J&J's Janssen to End Pimodivir Influenza Development Program
2020年9月2日 - 10:53PM
Dow Jones News
By Colin Kellaher
Johnson & Johnson's Janssen Pharmaceutical Cos. unit on
Wednesday said it is ending the development of pimodivir, an
investigational antiviral treatment for influenza A infection,
after disappointing study results.
Janssen said interim analyses of a Phase 3 study in hospitalized
patients with influenza A found that pimodivir in combination with
the standard of care was very unlikely to show an added benefit
compared to standard-of-care treatment alone.
The company said it is halting the trial in hospitalized
patients, along with a parallel study in outpatients with influenza
A.
Janssen licensed pimodivir in 2014 from Boston drug maker Vertex
Pharmaceuticals Inc. in a deal that included an upfront payment of
$30 million, along with contingent development and commercial
milestone payments.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
September 02, 2020 09:38 ET (13:38 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
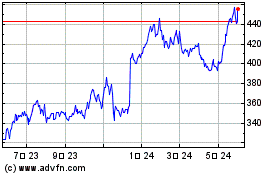
Vertex Pharmaceuticals (NASDAQ:VRTX)
過去 株価チャート
から 9 2024 まで 10 2024
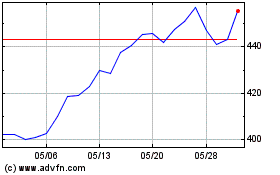
Vertex Pharmaceuticals (NASDAQ:VRTX)
過去 株価チャート
から 10 2023 まで 10 2024