PepGen Shares Tumble 18.4% After FDA Issues Hold Notice on Study
2023年5月31日 - 6:24AM
Dow Jones News
By Paul Ziobro
PepGen shares plunged in after-hours trading Tuesday after the
biotechnology company said the U.S. Food and Drug Administration
issued a clinical hold notice for the study of a drug in patients
with myotonic dystrophy, a neuromuscular disease.
PepGen shares were down 18.4% to $12.87 in late trading. Through
Tuesday's close, the stock was up about 18% this year.
The Phase 1 study was regarding PGN-EDODM1, which delivers a
peptide conjugated antisense oligonucleotide to restore cellular
function, according to PepGen.
"We are disappointed to receive a clinical hold notice on our
planned PGN-EDODM1 study in the U.S., and we will work closely with
the FDA to lift the hold as quickly as possible," PepGen Chief
Executive James McArthur said.
The Boston-based company said the FDA plans to provide an
official clinical hold letter stating the reasons for the hold
within 30 days.
Write to Paul Ziobro at paul.ziobro@wsj.com
(END) Dow Jones Newswires
May 30, 2023 17:09 ET (21:09 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
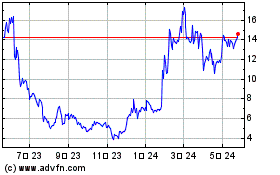
PepGen (NASDAQ:PEPG)
過去 株価チャート
から 4 2024 まで 5 2024
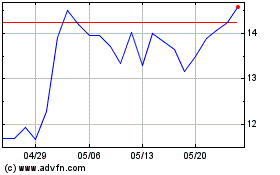
PepGen (NASDAQ:PEPG)
過去 株価チャート
から 5 2023 まで 5 2024