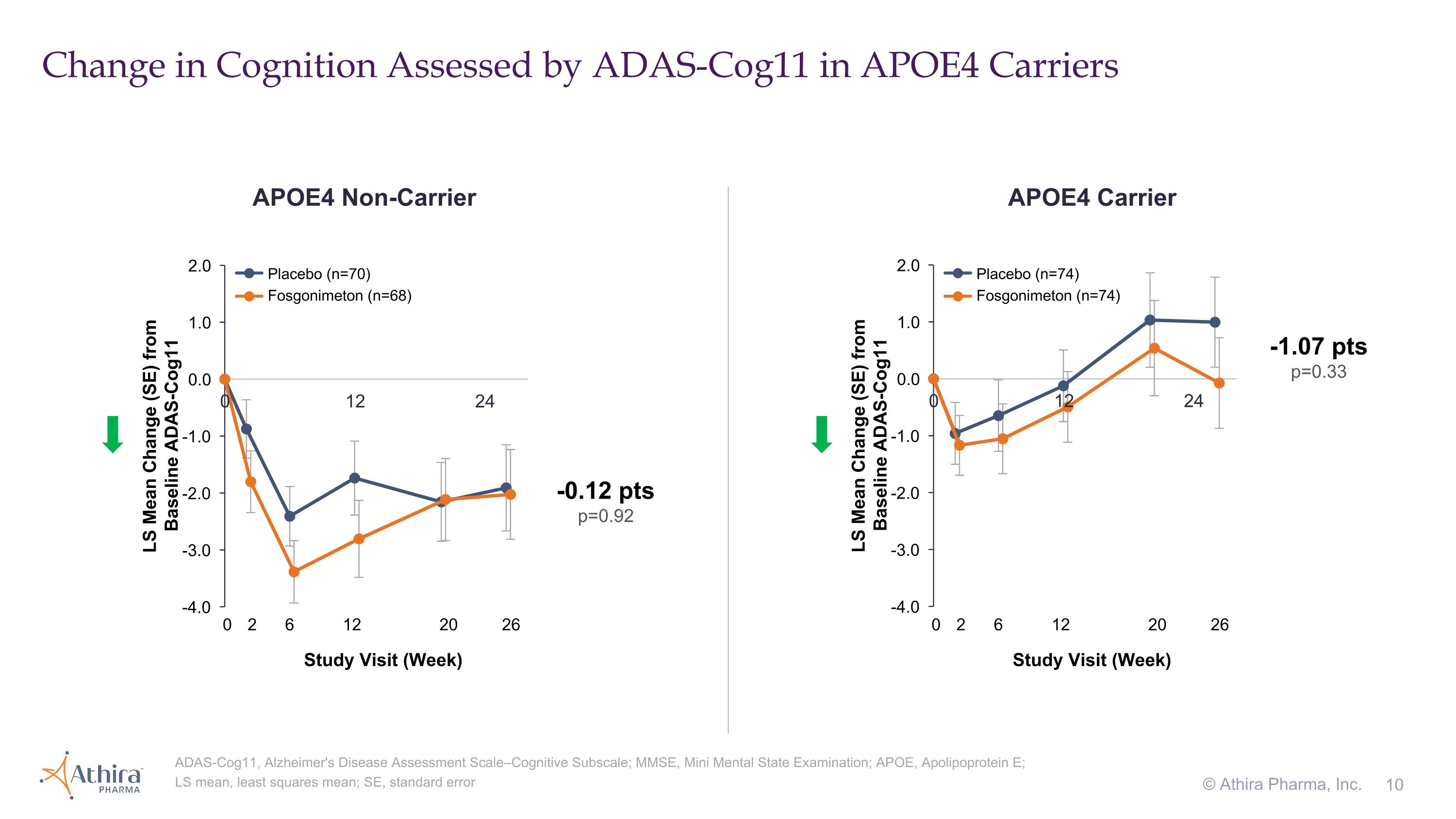
Change in Cognition Assessed by ADAS-Cog11 in APOE4 Carriers © Athira Pharma, Inc. ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; MMSE, Mini Mental State Examination; APOE, Apolipoprotein E; LS mean, least squares mean; SE, standard error APOE4 Non-Carrier APOE4 Carrier -1.07 pts p=0.33 -0.12 pts p=0.92 Placebo (n=70) Fosgonimeton (n=68) Placebo (n=74) Fosgonimeton (n=74) 0 2 6 12 20 26 Study Visit (Week) 0 2 6 12 20 26 Study Visit (Week)
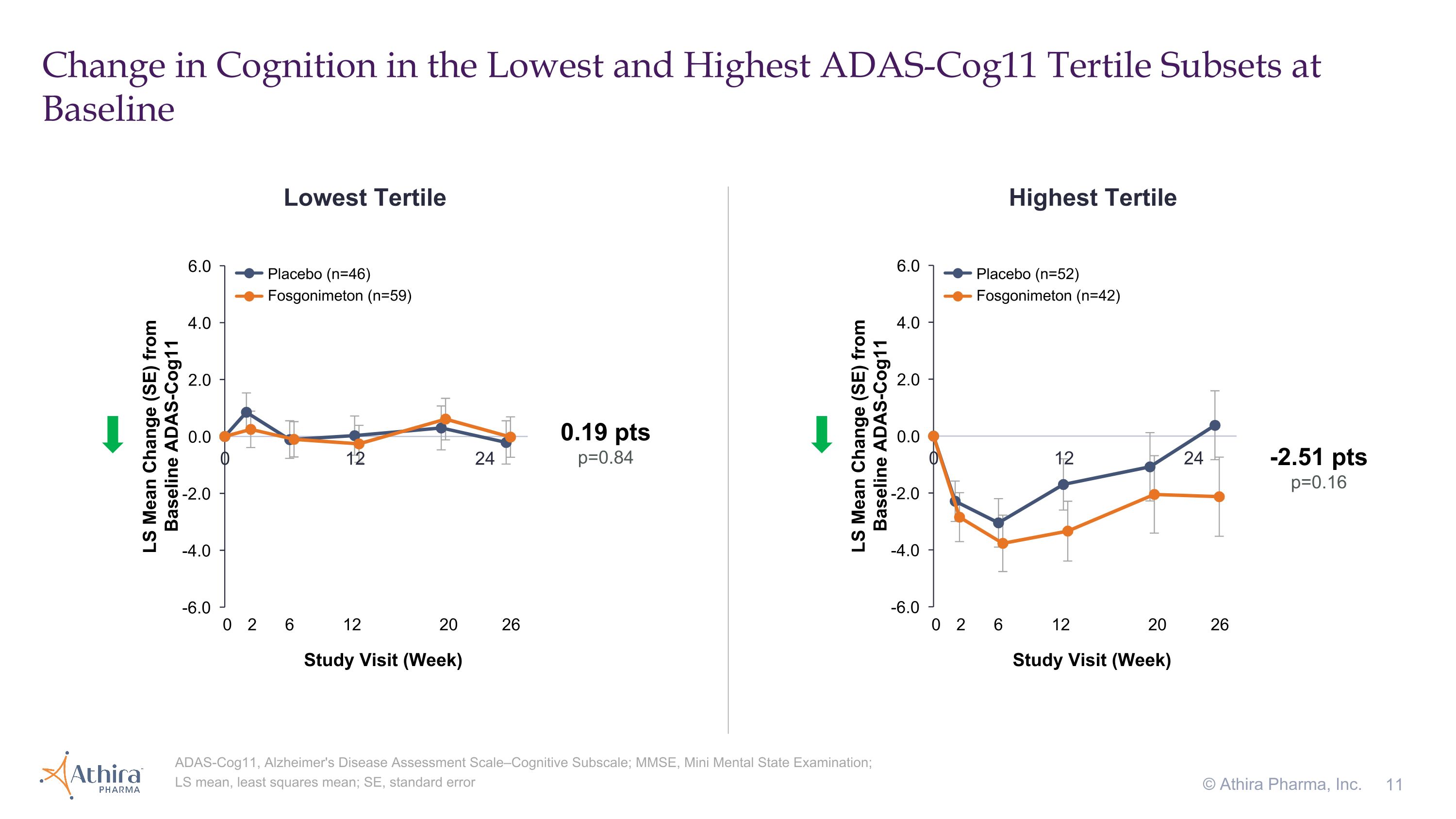
Change in Cognition in the Lowest and Highest ADAS-Cog11 Tertile Subsets at Baseline © Athira Pharma, Inc. ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; MMSE, Mini Mental State Examination; LS mean, least squares mean; SE, standard error Lowest Tertile Highest Tertile -2.51 pts p=0.16 0.19 pts p=0.84 Placebo (n=46) Fosgonimeton (n=59) Placebo (n=52) Fosgonimeton (n=42) 0 2 6 12 20 26 Study Visit (Week) 0 2 6 12 20 26 Study Visit (Week)
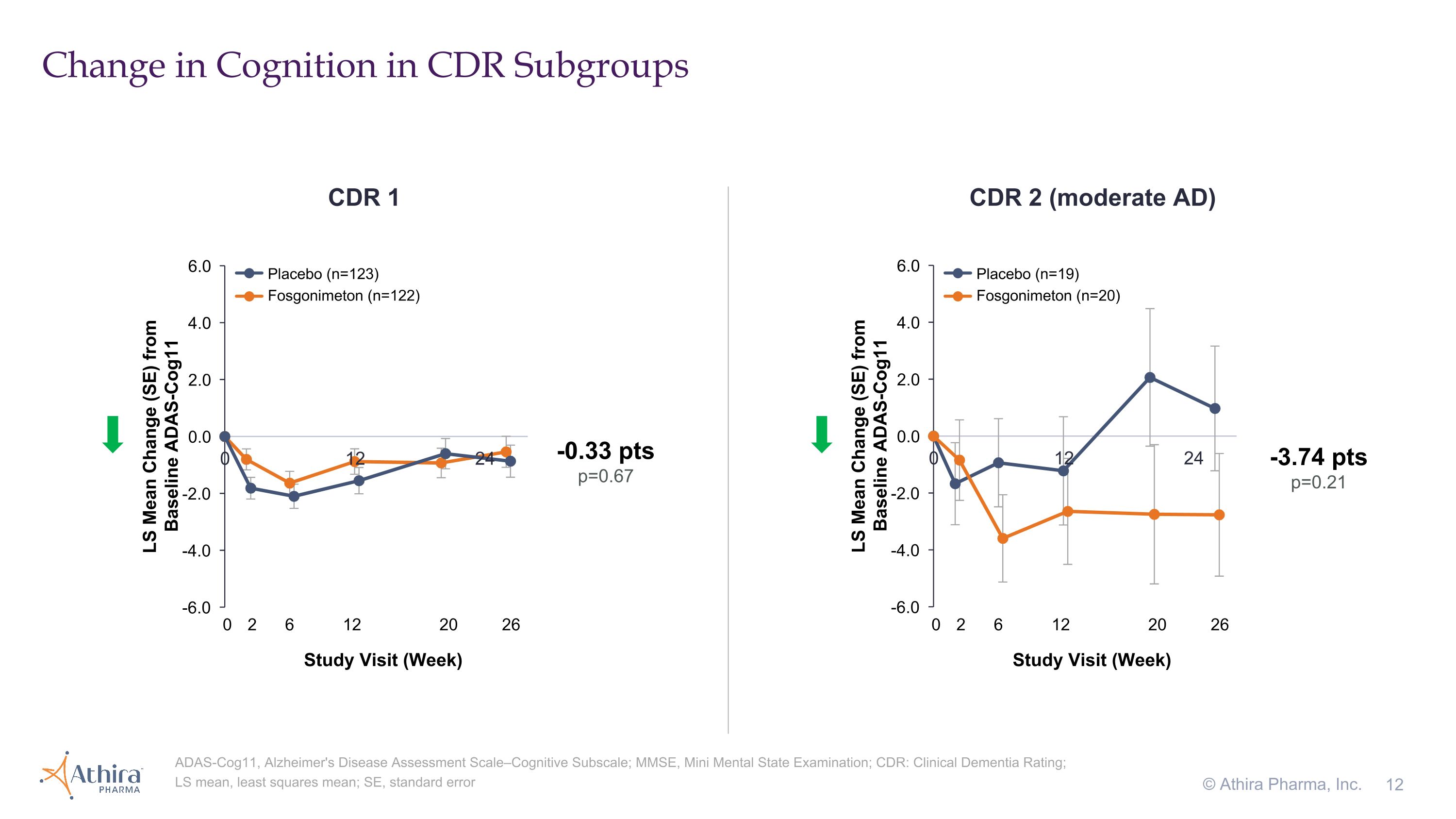
Change in Cognition in CDR Subgroups © Athira Pharma, Inc. ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive Subscale; MMSE, Mini Mental State Examination; CDR: Clinical Dementia Rating; LS mean, least squares mean; SE, standard error CDR 1 CDR 2 (moderate AD) -3.74 pts p=0.21 -0.33 pts p=0.67 Placebo (n=123) Fosgonimeton (n=122) Placebo (n=19) Fosgonimeton (n=20) 0 2 6 12 20 26 Study Visit (Week) 0 2 6 12 20 26 Study Visit (Week)
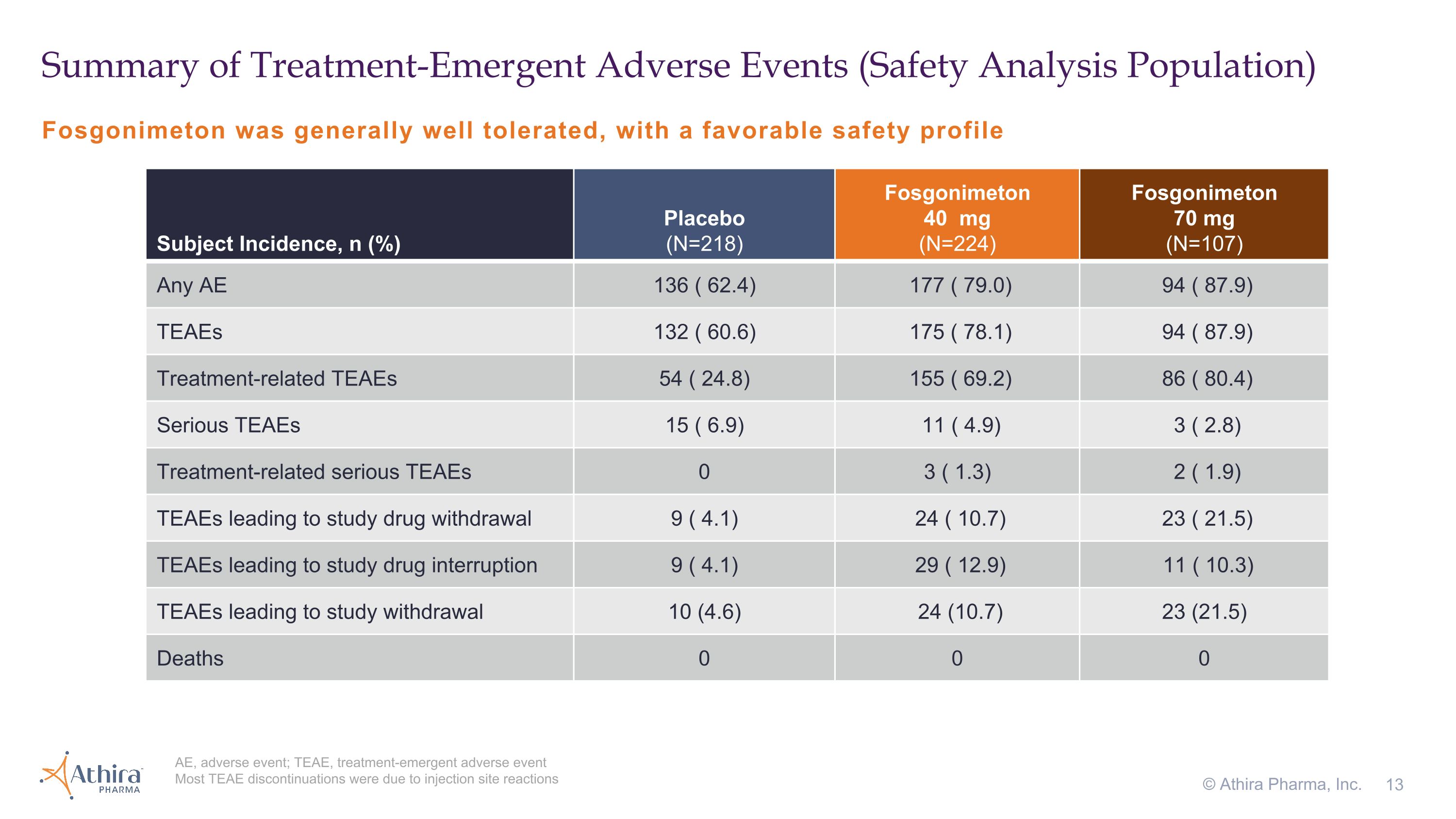
Fosgonimeton was generally well tolerated, with a favorable safety profile Summary of Treatment-Emergent Adverse Events (Safety Analysis Population) AE, adverse event; TEAE, treatment-emergent adverse event Most TEAE discontinuations were due to injection site reactions © Athira Pharma, Inc. Subject Incidence, n (%) Placebo �(N=218) Fosgonimeton�40 mg�(N=224) Fosgonimeton�70 mg�(N=107) Any AE 136 ( 62.4) 177 ( 79.0) 94 ( 87.9) TEAEs 132 ( 60.6) 175 ( 78.1) 94 ( 87.9) Treatment-related TEAEs 54 ( 24.8) 155 ( 69.2) 86 ( 80.4) Serious TEAEs 15 ( 6.9) 11 ( 4.9) 3 ( 2.8) Treatment-related serious TEAEs 0 3 ( 1.3) 2 ( 1.9) TEAEs leading to study drug withdrawal 9 ( 4.1) 24 ( 10.7) 23 ( 21.5) TEAEs leading to study drug interruption 9 ( 4.1) 29 ( 12.9) 11 ( 10.3) TEAEs leading to study withdrawal 10 (4.6) 24 (10.7) 23 (21.5) Deaths 0 0 0
LIFT-AD trial did not meet primary endpoint of GST and key secondary endpoints; fosgonimeton compares favorably to placebo numerically despite the very small decline in the placebo group In subgroups1 of patients with moderate AD, or APOE4 carriers or those with greatest impairment in cognition by ADAS-Cog11 fosgonimeton showed a larger effect size Fosgonimeton treatment was associated with changes in biomarkers of Alzheimer’s disease pathology consistent with the broad neuroprotective mechanism of HGF modulation Fosgonimeton was generally well tolerated, with a favorable safety profile Summary and Interpretation 1. Prespecified Analysis – MMSE and APOE4; Post Hoc – ADAS-Cog11 Tertiles and CDR © Athira Pharma, Inc. Totality of the data suggests that positive modulation of HGF signaling may have potential beneficial effects in neurodegenerative diseases

Thank You © Athira Pharma, Inc.
v3.24.2.u1
Document and Entity Information
|
Sep. 03, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 03, 2024
|
| Entity Registrant Name |
Athira Pharma, Inc.
|
| Entity Central Index Key |
0001620463
|
| Entity Incorporation, State or Country Code |
DE
|
| Securities Act File Number |
001-39503
|
| Entity Tax Identification Number |
45-3368487
|
| Entity Address, Address Line One |
18706 North Creek Parkway
|
| Entity Address, Address Line Two |
Suite 104
|
| Entity Address, City or Town |
Bothell
|
| Entity Address, State or Province |
WA
|
| Entity Address, Postal Zip Code |
98011
|
| City Area Code |
425
|
| Local Phone Number |
620-8501
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
ATHA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Athira Pharma (NASDAQ:ATHA)
過去 株価チャート
から 9 2024 まで 10 2024

Athira Pharma (NASDAQ:ATHA)
過去 株価チャート
から 10 2023 まで 10 2024
