UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
For the month of October 2023
Commission File Number: 001-37643
PURPLE BIOTECH LTD.
(Translation of registrant’s name into English)
4 Oppenheimer Street, Science Park, Rehovot
7670104, Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Purple Biotech Ltd. (the “Company”
or the “Registrant”) is announcing that it has made available an updated Company Presentation on its website. A copy of the
updated Company Presentation is attached hereto as Exhibit 99.1 and may be viewed at the Company’s website at www.purple-biotech.com.
Incorporation by Reference
This Report on Form 6-K, including all exhibits attached hereto, is
hereby incorporated by reference into each of the Registrant’s Registration Statement on Form
S-8 filed with the Securities and Exchange Commission on May 20, 2016 (Registration file number 333-211478), the Registrant’s
Registration Statement on Form S-8
filed with the Securities and Exchange Commission on June 6, 2017 (Registration file number 333-218538), the Registrant’s Registration
Statement on Form F-3, as
amended, originally filed with the Securities and Exchange Commission on July 16, 2018 (Registration file number 333-226195), the Registrant’s
Registration Statement on Form
S-8 filed with the Securities and Exchange Commission on March 28, 2019 (Registration file number 333-230584), the Registrant’s
Registration Statement on Form F-3
filed with the Securities and Exchange Commission on September 16, 2019 (Registration file number 333-233795), the Registrant’s
Registration Statement on Form
F-3 filed with the Securities and Exchange Commission on December 2, 2019 (Registration file number 333-235327), the Registrant’s
Registration Statement on Form
F-1 filed with the Securities and Exchange Commission on December 27, 2019 (Registration file number 333-235729), the Registrant’s
Registration Statement on Form
F-3 filed with the Securities and Exchange Commission on May 13, 2020 (Registration file number 333- 238229), the Registrant’s
Registration Statement on Form
S-8 filed with the Securities and Exchange Commission on May 18, 2020 (Registration file number 333-238481), each of the Registrant’s
Registration Statements on Form F-3 filed with the Securities and Exchange Commission on July 10, 2020 (Registration file numbers 333-239807
and 333-233793), the Registrant’s
Registration Statement on Form
S-8 filed with the Securities and Exchange Commission on April 4, 2022 (Registration file number 333-264107) and the Registrant’s
Registration Statement on Form F-3 filed with the Securities and Exchange Commission on March 23, 2023 (Registration file number 333-270769) and the Registrant’s
Registration Statement on Form
F-3, as amended, originally filed with the Securities and Exchange Commission on December 8, 2022 (Registration file number 333-268710),
to be a part thereof from the date on which this report is submitted, to the extent not superseded by documents or reports subsequently
filed or furnished.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| October 13, 2023 |
PURPLE BIOTECH LTD. |
| |
|
| |
By: |
/s/ Lior Fhima |
| |
|
Lior Fhima |
| |
|
Chief Financial Officer |
2
Exhibit
99.1

| 1 NASDAQ/TASE: PPBT October 2023 CORPORATE PRESENTATION

| 2 Forward - looking Statements and Safe Harbor Certain statements in this press release that are forward - looking and not statements of historical fact are forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . Such forward - looking statements include, but are not limited to, statements that are not statements of historical fact, and may be identified by words such as “ believe ” , “ expect ” , “ intend ” , “ plan ” , “ may ” , “ should ” , “ could ” , “ might ” , “ seek ” , “ target ” , “ will ” , “ project ” , “ forecast ” , “ continue ” or “ anticipate ” or their negatives or variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical matters . You should not place undue reliance on these forward - looking statements, which are not guarantees of future performance . Forward - looking statements reflect our current views, expectations, beliefs or intentions with respect to future events, and are subject to a number of assumptions, involve known and unknown risks, many of which are beyond our control, as well as uncertainties and other factors that may cause our actual results, performance or achievements to be significantly different from any future results, performance or achievements expressed or implied by the forward - looking statements . Important factors that could cause or contribute to such differences include, among others, risks relating to : the expected benefits, synergies and costs of the transaction ; management plans relating to the transaction ; the potential future financial impact of the transaction ; and any assumptions underlying any of the foregoing ; the expected timing of the completion of the transaction and the parties ’ ability to complete the transaction ; the plans, strategies and objectives of management for future operations ; product development for NT 219 and CM 24 as well as Immunorizon Ltd . ’ s portfolio of investigational tri - specific antibody compounds to be acquired ; the process by which such early stage therapeutic candidates could potentially lead to an approved drug product is long and subject to highly significant risks, particularly with respect to a joint development collaboration ; the fact that drug development and commercialization involves a lengthy and expensive process with uncertain outcomes ; our ability to successfully develop and commercialize our pharmaceutical products ; the expense, length, progress and results of any clinical trials ; the impact of any changes in regulation and legislation that could affect the pharmaceutical industry ; the difficulty in receiving the regulatory approvals necessary in order to commercialize our products ; the difficulty of predicting actions of the U . S . Food and Drug Administration or any other applicable regulator of pharmaceutical products ; the regulatory environment and changes in the health policies and regimes in the countries in which we operate ; the uncertainty surrounding the actual market reception to our pharmaceutical products once cleared for marketing in a particular market ; the introduction of competing products ; patents obtained by competitors ; dependence on the effectiveness of our patents and other protections for innovative products ; our ability to obtain, maintain and defend issued patents ; the commencement of any patent interference or infringement action against our patents, and our ability to prevail, obtain a favorable decision or recover damages in any such action ; and the exposure to litigation, including patent litigation, and/or regulatory actions, and other factors that are discussed in our Annual Report on Form 20 - F for the year ended December 31 , 202 Ϯ and in our other filings with the U . S . Securities and Exchange Commission ( “ SEC ” ), including our cautionary discussion of risks and uncertainties under “ Risk Factors ” in our Registration Statements and Annual Reports . These are factors that we believe could cause our actual results to differ materially from expected results . Other factors besides those we have listed could also adversely affect us . Any forward - looking statement in this press release speaks only as of the date which it is made . We disclaim any intention or obligation to publicly update or revise any forward - looking statement or other information contained herein, whether as a result of new information, future events or otherwise, except as required by applicable law . You are advised, however, to consult any additional disclosures we make in our reports to the SEC, which are available on the SEC ’ s website, https : //www . sec . gov .
| 3 Corporate Highlights Purple Biotech (NASDAQ/TASE: PPBT) As of June 30, 2023 • ADS Outstanding: 21.4 M • Cash Balance : $18 M Strong position to reach short and mid term value creating clinical data catalysts • Multiple data read - outs expected in 2023 & 2024 • Two First - in - Class clinical stage drugs • A preclinical tri - specific immuno - engagers platform • Lean & global operation • Cash runway into 1H25 Purple Biotech identifies promising first - in - class drug candidates to treat cancers with high unmet medical need

| 4 Leadership Team Hadas Reuveni, PhD VP Research & Development Formerly at Keryx (NASDAQ:KERX) Michael Schickler, PhD Head of Clinical and Regulatory Affairs Formerly at Hoffmann - La Roche, CEO at CureTech Gil Efron Chief Executive Officer Former Deputy CEO & CFO at Kamada (NASDAQ:KMDA) Fabien Sebille, PhD Chief Business Officer Formerly at Debiopharm Lior Fhima Chief Financial Officer Formerly at Kamada (NASDAQ:KMDA) Eric K. Rowinsky , MD Chairman of the Board Former CMO at ImClone, Stemline , Board member at Biogen Inc.

| 5 A Pipeline Dedicated to Advancing Oncology Therapies *Clinical collaboration and supply agreement with: Multiple data read - outs expected in the next 12 months Value Drivers Development Stage Indications Target Project Phase III Phase II Phase I Pre - Clinical □ Phase 2 interim analysis 2 H 23 & 1 H 24 □ Phase 2 top line results 2 H 24 Pancreatic Cancer (+ nivolumab+SoC ) CEACAM1 mAb CM24 * □ Initiation of Phase 2 1H24 Solid tumors (monotherapy) STAT3xIRS1/2 Dual Inhibitor NT219 Head and Neck & Colorectal Cancer (+Cetuximab) Solid Tumors CD3x5T4xNKG2A Tri - specific Ab IM1240

Advancing First - in - Class Oncology Therapies CM 24 : an α - CEACAM 1 * mAb Lead indication: Pancreatic Ductal Adenocarcinoma (PDAC) *Carcinoembryonic Antigen Cell Adhesion Molecule
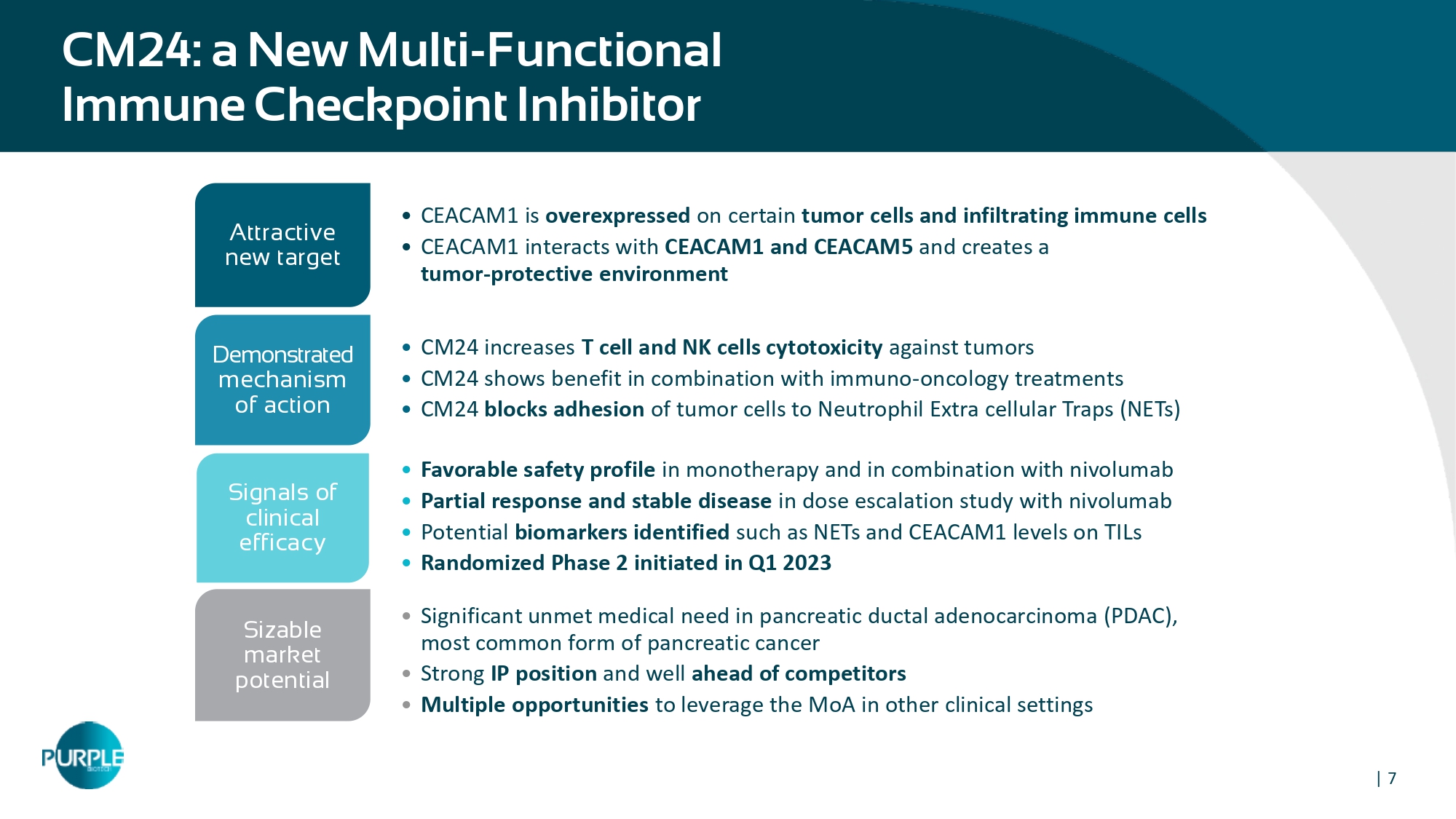
| 7 CM 24 : a New Multi - Functional Immune Checkpoint Inhibitor • CEACAM1 is overexpressed on certain tumor cells and infiltrating immune cells • CEACAM1 interacts with CEACAM1 and CEACAM5 and creates a tumor - protective environment Attractive new target • C M24 increases T cell and NK cells cytotoxicity against tumors • CM24 shows benefit in combination with immuno - oncology treatments • CM24 blocks adhesion of tumor cells to Neutrophil Extra cellular Traps (NETs) Demonstrated mechanism of action • Favorable safety profile in monotherapy and in combination with nivolumab • Partial response and stable disease in dose escalation study with nivolumab • Potential biomarkers identified such as NETs and CEACAM 1 levels on TILs • Randomized Phase 2 initiated in Q 1 2023 Signals of clinical efficacy • Significant unmet medical need in pancreatic ductal adenocarcinoma (PDAC), most common form of pancreatic cancer • Strong IP position and well ahead of competitors • Multiple opportunities to leverage the MoA in other clinical settings Sizable market potential
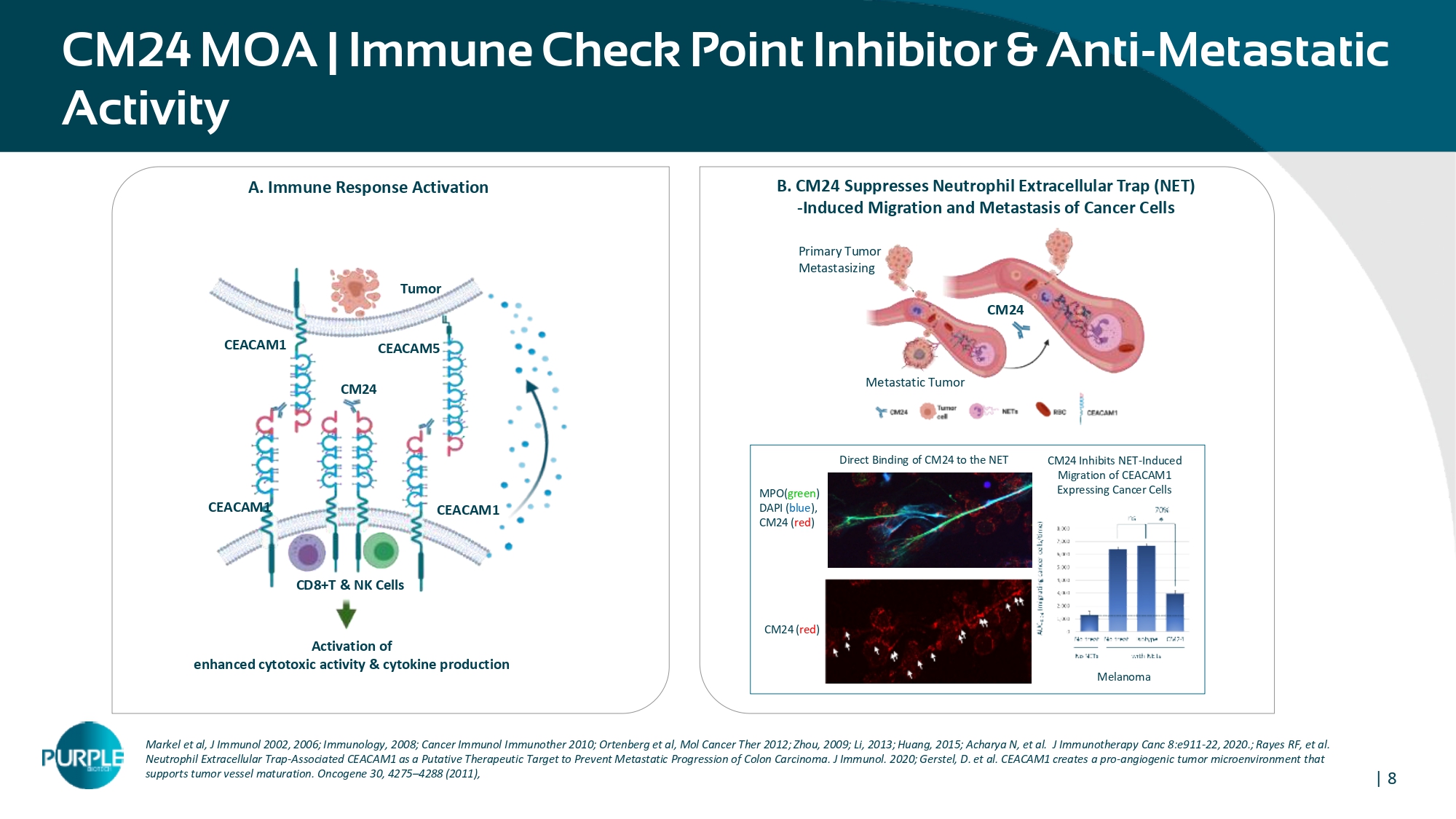
| 8 CM24 MOA | Immune Check Point Inhibitor & Anti - Metastatic Activity Markel et al, J Immunol 2002, 2006; Immunology, 2008; Cancer Immunol Immunother 2010; Ortenberg et al, Mol Cancer Ther 2012; Zhou, 2009; Li, 2013; Huang, 2015; Acharya N, et al. J Immunotherapy Canc 8:e911 - 22, 2020. ; Rayes RF, et al. Neutrophil Extracellular Trap - Associated CEACAM1 as a Putative Therapeutic Target to Prevent Metastatic Progression of Colon Car cinoma. J Immunol. 2020; Gerstel, D. et al. CEACAM1 creates a pro - angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene 30, 4275 – 4288 (2011), Tumor CD 8 +T & NK Cells CEACAM 1 CEACAM1 CEACAM 1 CEACAM5 Activation of enhanced cytotoxic activity & cytokine production CM24 A. Immune Response Activation B. CM 24 Suppresses Neutrophil Extracellular Trap (NET) - Induced Migration and Metastasis of Cancer Cells Primary Tumor Metastasizing CM24 Metastatic Tumor CM24 Inhibits NET - Induced Migration of CEACAM1 Expressing Cancer Cells Direct Binding of CM24 to the NET Structure MPO( green ) DAPI ( blue ), CM24 ( red ) CM24 ( red ) Melanoma
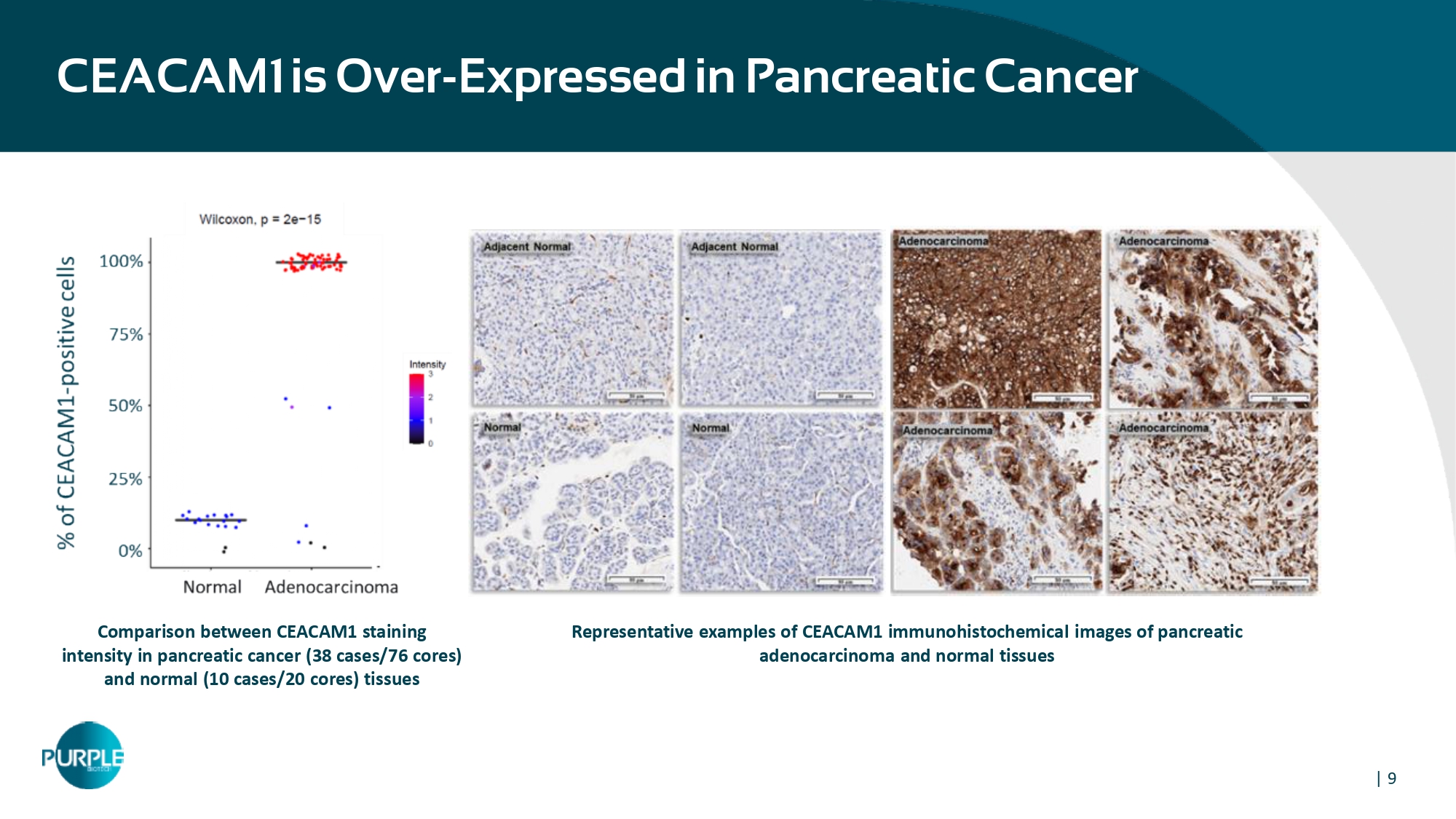
| 9 Representative examples of CEACAM1 immunohistochemical images of pancreatic adenocarcinoma and normal tissues Comparison between CEACAM 1 staining intensity in pancreatic cancer ( 38 cases/ 76 cores) and normal ( 10 cases/ 20 cores) tissues CEACAM1 is Over - Expressed in Pancreatic Cancer
| 10 Large Market Opportunity in Pancreatic Cancer • Pancreatic Cancer accounts for ~60K new cases/year in the US alone; with a 5 - year relative survival rate of 11.5% 1 • Immuno - oncology approaches have been limited to patients with high microsatellite instability (MSI - H) or high tumor mutational b urden (TMB - H) • 5 - year overall survival rate with chemotherapy in 2 nd line patients is 3% 1 • 3L standard of care regimens efficacy data: patients treated without chemotherapy: Overall Survival (OS) 2 months, OS of 3 - 4 months with chemotherapy • 2L standard of care regimens efficacy data: Gemcitabine/Nab - paclitaxel 3 : OS 7.9 months, Progression Free Survival ( PFS ) 4.3 months or Nal - IRI/5FU/LV 4 : OS 6.2 months, PFS 3.1 months • CEACAM1 expression correlates with poor prognosis in Pancreatic cancer 2 • Preclinical data support significant synergy of CM24 with currently marketed immuno - oncology therapies Combining nivolumab with CM24 in a clinical collaboration with Bristol Myers Squibb 1 . American Cancer Society, Cancer Facts & Figures 2019 , and the ACS website,https ://seer.cancer.gov/statfacts/html/pancreas.html 2 . Calinescu et al, Journal of Immunology Research 2018 : 7169081 ; Carcinoembryonic antigen - related cell adhesion molecules (CEACAM) 1 , 5 and 6 as biomarkers in pancreatic cancer, DOI: 10.1371 /journal.pone. 0113023 3 . De Jesus VHF, Camandaroba MPG, Calsavara VF, Riechelmann RP. Systematic review and meta - analysis of gemcitabine - based chemotherapy after FOLFIRINOX in advanced pancreat ic cancer. Therapeutic Advances in Medical Oncology. 2020 ; 12 . doi: 10.1177 / 1758835920905408 4 . Wang - Gillam A, Hubner RA, Siveke JT, et al. NAPOLI - 1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long - term survivors. Eur J Cancer. 2019 ; 108:78 - 87 . doi: 10.1016 /j.ejca. 2018.12.007
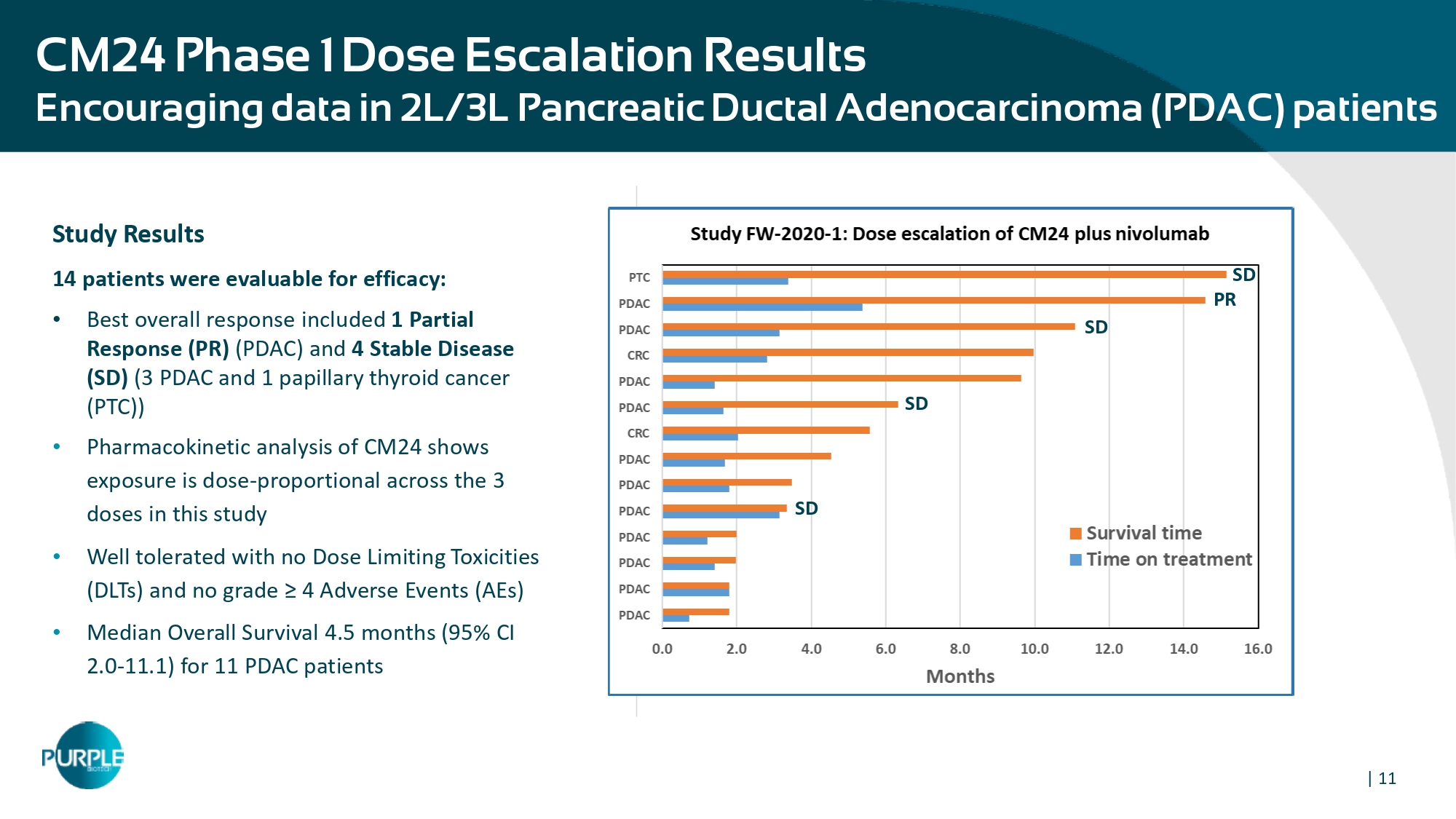
| 11 CM24 Phase 1 Dose Escalation Results E ncouraging data in 2L/3L Pancreatic Ductal Adenocarcinoma (PDAC) patients Study Results 14 patients were evaluable for efficacy: • Best overall response included 1 Partial Response (PR) (PDAC) and 4 Stable Disease (SD) ( 3 PDAC and 1 papillary thyroid cancer (PTC)) • Pharmacokinetic analysis of CM 24 shows exposure is dose - proportional across the 3 doses in this study • Well tolerated with no Dose Limiting Toxicities (DLTs) and no grade ≥ 4 Adverse Events (AEs) • Median Overall Survival 4.5 months ( 95 % CI 2.0 - 11.1 ) for 11 PDAC patients PR SD SD SD SD
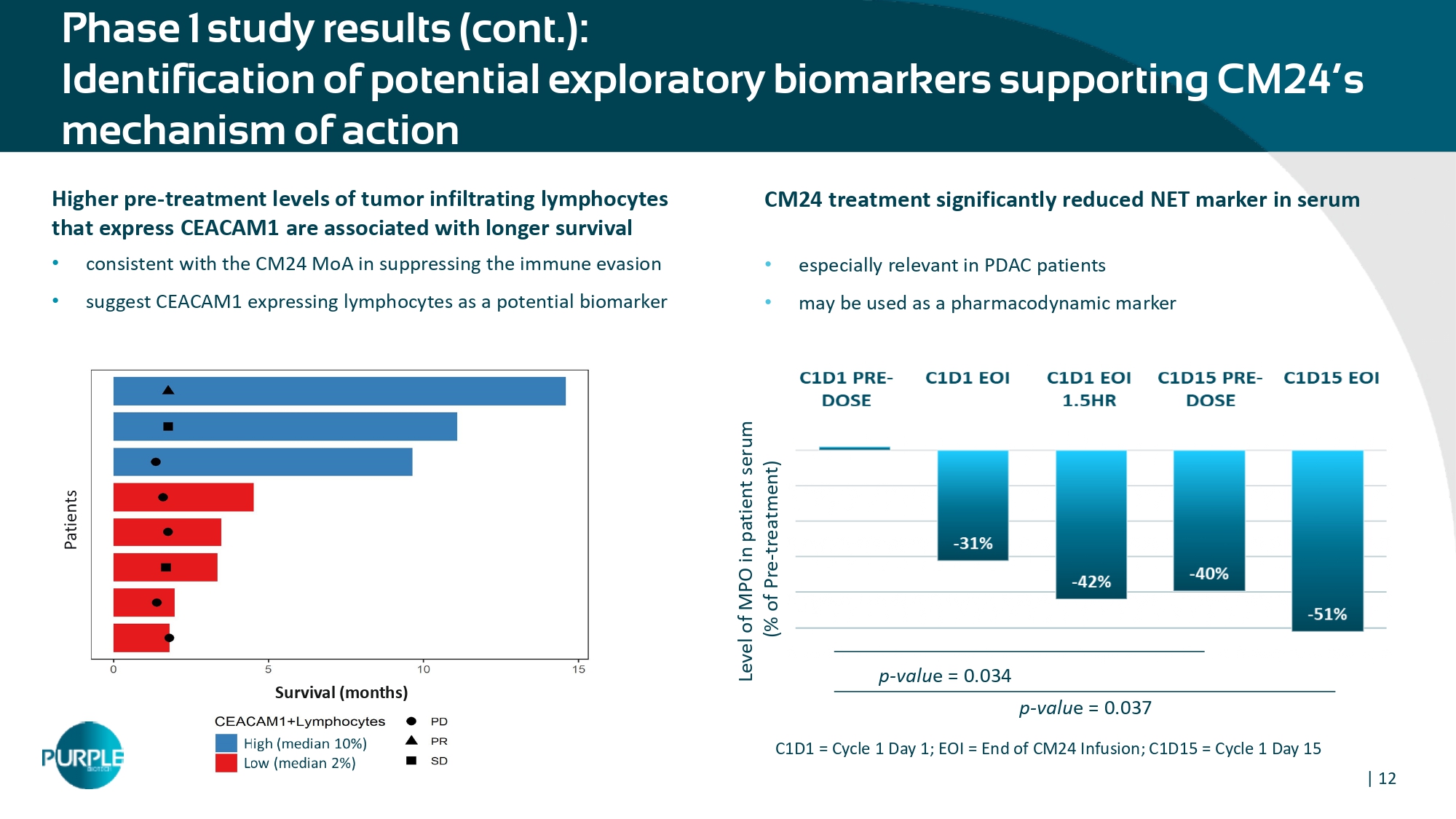
| 12 Phase 1 study results (cont.): Identification of potential exploratory biomarkers supporting CM24’s mechanism of action Higher pre - treatment level s of tumor infiltrating lymphocytes that express CEACAM 1 are associated with longer s urvival • consistent with the CM 24 MoA in suppressing the immune evasion • suggest CEACAM 1 expressing lymphocytes as a potential biomarker CM24 treatment s ignificantly r educed NET marker in serum • especially relevant in PDAC patients • may be used as a pharmacodynamic marker Level of MPO in patient serum (% of Pre - treatment) p - valu e = 0.034 p - valu e = 0.037 C 1 D 1 = Cycle 1 Day 1 ; EOI = End of CM 24 Infusion; C 1 D 15 = Cycle 1 Day 15 Survival (months)
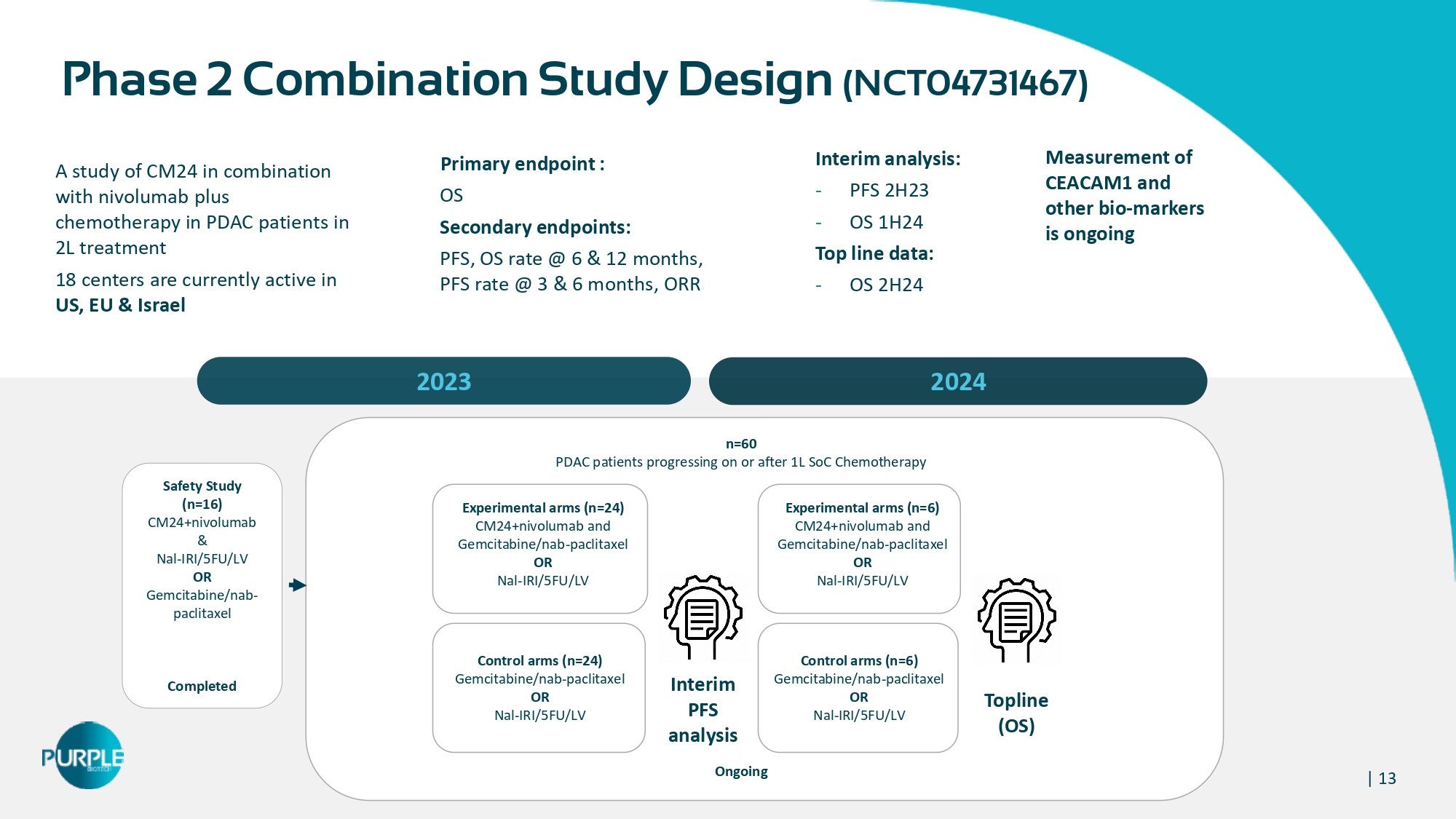
| 13 A study of CM 24 in combination with nivolumab plus chemotherapy in PDAC patients in 2 L treatment 18 centers are currently active in US, EU & Israel Measurement of CEACAM1 and other bio - markers is ongoing 2023 2024 Primary endpoint : OS Secondary endpoints: PFS, OS rate @ 6 & 12 months, PFS rate @ 3 & 6 months, ORR n= 60 PDAC patients progressing on or after 1 L SoC Chemotherapy Ongoing Experimental arms (n= 24 ) CM 24 +nivolumab and Gemcitabine/nab - paclitaxel OR Nal - IRI/ 5 FU/LV Control arms (n=24) Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Phase 2 Combination Study Design (NCT 04731467 ) – Interim analysis : - PFS 2H23 - OS 1H24 Top line data : - OS 2H24 Safety Study (n=16) CM24+nivolumab & Nal - IRI/5FU/LV OR Gemcitabine/nab - paclitaxel Completed Experimental arms (n= 6 ) CM24+nivolumab and Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Control arms (n= 6 ) Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Interim PFS a nalysis Topline (OS)

Advancing First - in - Class Oncology Therapies NT 219 : A Small Molecule Dual Inhibitor of IRS 1 / 2 and STAT 3 Lead indication: Recurrent/Metastatic Head & Neck Cancer (SCCHN)
| 15 NT219, a new solution to improve treatment outcome for cancer patients Confidential • NT219 is a First - in - Class , small molecule • Dual inhibitor of IRS1/2 and STAT3 Innovative MOA • Outstanding efficacy in various PDX models in monotherapy and in combination • Uniquely positioned to tackle resistance to EGFRi , MAPKi and ICI Robust preclinical package • No DLTs in monotherapy or in combination • Early activity demonstrated Clinical Stage • Opportunity to establish a Standard of Care in 2L r/m SCCHN patients • Multiple market upsides in combination with major cancer treatments Broad Market Potential
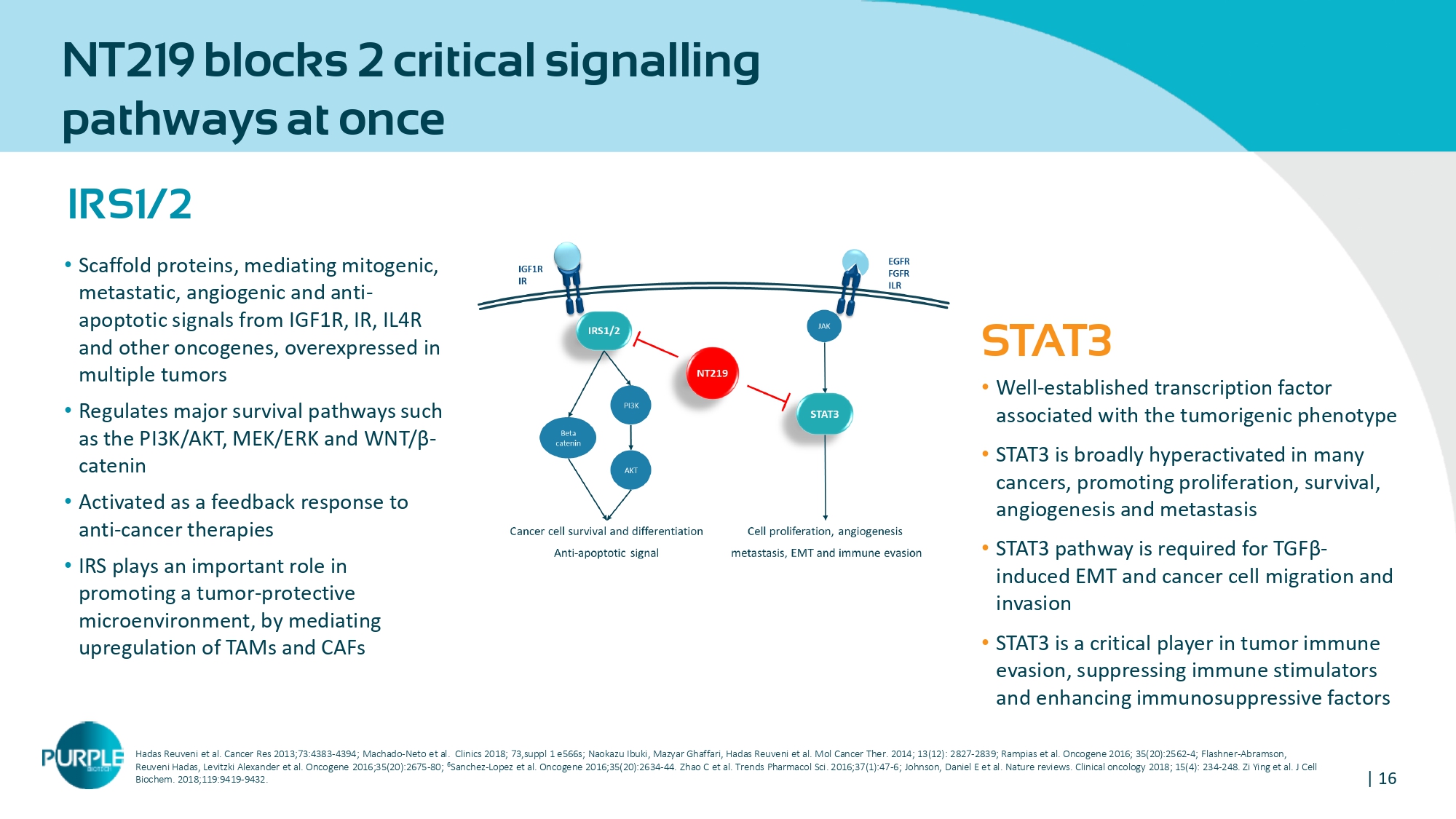
| 16 NT 219 blocks 2 critical signalling pathways at once IRS 1 / 2 • Scaffold proteins, mediating mitogenic, metastatic, angiogenic and anti - apoptotic signals from IGF1R, IR, IL4R and other oncogenes, overexpressed in multiple tumors • Regulates major survival pathways such as the PI3K/AKT, MEK/ERK and WNT/ β - catenin • Activated as a feedback response to anti - cancer therapies • IRS plays an important role in promoting a tumor - protective microenvironment, by mediating upregulation of TAMs and CAFs STAT 3 • Well - established transcription factor associated with the tumorigenic phenotype • STAT3 is broadly hyperactivated in many cancers, promoting proliferation, survival, angiogenesis and metastasis • STAT3 pathway is required for TGFβ - induced EMT and cancer cell migration and invasion • STAT3 is a critical player in tumor immune evasion, suppressing immune stimulators and enhancing immunosuppressive factors Hadas Reuveni et al. Cancer Res 2013;73:4383 - 4394 ; Machado - Neto et al. Clinics 2018; 73,suppl 1 e566s; Naokazu Ibuki , Mazyar Ghaffari , Hadas Reuveni et al. Mol Cancer Ther . 2014; 13(12): 2827 - 2839 ; Rampias et al . Oncogene 2016; 35(20):2562 - 4; Flashner - Abramson, Reuveni Hadas, Levitzki Alexander et al. Oncogene 2016;35(20):2675 - 80 ; 6 Sanchez - Lopez et al. Oncogene 2016;35(20):2634 - 44. Zhao C et al. Trends Pharmacol Sci. 2016;37(1):47 - 6; Johnson, Daniel E et al. Nature reviews. Clinical oncology 2018; 15(4): 234 - 248. Zi Ying et al. J Cell Biochem . 2018;119:9419 - 9432.
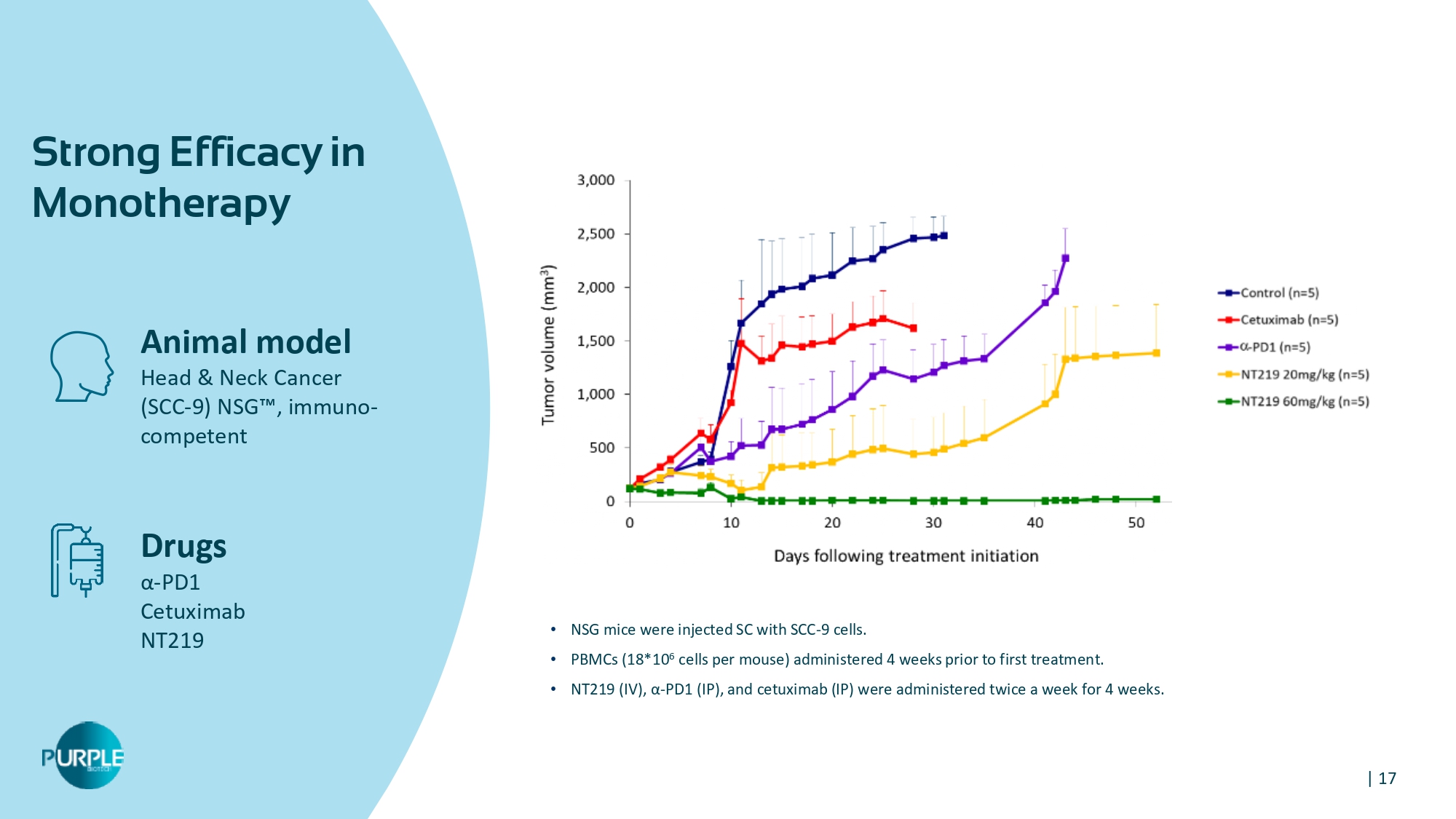
| 17 Animal model Head & Neck Cancer (SCC - 9) NSG Ρ , immuno - competent Drugs α - PD 1 Cetuximab NT 219 Strong Efficacy in Monotherapy • NSG mice were injected SC with SCC - 9 cells . • PBMCs (18*10 6 cells per mouse) administered 4 weeks prior to first treatment . • NT219 (IV), α - PD1 (IP), and cetuximab (IP) were administered twice a week for 4 weeks .
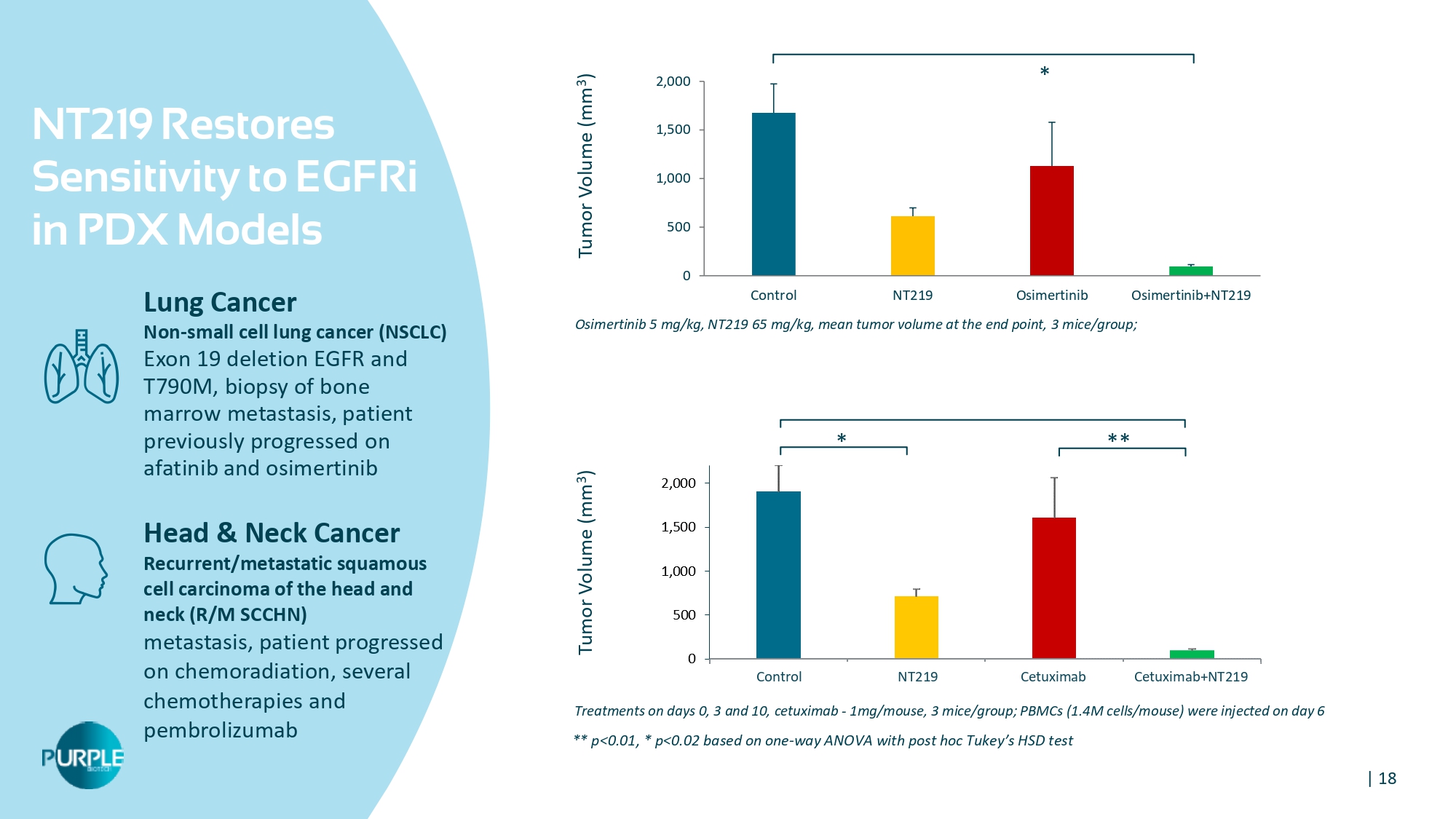
| 18 NT 219 Restores Sensitivity to EGFRi in PDX Models Lung Cancer Non - small cell lung cancer (NSCLC) Exon 19 deletion EGFR and T790M, biopsy of bone marrow metastasis, patient previously progressed on afatinib and osimertinib Head & Neck Cancer Recurrent/metastatic squamous cell carcinoma of the head and neck ( R/ M SCCHN) metastasis, patient progressed on chemoradiation, several chemotherapies and pembrolizumab Treatments on days 0, 3 and 10, cetuximab - 1mg/mouse, 3 mice/group; PBMCs (1.4M cells/mouse) were injected on day 6 Osimertinib 5 mg/kg, NT 219 65 mg/kg, mean tumor volume at the end point, 3 mice/group; 0 500 1,000 1,500 2,000 Control NT219 Osimertinib Osimertinib+NT219 Tumor Volume (mm 3 ) Tumor Volume (mm 3 ) ** p< 0.01 , * p< 0.02 based on one - way ANOVA with post hoc Tukey ’ s HSD test * ** * Control NT 219 Cetuximab Cetuximab+NT 219
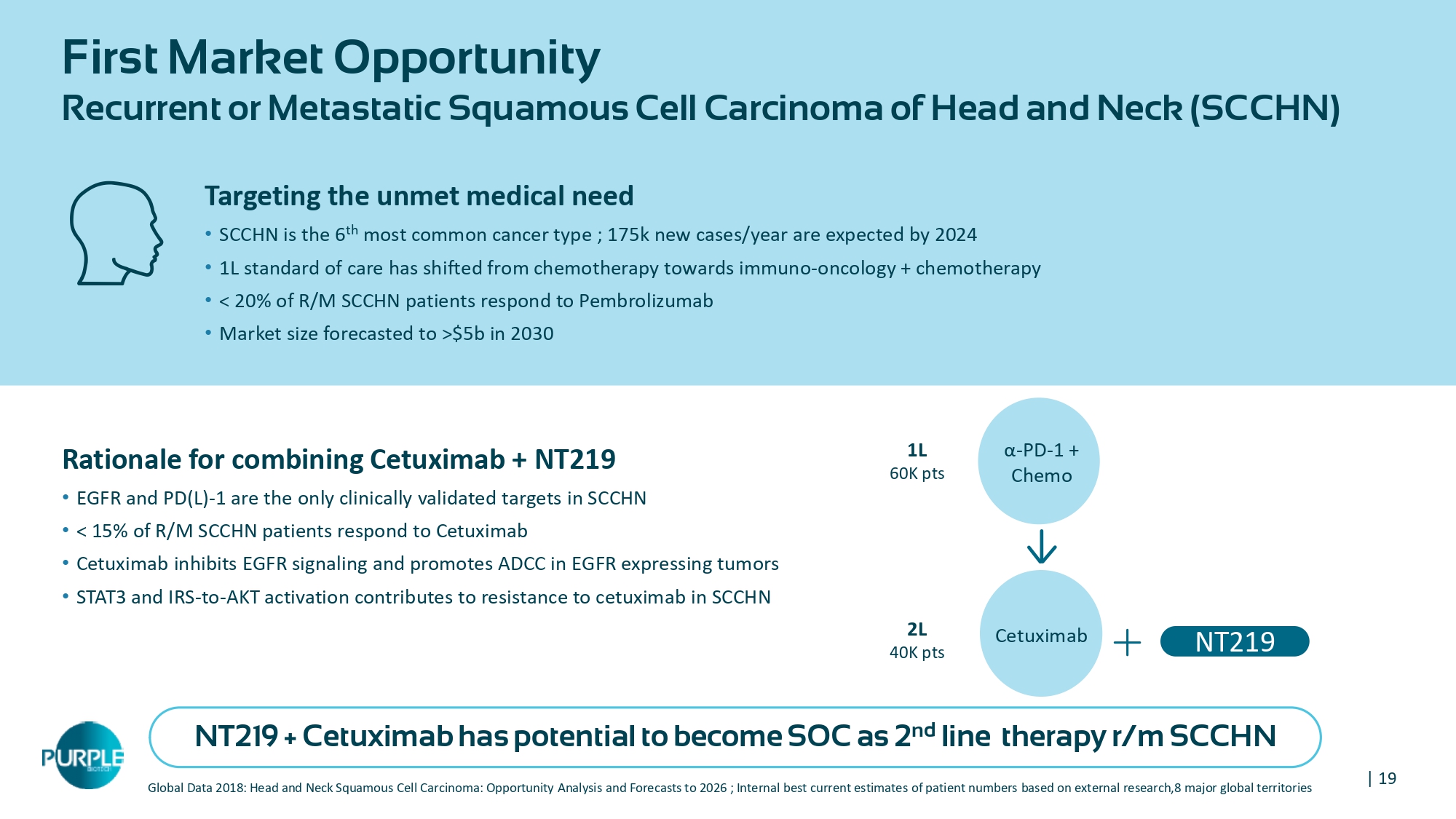
| 19 First Market Opportunity Recurrent or Metastatic Squamous Cell Carcinoma of Head and Neck (SCCHN) Global Data 2018: Head and Neck Squamous Cell Carcinoma: Opportunity Analysis and Forecasts to 2026 ; Internal best current e sti mates of patient numbers based on external research,8 major global territories Rationale for combining Cetuximab + NT 219 • EGFR and PD(L) - 1 are the only clinically validated targets in SCCHN • < 15 % of R/M SCCHN patients respond to Cetuximab • Cetuximab inhibits EGFR signaling and promotes ADCC in EGFR expressing tumors • STAT 3 and IRS - to - AKT activation contributes to resistance to cetuximab in SCCHN Targeting the unmet medical need • SCCHN is the 6 th most common cancer type ; 175 k new cases/year are expected by 2024 • 1 L standard of care has shifted from chemotherapy towards immuno - oncology + chemotherapy • < 20 % of R/M SCCHN patients respond to Pembrolizumab • Market size forecasted to >$ 5 b in 2030 α - PD - 1 + Chemo 1L 60K pts Cetuximab 2 L 40 K pts NT 219 NT219 + Cetuximab has potential to become SOC as 2 nd line therapy r/m SCCHN
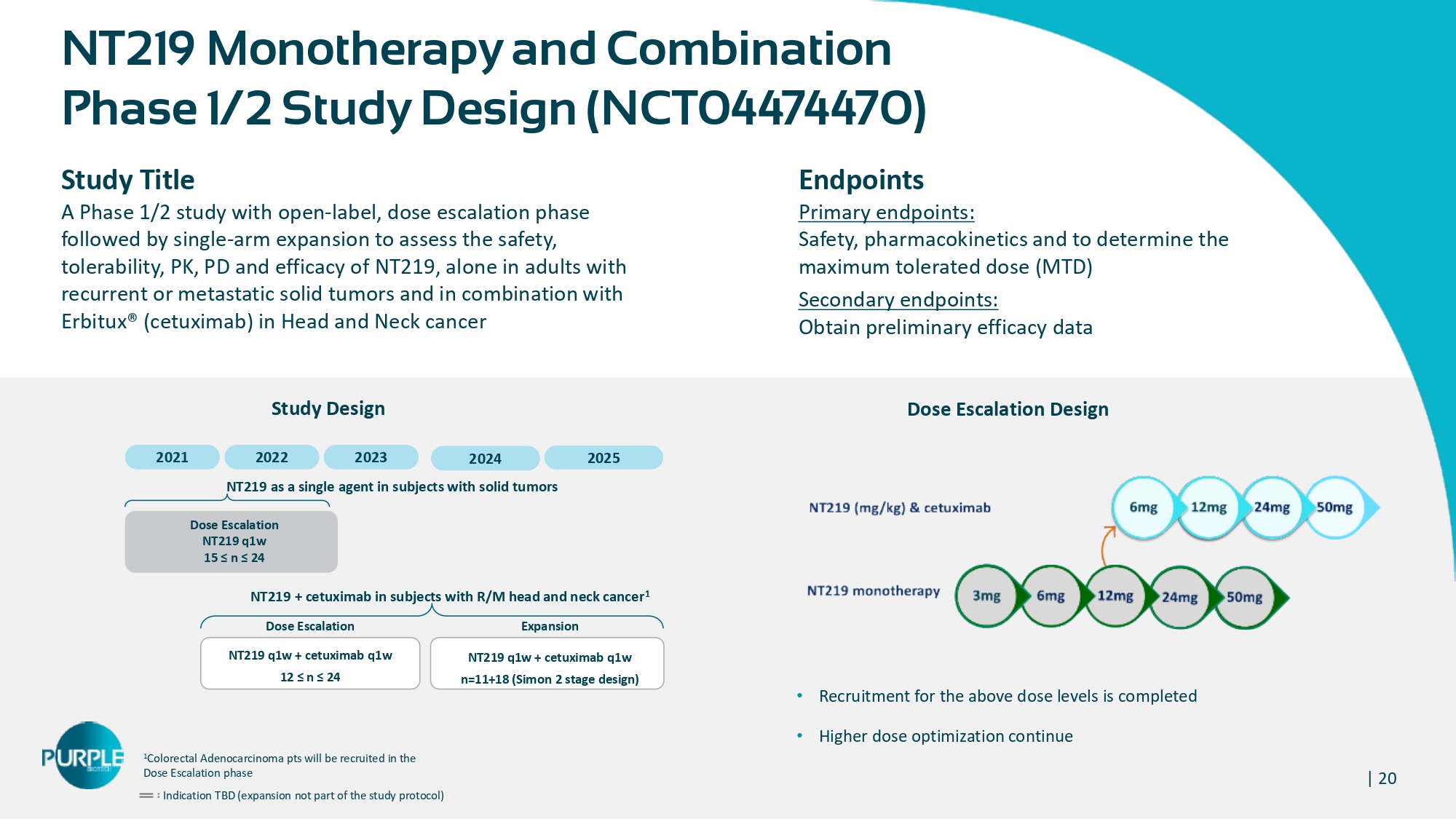
| 20 NT 219 Monotherapy and Combination Phase 1 / 2 Study Design ( NCT 04474470 ) Study Title A Phase 1 / 2 study with open - label, dose escalation p hase followed by single - arm expansion to assess the safety, tolerability, PK, PD and efficacy of NT 219 , alone in adults with recurrent or metastatic solid tumors and in combination with Erbitux® (cetuximab) in Head and Neck cancer Endpoints Primary endpoints: Safety, pharmacokinetics and to determine the maximum tolerated dose ( MTD ) Secondary endpoints: Obtain preliminary efficacy data 1 Colorectal Adenocarcinoma pts will be recruited in the Dose Escalation phase Indication TBD (expansion not part of the study protocol) 2021 2022 2023 Dose Escalation NT 219 q 1 w 15 ≤ n ≤ 24 Dose Escalation NT 219 q 1 w + cetuximab q 1 w 12 ≤ n ≤ 24 NT219 + cetuximab in subjects with R/M head and neck cancer 1 Expansion NT 219 q 1 w + cetuximab q 1 w n= 11 + 18 (Simon 2 stage design) NT 219 as a single agent in subjects with solid tumors Dose Escalation Design Study Design • Recruitment for the above dose levels is completed • Higher dose optimization continue 2024 2025
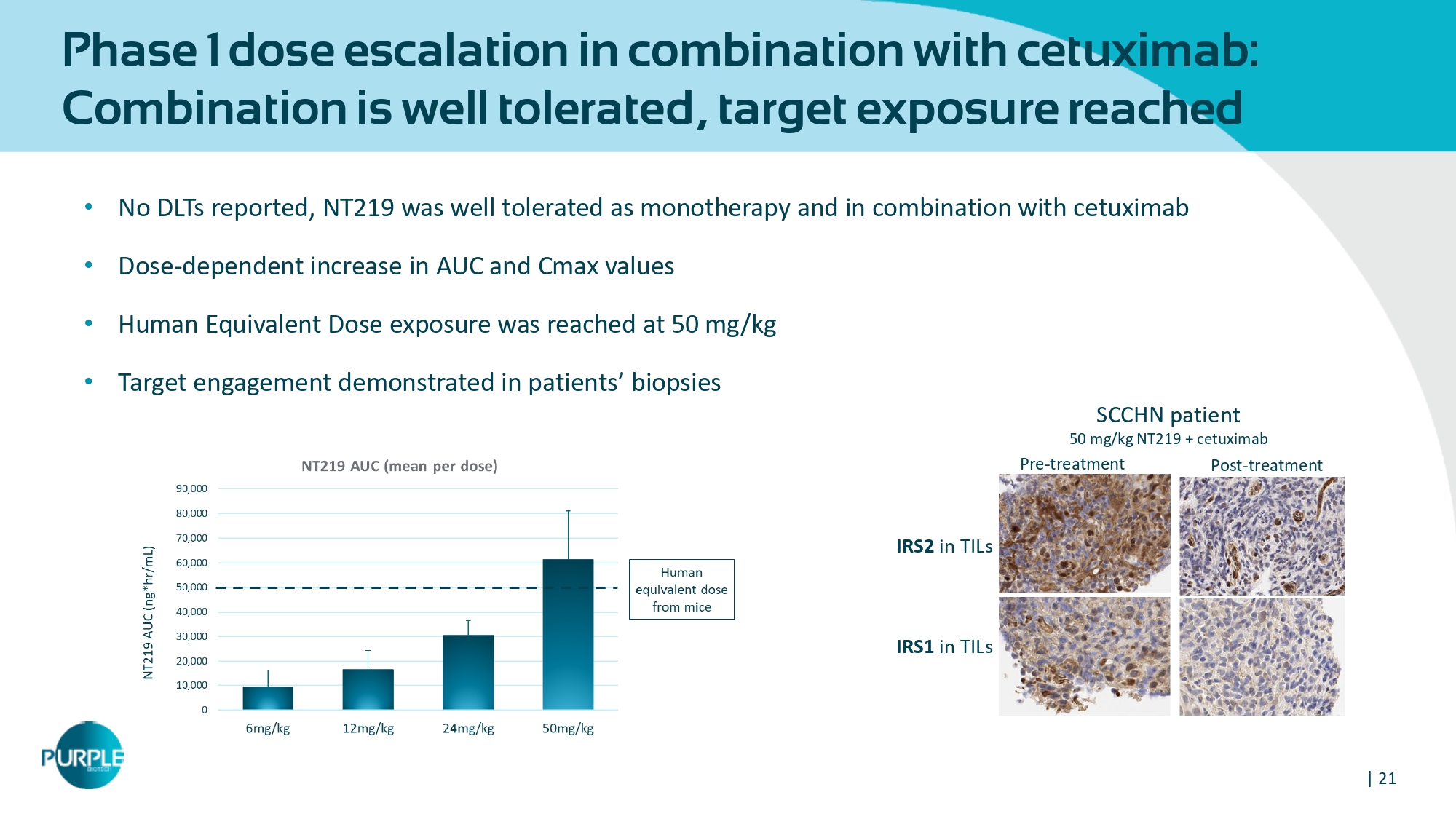
| 21 Phase 1 dose escalation in combination with cetuximab: Combination is well tolerated, target exposure reached • No DLTs reported, NT 219 was well tolerated as monotherapy and in combination with cetuximab • Dose - dependent increase in AUC and Cmax values • Human Equivalent Dose exposure was reached at 50 mg/kg • Target engagement demonstrated in patients ’ biopsies Pre - treatment Post - treatment SCCHN patient 50 mg/kg NT 219 + cetuximab IRS 2 in TILs IRS 1 in TILs
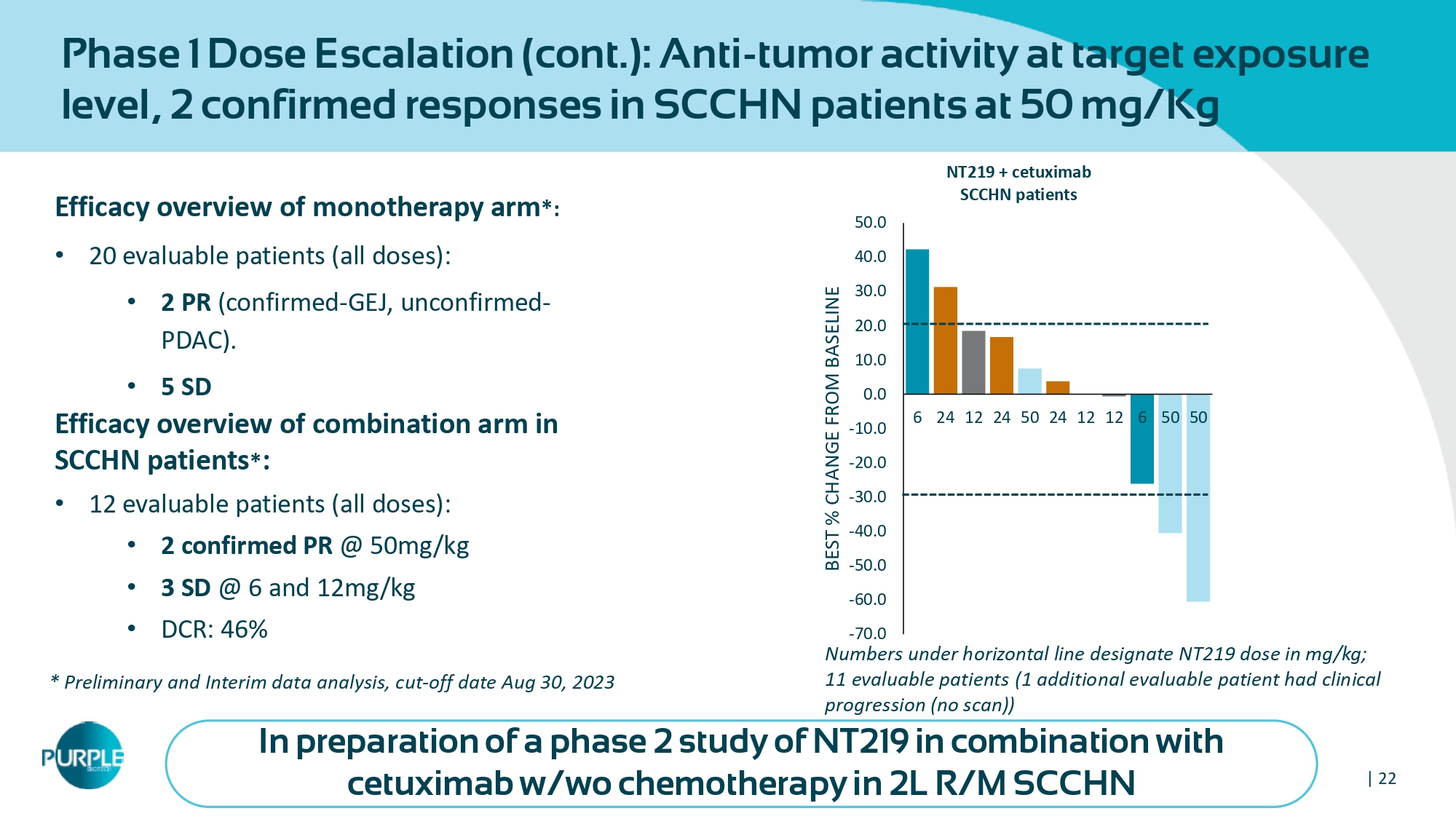
| 22 Efficacy overview of m onotherapy arm *: • 20 evaluable patients (all doses): • 2 PR (confirmed - GEJ, unconfirmed - PDAC). • 5 SD Efficacy overview of combination arm in SCCHN patients * : • 12 evaluable patients (all doses) : • 2 confirmed PR @ 50 mg/kg • 3 SD @ 6 and 12 mg/kg • DCR: 46 % In preparation of a phase 2 study of NT219 in combination with cetuximab w/wo chemotherapy in 2L R/M SCCHN Phase 1 Dose Escalation (cont.): Anti - tumor activity at target exposure level, 2 confirmed responses in SCCHN patients at 50 mg/Kg Numbers under horizontal line designate NT 219 dose in mg/kg; 11 evaluable patients ( 1 additional evaluable patient had clinical progression (no scan)) * Preliminary and Interim data analysis, cut - off date Aug 30 , 2023 -70.0 -60.0 -50.0 -40.0 -30.0 -20.0 -10.0 0.0 10.0 20.0 30.0 40.0 50.0 6 24 12 24 50 24 12 12 6 50 50 BEST % CHANGE FROM BASELINE NT 219 + cetuximab SCCHN patients

Advancing First - in - Class Oncology Therapies IM1240: CD3x5T4xNKG2A Conditionally - Activated Tri - Specific Antibody
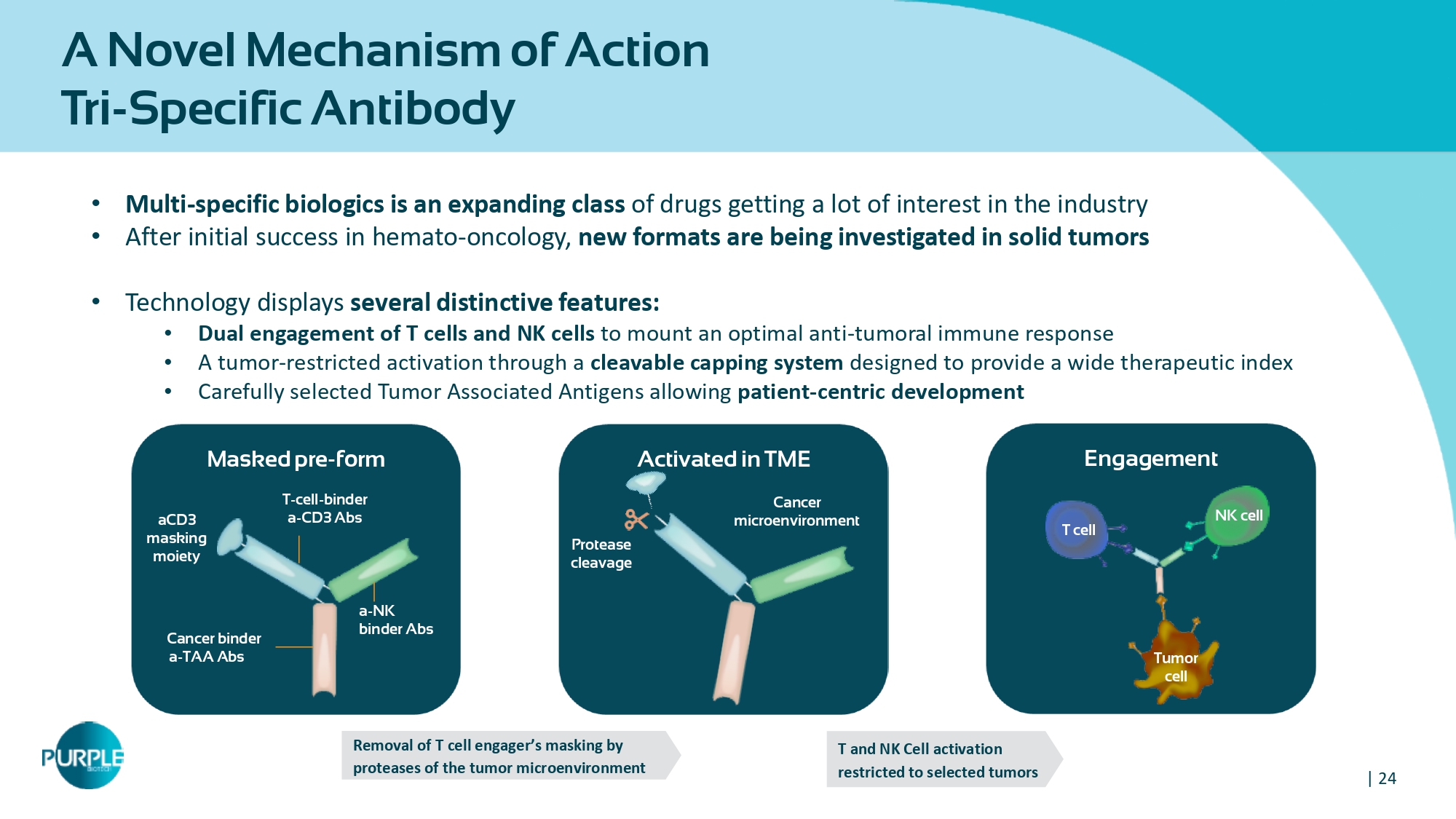
| 24 A Novel Mechanism of Action Tri - Specific Antibody Engagement Activated in TME Masked pre - form Cancer binder a - TAA Abs a - NK binder Abs T - cell - binder a - CD3 Abs aCD 3 masking moiety Tumor cell T cell NK cell Cancer microenvironment Protease cleavage Removal of T cell engager ’ s masking by proteases of the tumor microenvironment T and NK Cell activation restricted to selected tumors • Multi - specific biologics is an expanding class of drugs getting a lot of interest in the industry • After initial success in hemato - oncology, new formats are being investigated in solid tumors • Technology displays several distinctive features: • Dual engagement of T cells and NK cells to mount an optimal anti - tumoral immune response • A tumor - restricted activation through a cleavable capping system designed to provide a wide therapeutic index • Carefully selected Tumor Associated Antigens allowing patient - centric development
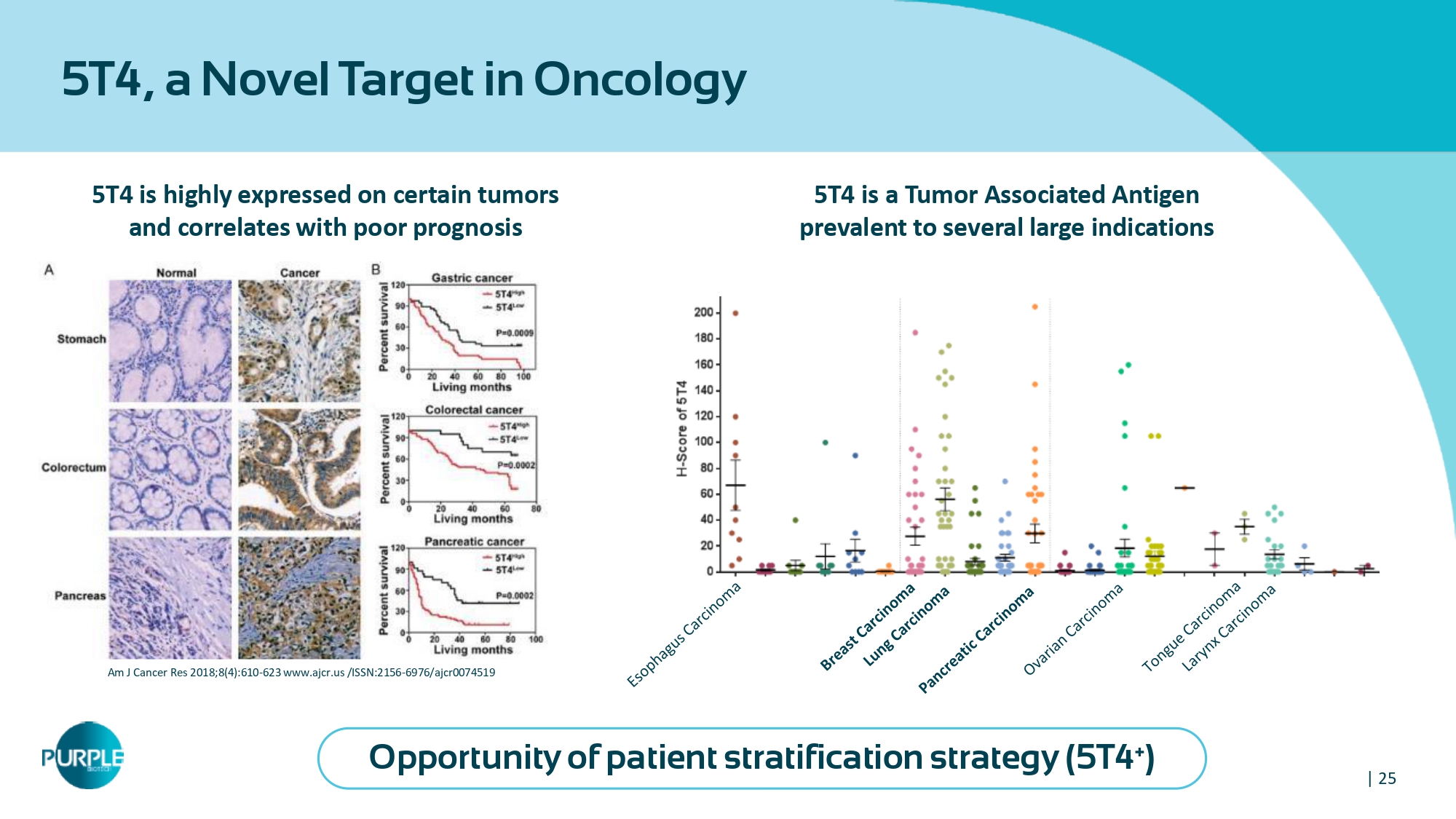
| 25 5 T 4 , a Novel Target in Oncology 5 T 4 is highly expressed on certain tumors and correlates with poor prognosis Am J Cancer Res 2018 ; 8 ( 4 ): 610 - 623 www.ajcr.us /ISSN: 2156 - 6976 /ajcr 0074519 5 T 4 is a Tumor Associated Antigen prevalent to several large indications Opportunity of patient stratification strategy ( 5 T 4 + )
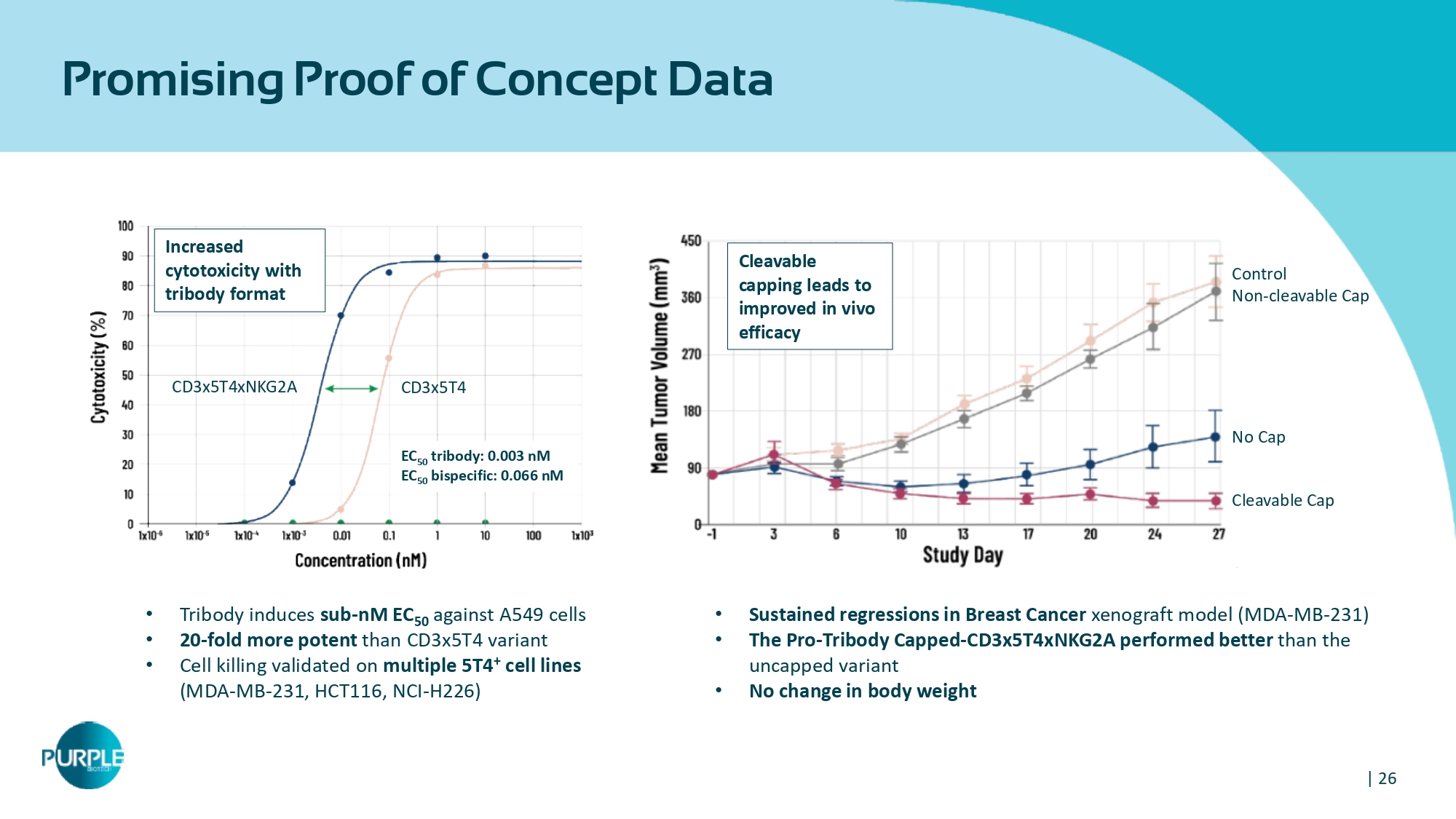
| 26 Promising Proof of Concept Data • Tribody induces sub - nM EC 50 against A 549 cells • 20 - fold more potent than CD 3 x 5 T 4 variant • Cell killing validated on multiple 5 T 4 + cell lines (MDA - MB - 231 , HCT 116 , NCI - H 226 ) • Sustained regressions in Breast Cancer xenograft model ( MDA - MB - 231 ) • T he Pro - Tribody Capped - CD 3 x 5 T 4 xNKG 2 A performed better than the uncapped variant • No change in body weight CD 3 x 5 T 4 xNKG 2 A CD 3 x 5 T 4 Cleavable Cap No Cap Control Non - cleavable Cap Cleavable capping leads to improved in vivo efficacy Increased cytotoxicity with tribody format EC 50 tribody : 0.003 nM EC 50 bispecific: 0.066 nM
| 27 Corporate Highlights Purple Biotech (NASDAQ/TASE: PPBT) As of June 30 , 2023 • ADS Outstanding: 21.4 M • Cash Balance : $ 18 M Strong position to reach short and mid term value creating clinical data catalysts • Multiple data read - outs expected in 2023 & 2024 • Two First - in - Class clinical stage drugs • A preclinical tri - specific immuno - engagers platform • Lean & global operation • Cash runway into 1 H 25 Purple Biotech identifies promising first - in - class drug candidates to treat cancers with high unmet medical need

| 28 Contact Us: ir@purple - biotech.com THANK YOU

Appendix A | CM 24
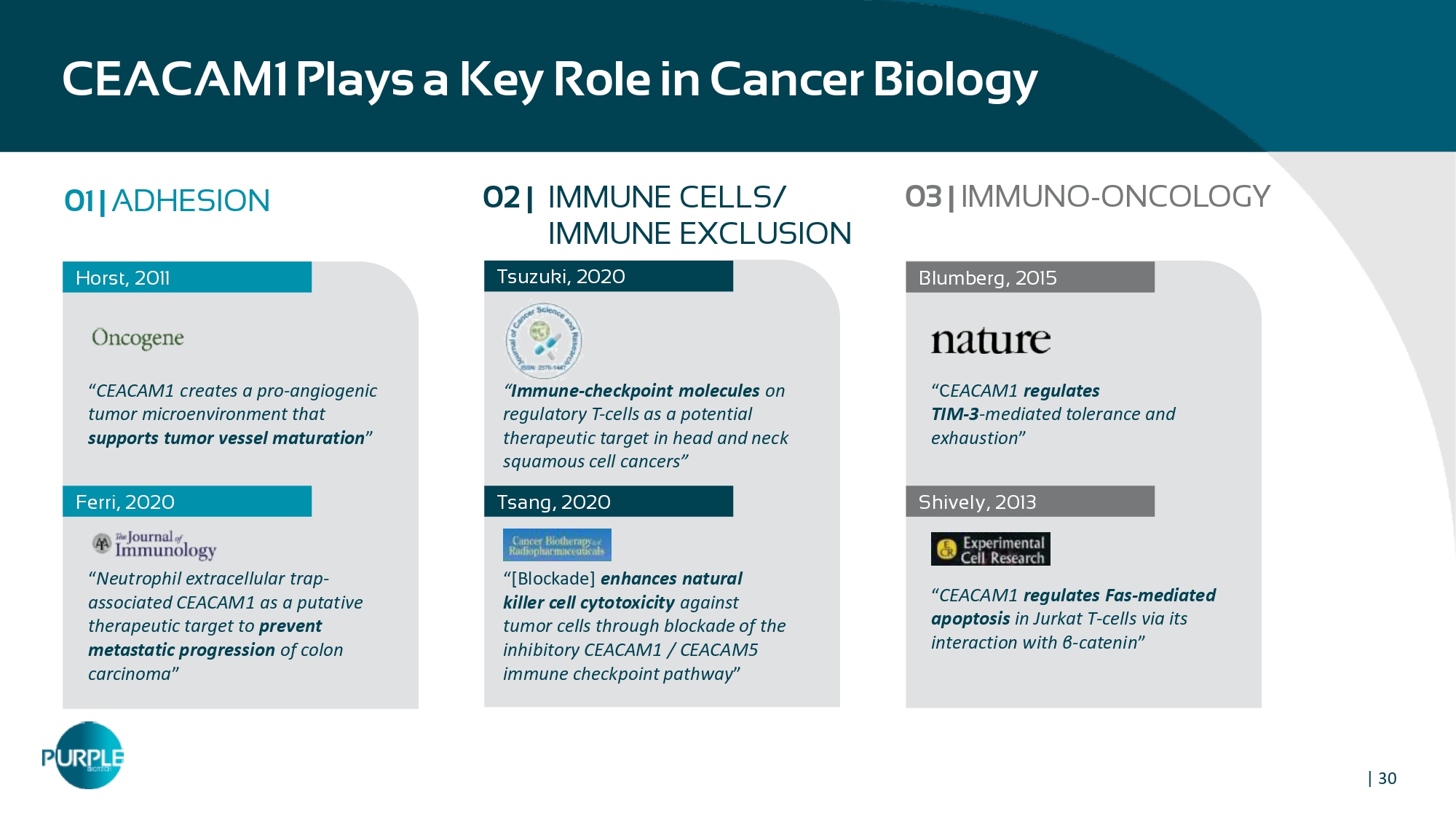
| 30 CEACAM 1 Plays a Key Role in Cancer Biology 01 | ADHESION Horst, 2011 “ CEACAM 1 creates a pro - angiogenic tumor microenvironment that supports tumor vessel maturation ” “ Neutrophil extracellular trap - associated CEACAM 1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma ” Ferri , 2020 Tsuzuki, 2020 Tsang, 2020 Blumberg, 2015 “C EACAM1 regulates TIM - 3 - mediated tolerance and exhaustion ” “ CEACAM 1 regulates Fas - mediated apoptosis in Jurkat T - cells via its interaction with β - catenin ” Shively, 2013 02 | IMMUNE CELLS/ IMMUNE EXCLUSION 03 | IMMUNO - ONCOLOGY “ Immune - checkpoint molecules on regulatory T - cells as a potential therapeutic target in head and neck squamous cell cancers ” “[Blockade] enhances natural killer cell cytotoxicity against tumor cells through blockade of the inhibitory CEACAM1 / CEACAM5 immune checkpoint pathway ”
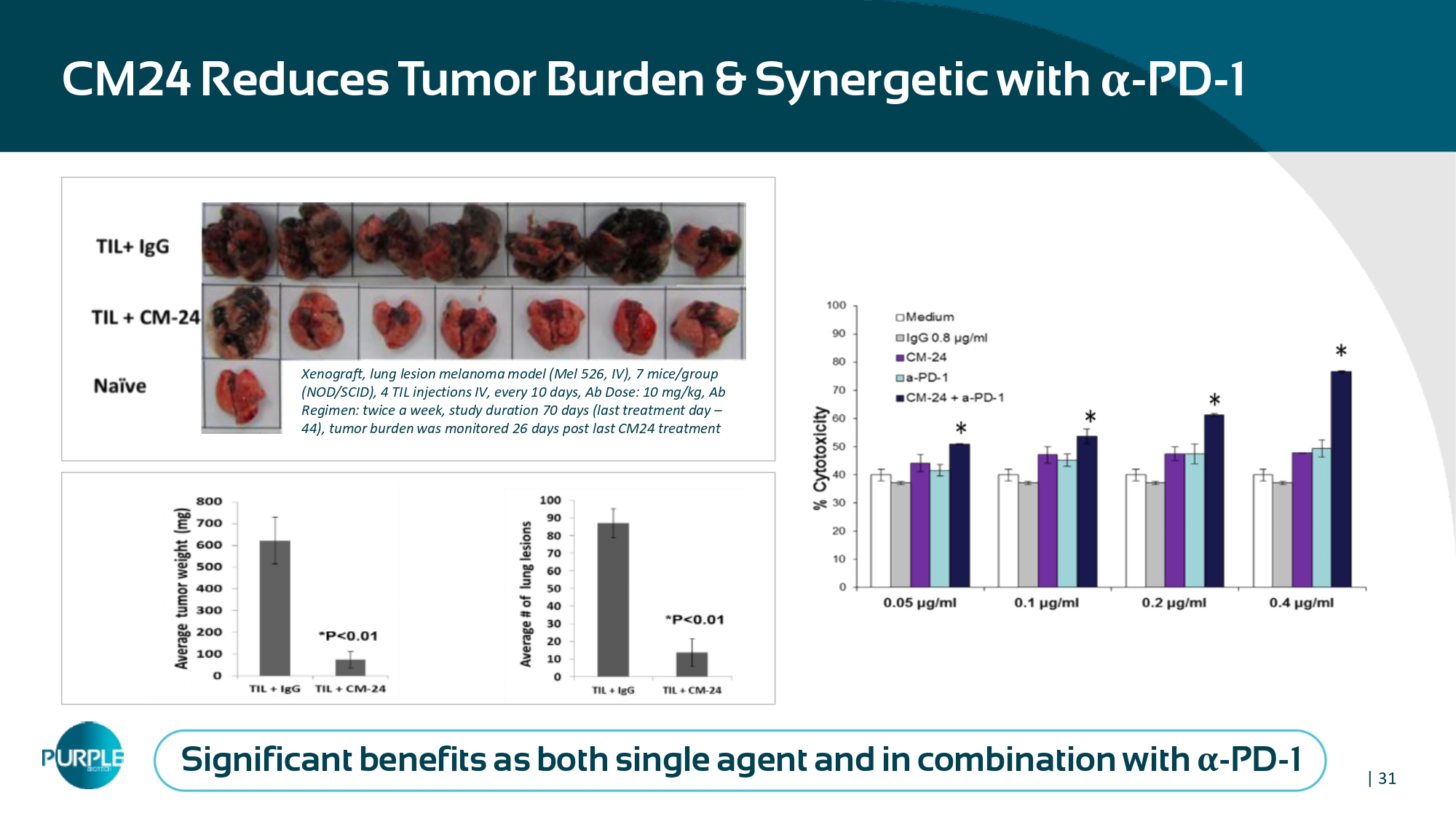
| 31 CM 24 Reduces Tumor Burden & Synergetic with α - PD - 1 Xenograft, lung lesion melanoma model (Mel 526 , IV), 7 mice/group (NOD/SCID), 4 TIL injections IV, every 10 days, Ab Dose: 10 mg/kg, Ab Regimen: twice a week, study duration 70 days (last treatment day – 44 ), tumor burden was monitored 26 days post last CM 24 treatment Significant benefits as both single agent and in combination with α - PD - 1
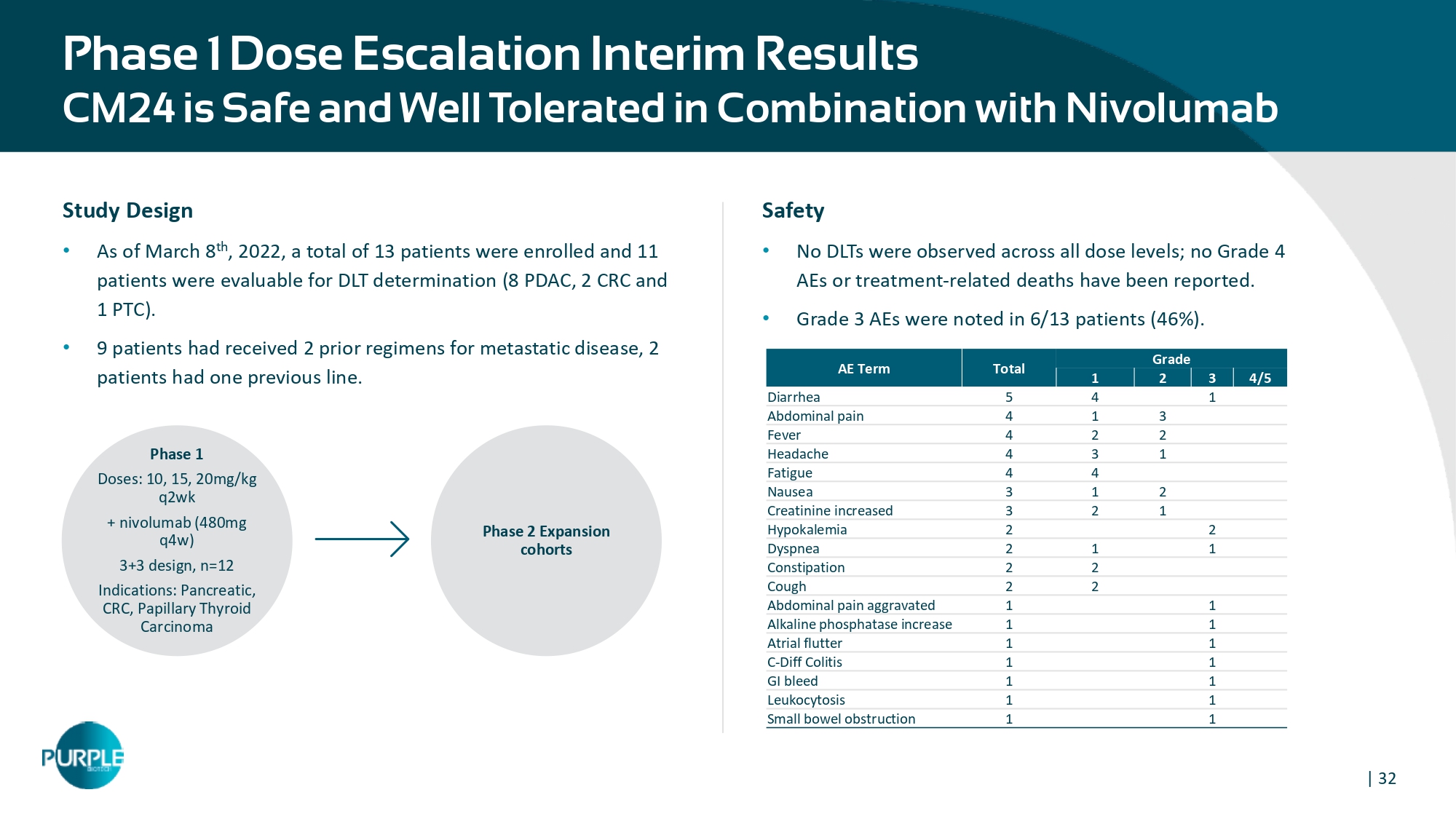
| 32 Phase 1 Dose Escalation Interim Results CM24 is Safe and Well Tolerated in Combi na tion with Nivolumab Grade Total AE Term 4/5 3 2 1 1 4 5 Diarrhea 3 1 4 Abdominal pain 2 2 4 Fever 1 3 4 Headache 4 4 Fatigue 2 1 3 Nausea 1 2 3 Creatinine increased 2 2 Hypokalemia 1 1 2 Dyspnea 2 2 Constipation 2 2 Cough 1 1 Abdominal pain aggravated 1 1 Alkaline phosphatase increase 1 1 Atrial flutter 1 1 C - Diff Colitis 1 1 GI bleed 1 1 Leukocytosis 1 1 Small bowel obstruction Study Design • As of March 8 th , 2022, a total of 13 patients were enrolled and 11 patients were evaluable for DLT determination (8 PDAC, 2 CRC and 1 PTC). • 9 patients had received 2 prior regimens for metastatic disease, 2 patients had one previous line. Phase 1 Doses: 10, 15, 20mg/kg q2wk + nivolumab (480mg q4w) 3+3 design, n=12 Indications: Pancreatic, CRC, Papillary Thyroid Carcinoma Phase 2 Expansion cohorts Safety • No DLTs were observed across all dose levels; no Grade 4 AEs or treatment - related deaths have been reported. • Grade 3 AEs were noted in 6/13 patients (46%).
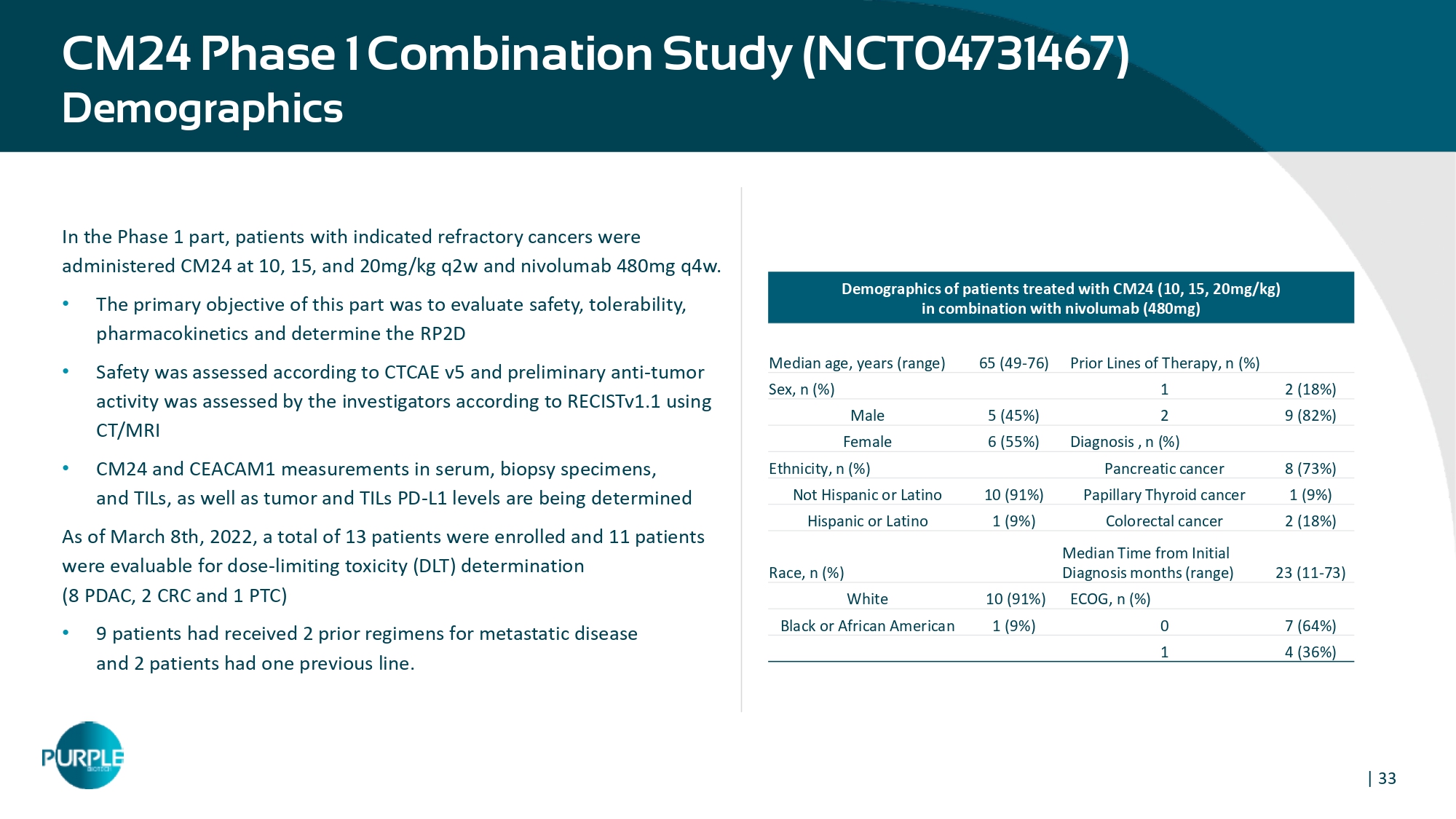
| 33 CM24 Phase 1 Combination Study (NCT04731467) Demographics In the Phase 1 part, patients with indicated refractory cancers were administered CM24 at 10, 15, and 20mg/kg q2w and nivolumab 480mg q4w. • The primary objective of this part was to evaluate safety, tolerability, pharmacokinetics and determine the RP2D • Safety was assessed according to CTCAE v5 and preliminary anti - tumor activity was assessed by the investigators according to RECISTv1.1 using CT/MRI • CM24 and CEACAM1 measurements in serum, biopsy specimens, and TILs, as well as tumor and TILs PD - L1 levels are being determined As of March 8th, 2022, a total of 13 patients were enrolled and 11 patients were evaluable for dose - limiting toxicity (DLT) determination (8 PDAC, 2 CRC and 1 PTC) • 9 patients had received 2 prior regimens for metastatic disease and 2 patients had one previous line. Demographics of patients treated with CM24 (10, 15, 20mg/kg) in combination with nivolumab (480mg) Prior Lines of Therapy, n (%) 65 (49 - 76) Median age, years (range) 2 (18 % ) 1 Sex, n (%) 9 (82 % ) 2 5 (45 % ) Male Diagnosis , n (%) 6 (55 % ) Female 8 ( 7 3 % ) Pancreatic cancer Ethnicity, n (%) 1 (9 % ) Papillary Thyroid cancer 10 (91 % ) Not Hispanic or Latino 2 (18 % ) Colorectal cancer 1 (9 % ) Hispanic or Latino 2 3 (11 - 73) Median Time from Initial Diagnosis months (range) Race, n (%) ECOG, n (%) 10 ( 9 1 % ) White 7 ( 64 %) 0 1 (9 % ) Black or African American 4 ( 36 %) 1
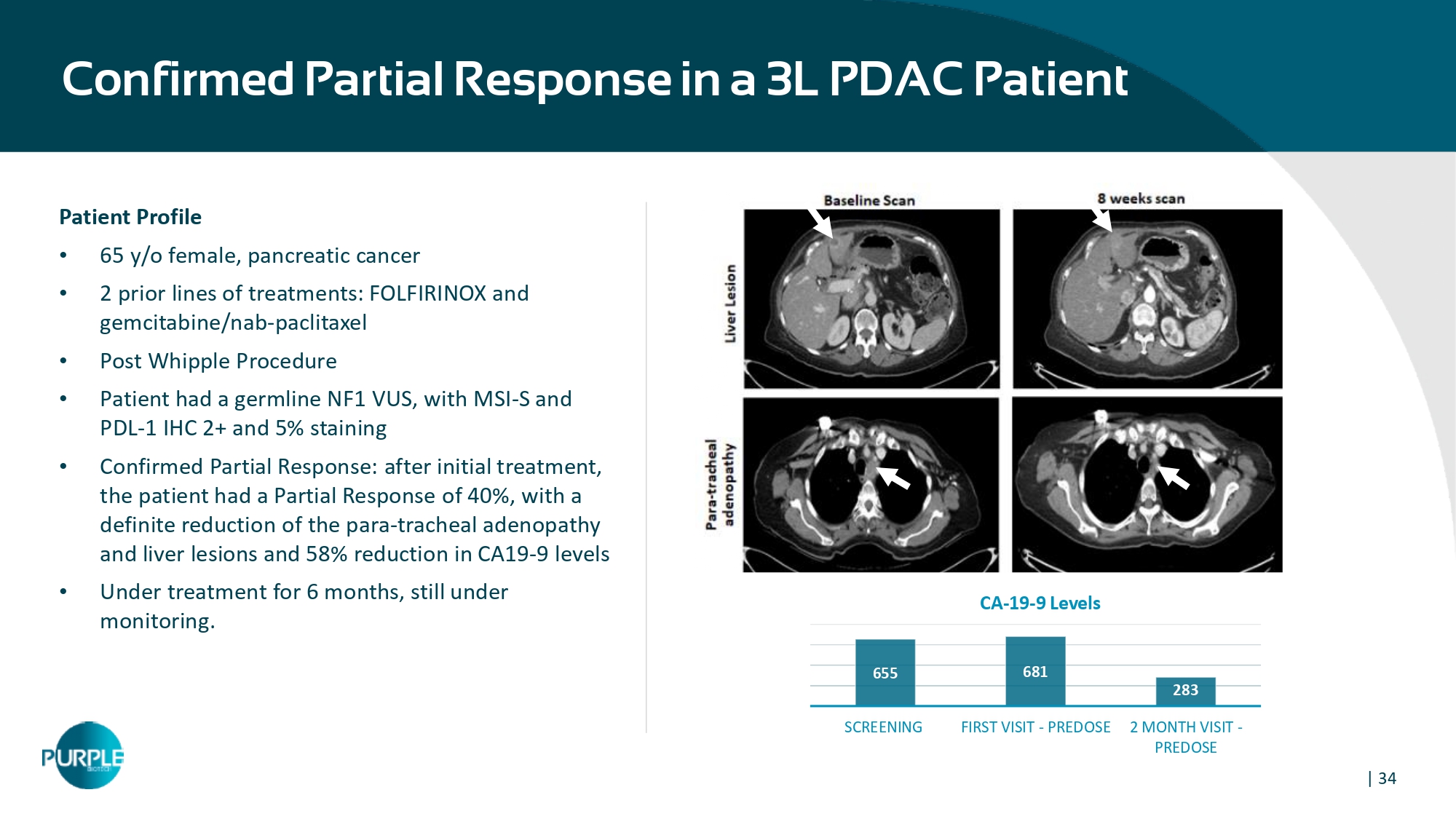
| 34 Confirmed Partial Response in a 3L PDAC Patient Patient Profile • 65 y/o female, pancreatic cancer • 2 prior lines of treatments: FOLFIRINOX and gemcitabine/nab - paclitaxel • Post Whipple Procedure • Patient had a germline NF1 VUS, with MSI - S and PDL - 1 IHC 2+ and 5% staining • Confirmed Partial Response: a fter initial treatment, the patient had a Partial Response of 40%, with a definite reduction of the para - tracheal adenopathy and liver lesions and 58% reduction in CA19 - 9 levels • Under treatment for 6 months, still under monitoring. 655 681 283 SCREENING FIRST VISIT - PREDOSE 2 MONTH VISIT - PREDOSE CA - 19 - 9 Levels

Appendix B | NT219
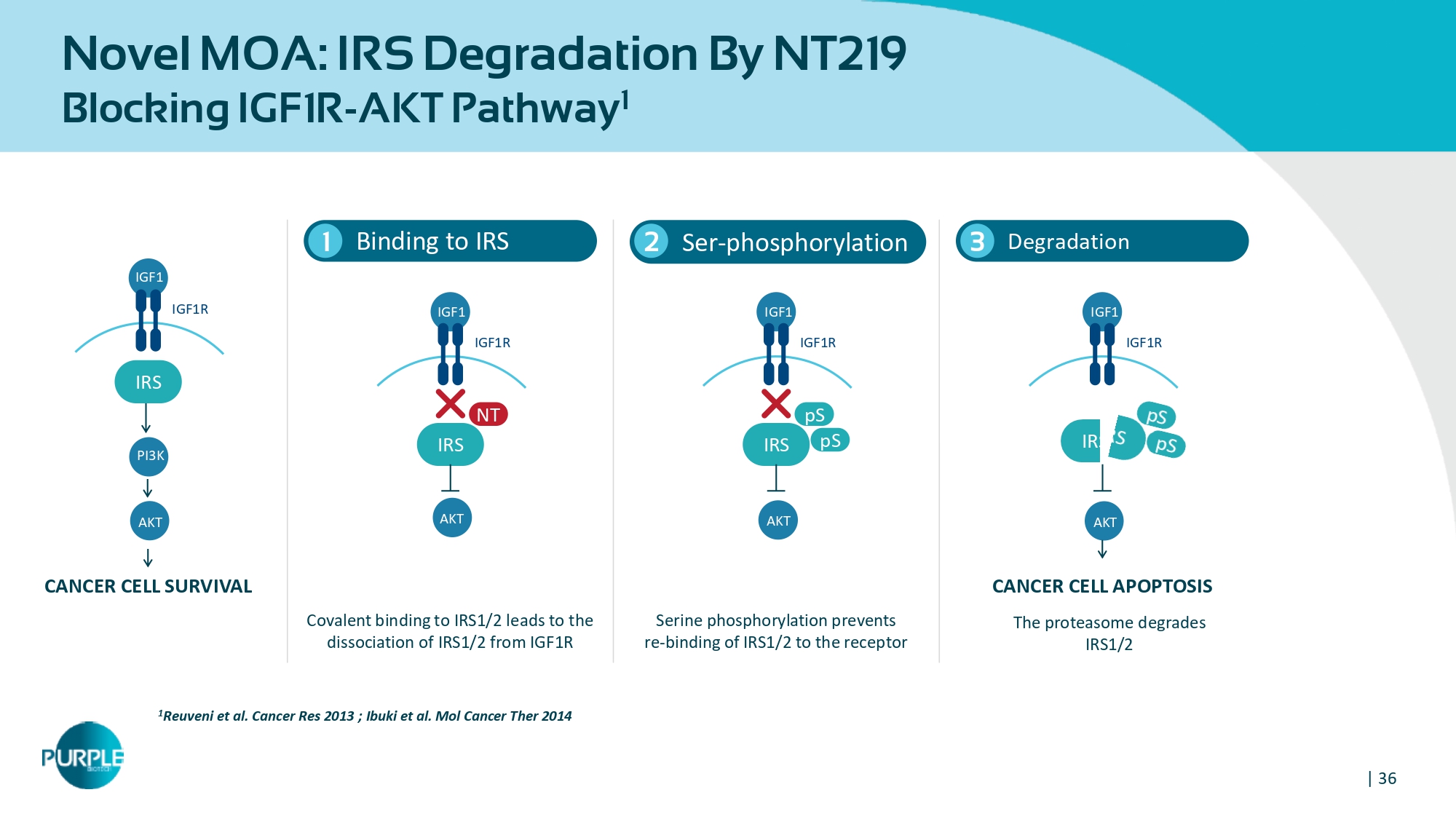
| 36 Novel MOA: IRS Degradation By NT219 Blocking IGF1R - AKT Pathway 1 Binding to IRS 1 1 Reuveni et al. Cancer Res 2013 ; Ibuki et al. Mol Cancer Ther 2014 Ser - phosphorylation 2 Degradation 3 Covalent binding to IRS1/2 leads to the dissociation of IRS1/2 from IGF1R Serine phosphorylation prevents re - binding of IRS1/2 to the receptor CANCER CELL SURVIVAL CANCER CELL APOPTOSIS The proteasome degrades IRS1/2 IGF 1 IGF 1 R IRS AKT PI 3 K IGF 1 IGF 1 R IRS NT AKT IGF 1 IGF 1 R IRS pS pS AKT IGF 1 IGF 1 R AKT
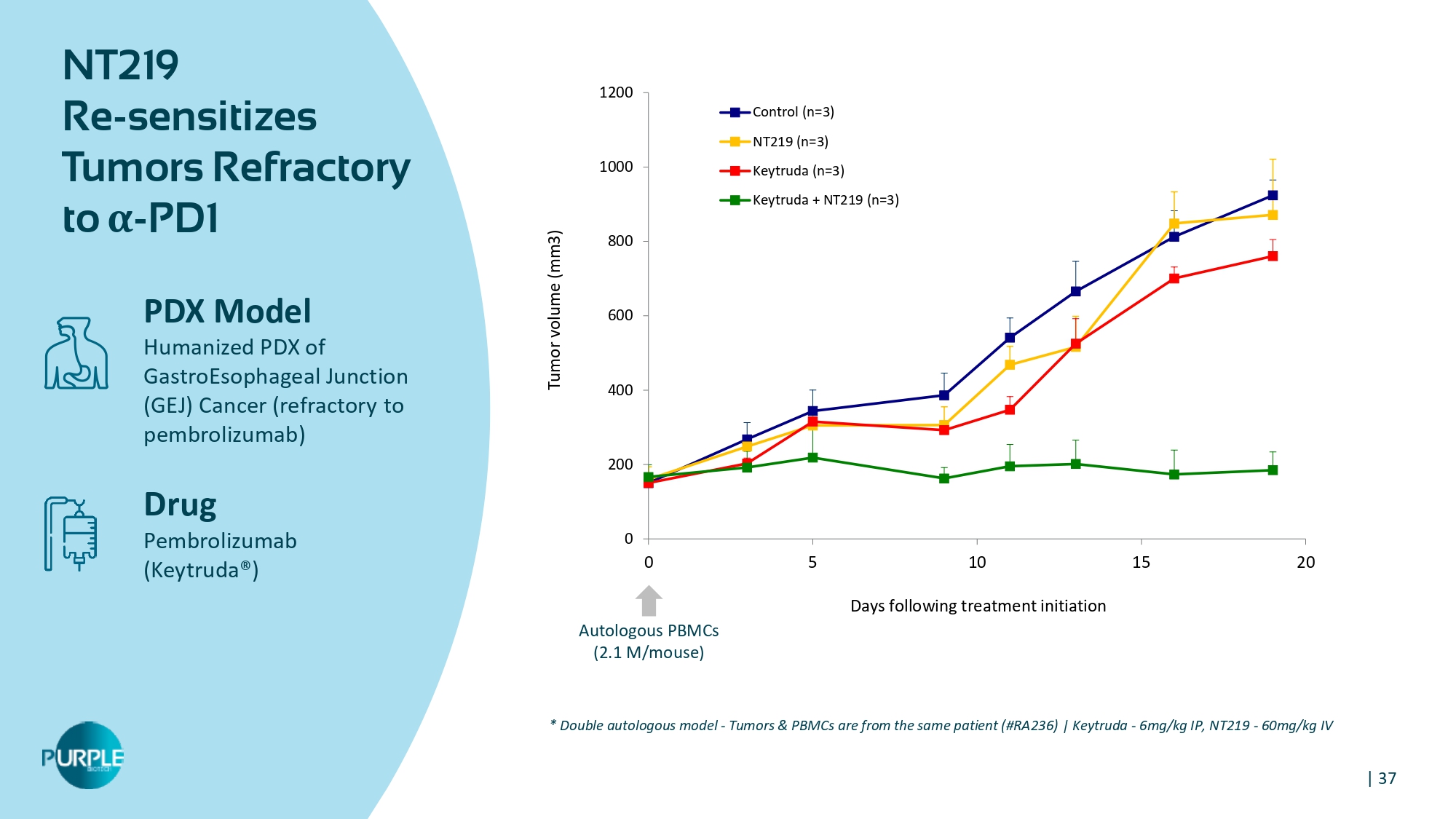
| 37 0 200 400 600 800 1000 1200 0 5 10 15 20 Tumor volume (mm 3 ) Days following treatment initiation Control (n=3) NT219 (n=3) Keytruda (n=3) Keytruda + NT219 (n=3) Autologous PBMCs ( 2.1 M/mouse) NT 219 Re - sensitizes Tumors Refractory to α - PD 1 PDX Model Humanized PDX of GastroEsophageal Junction (GEJ) Cancer (refractory to pembrolizumab) Drug Pembrolizumab (Keytruda®) * Double autologous model - Tumors & PBMCs are from the same patient (#RA 236 ) | Keytruda - 6 mg/kg IP, NT 219 - 60 mg/kg IV
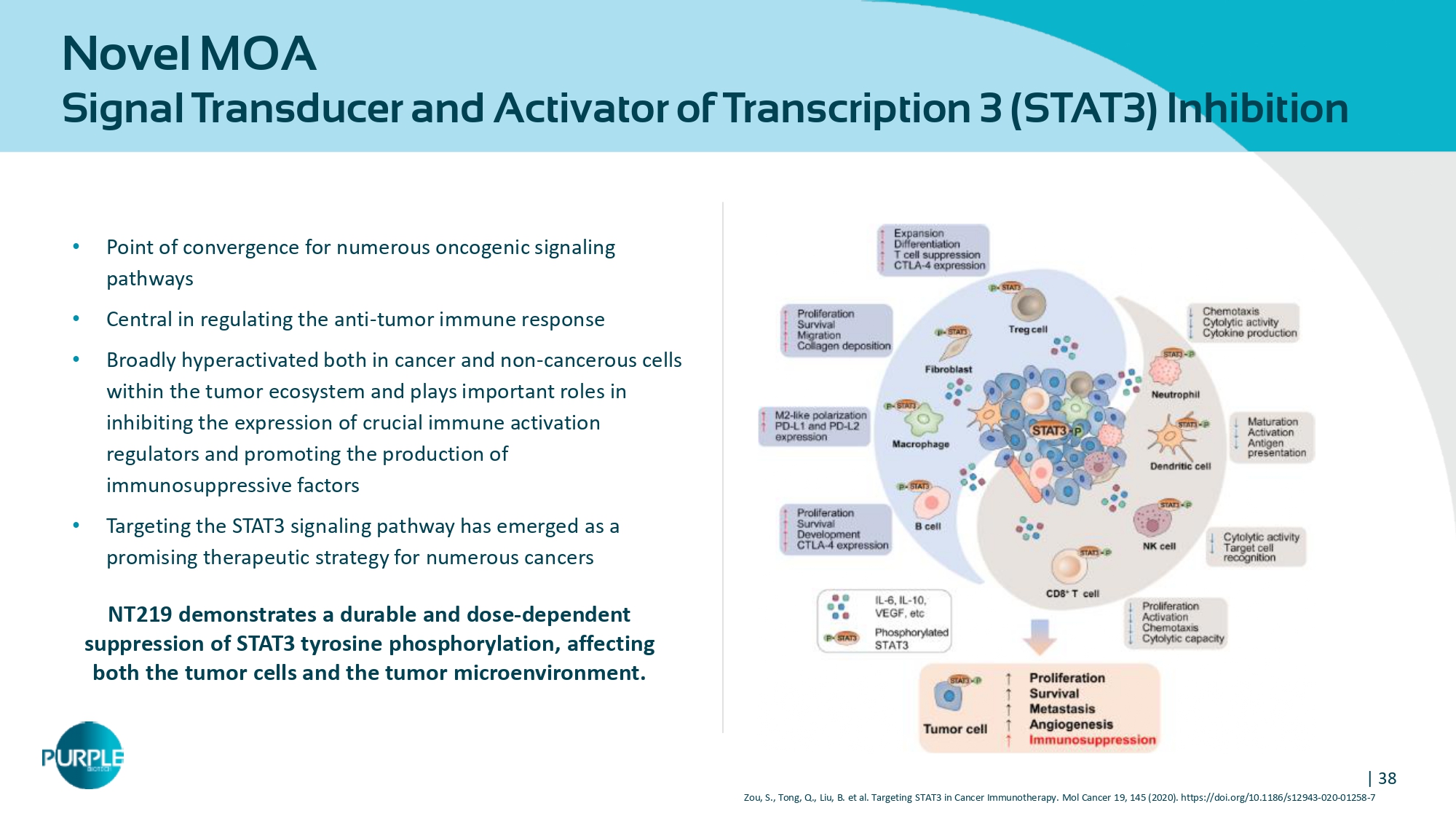
| 38 Novel MOA Signal Transducer and Activator of Transcription 3 ( STAT 3 ) Inhibition • Point of convergence for numerous oncogenic signaling pathways • Central in regulating the anti - tumor immune response • Broadly hyperactivated both in cancer and non - cancerous cells within the tumor ecosystem and plays important roles in inhibiting the expression of crucial immune activation regulators and promoting the production of immunosuppressive factors • Targeting the STAT 3 signaling pathway has emerged as a promising therapeutic strategy for numerous cancers NT 219 demonstrates a durable and dose - dependent suppression of STAT 3 tyrosine phosphorylation, affecting both the tumor cells and the tumor microenvironment. Zou, S., Tong, Q., Liu, B. et al. Targeting STAT 3 in Cancer Immunotherapy. Mol Cancer 19 , 145 ( 2020 ). https://doi.org/ 10.1186 /s 12943 - 020 - 01258 - 7
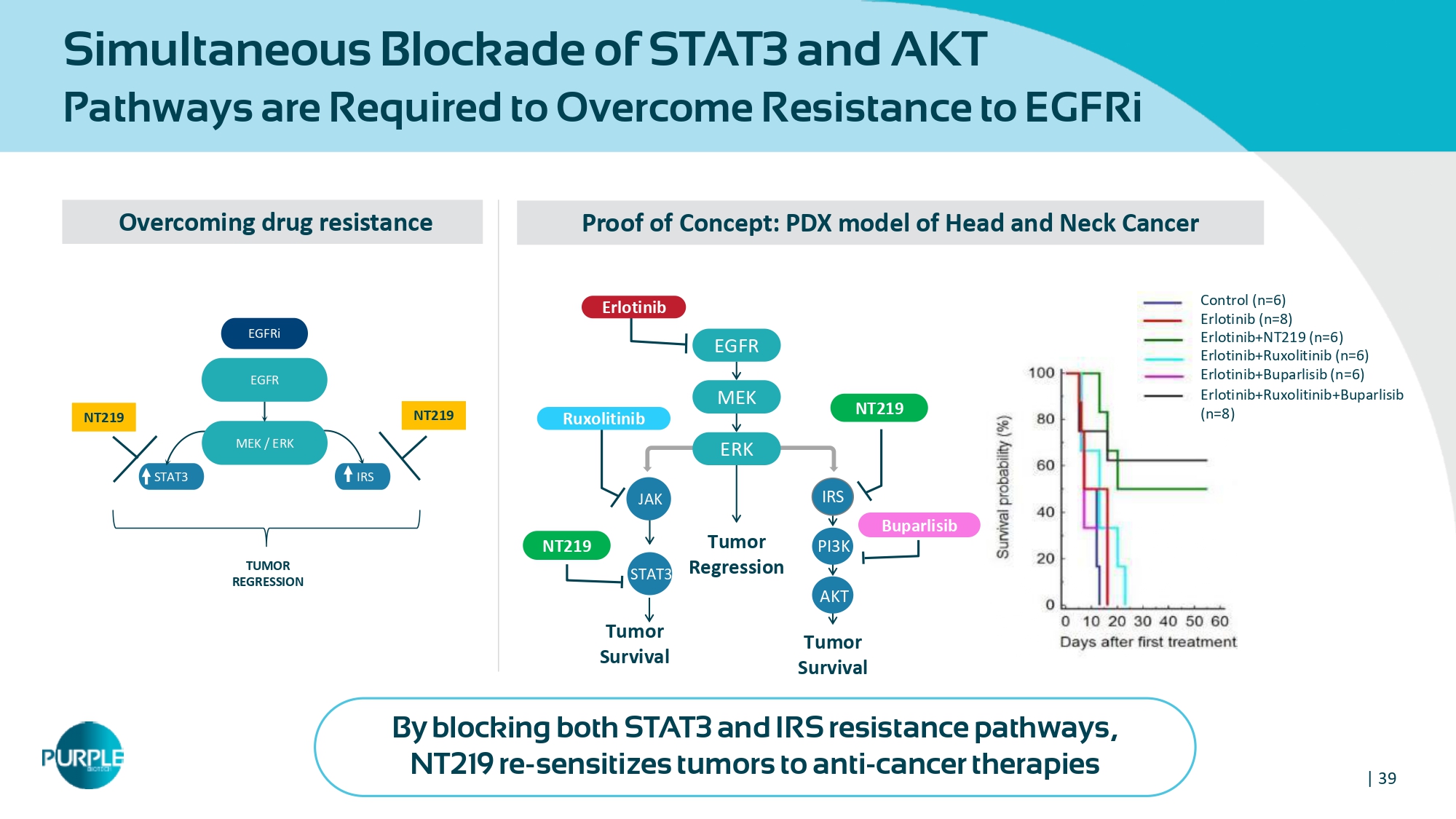
| 39 Simultaneous Blockade of STAT 3 and AKT Pathways are Required to Overcome Resistance to EGFRi Overcoming drug resistance NT 219 NT 219 STAT 3 IRS EGFR EGFRi MEK / ERK TUMOR REGRESSION Proof of Concept: PDX model of Head and Neck Cancer Erlotinib+Ruxolitinib (n= 6 ) Erlotinib+Buparlisib (n= 6 ) Control (n= 6 ) Erlotinib (n= 8 ) Erlotinib+NT 219 (n= 6 ) Erlotinib+Ruxolitinib+Buparlisib (n= 8 ) STAT 3 IRS EGFR MEK Tumor Regression ERK PI 3 K AKT Tumor Survival Tumor Survival Buparlisib Ruxolitinib Erlotinib NT 219 NT 219 JAK By blocking both STAT 3 and IRS resistance pathways, NT 219 re - sensitizes tumors to anti - cancer therapies

| 40 Selected Publications Michael Karin Alexander Levitzki Menashe Bar - Eli Michael Cox
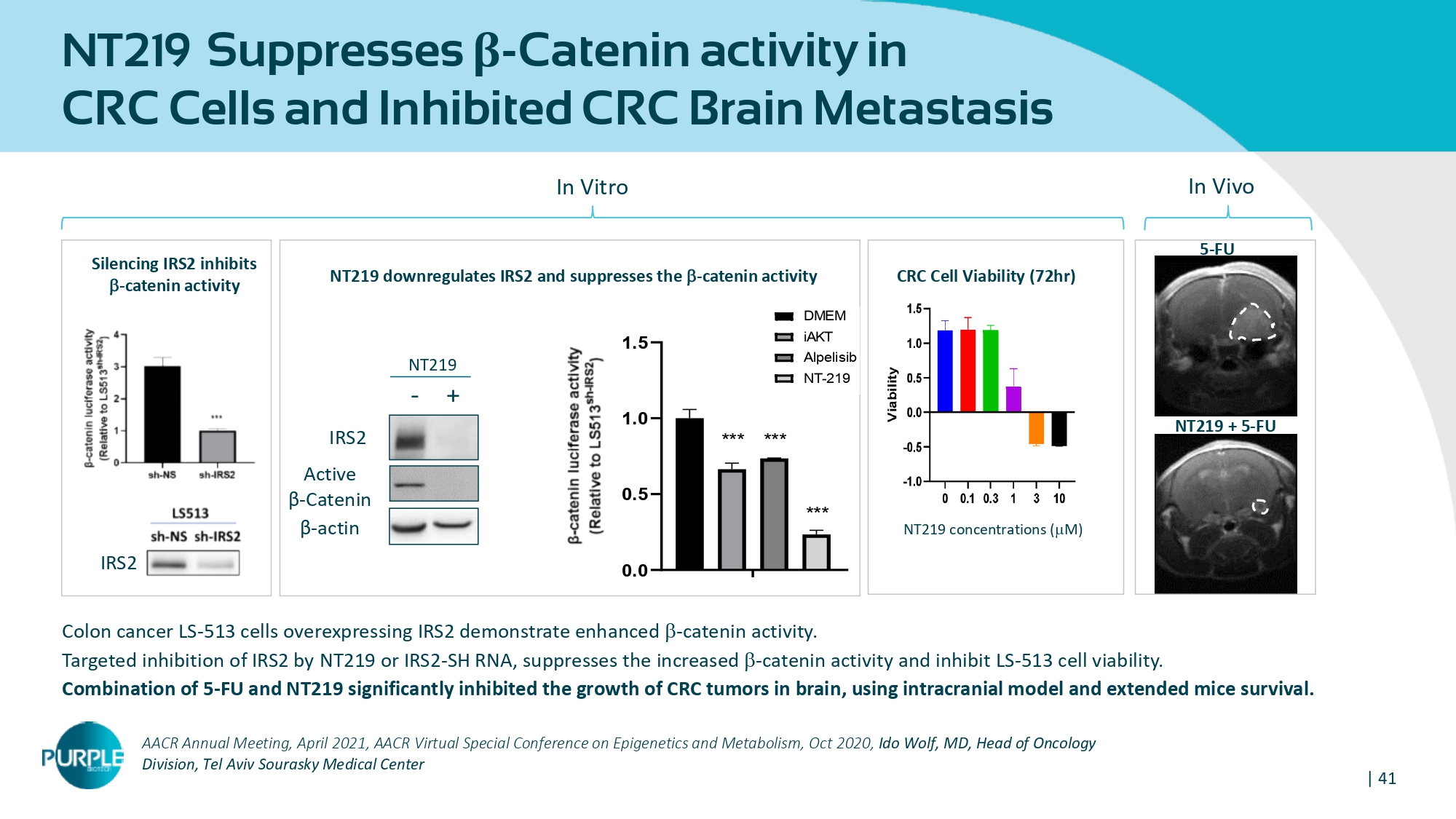
| 41 AACR Annual Meeting, April 2021 , AACR Virtual Special Conference on Epigenetics and Metabolism, Oct 2020 , Ido Wolf, MD, Head of Oncology Division, Tel Aviv Sourasky Medical Center Colon cancer LS - 513 cells overexpressing IRS 2 demonstrate enhanced b - catenin activity. Targeted inhibition of IRS 2 by NT 219 or IRS 2 - SH RNA, suppresses the increased b - catenin activity and inhibit LS - 513 cell viability. Combination of 5 - FU and NT 219 significantly inhibited the growth of CRC tumors in brain , using intracranial model and extended mice survival. Active β - Catenin IRS 2 β - actin NT 219 + - NT 219 concentrations ( m M) CRC Cell Viability ( 72 hr) IRS 2 NT 219 downregulates IRS 2 and suppresses the b - catenin activity Silencing IRS 2 inhibits b - catenin activity NT 219 Suppresses β - Catenin activity in CRC Cells and Inhibited CRC Brain Metastasis 5 - FU NT 219 + 5 Ͳ FU In Vitro In Vivo
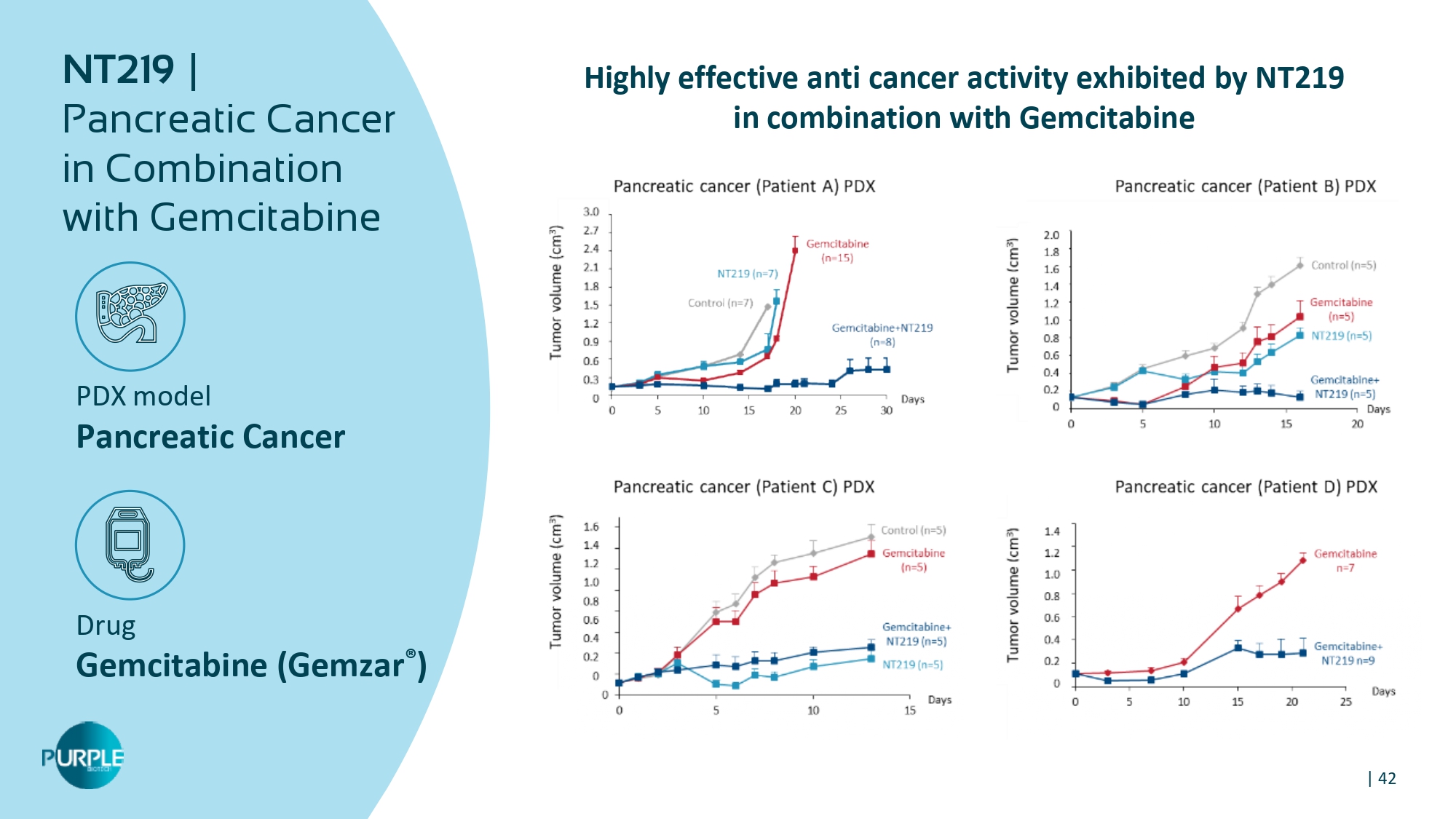
| 42 PDX model Pancreatic Cancer Drug Gemcitabine ( Gemzar ® ) NT 219 | Pancreatic Cancer in Combination with Gemcitabine Highly effective anti cancer activity exhibited by NT 219 in combination with Gemcitabine
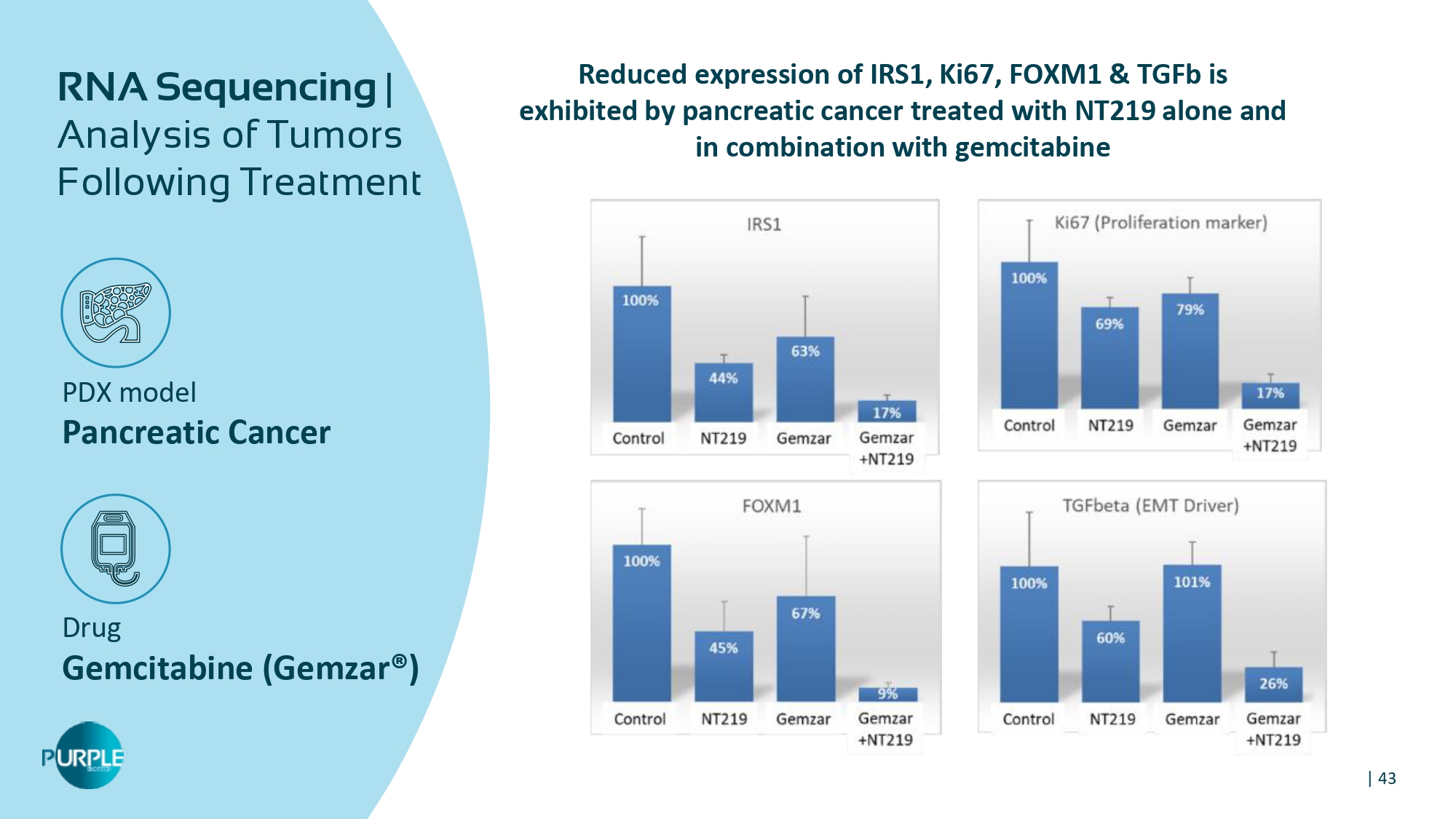
| 43 PDX model Pancreatic Cancer Drug Gemcitabine ( Gemzar ®) RNA Sequencing | Analysis of Tumors Following Treatment Reduced expression of IRS 1 , Ki 67 , FOXM 1 & TGFb is exhibited by pancreatic cancer treated with NT 219 alone and in combination with gemcitabine
Purple Biotech (NASDAQ:PPBT)
過去 株価チャート
から 4 2024 まで 5 2024

Purple Biotech (NASDAQ:PPBT)
過去 株価チャート
から 5 2023 まで 5 2024
