FDA Lifts Hold on PepGen's New Drug Application for Dystrophy Treatment
2023年10月12日 - 8:52PM
Dow Jones News
By Dean Seal
Federal regulators have lifted a clinical hold on PepGen's
investigational new drug application for a myotonic dystrophy
treatment.
The clinical-stage biotechnology company said Thursday the U.S.
Food and Drug Administration has cleared it to launch a phase 1
study of the treatment, PGN-EDODM1, in U.S.-based patients with
myotonic dystrophy type 1.
PepGen worked closely with the FDA to resolve questions about
the treatment, Chief Executive James McArthur said.
The company expects to obtain proof-of-concept data and safety
data for the study patients in 2024.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
October 12, 2023 07:37 ET (11:37 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
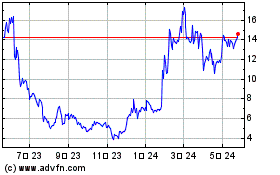
PepGen (NASDAQ:PEPG)
過去 株価チャート
から 4 2024 まで 5 2024
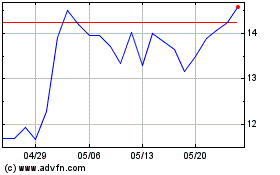
PepGen (NASDAQ:PEPG)
過去 株価チャート
から 5 2023 まで 5 2024