00019359792024Q2false--12-31xbrli:sharesiso4217:USDiso4217:USDxbrli:sharesbhvn:investmentxbrli:purebhvn:prodrugbhvn:claimbhvn:term00019359792024-01-012024-06-3000019359792024-08-0600019359792024-06-3000019359792023-12-3100019359792024-04-012024-06-3000019359792023-04-012023-06-3000019359792023-01-012023-06-3000019359792022-12-3100019359792023-06-300001935979us-gaap:USTreasurySecuritiesMember2024-06-300001935979us-gaap:DomesticCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:ForeignCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:USTreasurySecuritiesMember2023-12-310001935979us-gaap:CashAndCashEquivalentsMember2024-06-300001935979us-gaap:CashAndCashEquivalentsMember2023-12-310001935979bhvn:MarketableSecuritiesCurrentMember2024-06-300001935979bhvn:MarketableSecuritiesCurrentMember2023-12-310001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-06-300001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-06-300001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-06-300001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2024-06-300001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2024-06-300001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2024-06-300001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueInputsLevel1Memberus-gaap:PutOptionMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueInputsLevel2Memberus-gaap:PutOptionMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberus-gaap:PutOptionMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:PutOptionMember2024-06-300001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:DomesticCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:DomesticCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:DomesticCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:DomesticCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignCorporateDebtSecuritiesMember2023-12-310001935979us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001935979us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001935979us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001935979us-gaap:FairValueMeasurementsRecurringMember2023-12-310001935979bhvn:ForwardContractLiability2025AddtionalConsiderationMember2024-01-012024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMember2024-05-010001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMember2024-05-022024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputExpectedTermMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputExpectedTermMember2024-05-010001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputPriceVolatilityMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputPriceVolatilityMember2024-05-010001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputDiscountRateMember2024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractAndDerivativeLiabilitiesMemberus-gaap:MeasurementInputDiscountRateMember2024-05-010001935979bhvn:ForwardContractAndDerivativeLiabilitiesMember2024-01-012024-06-300001935979us-gaap:LandAndBuildingMember2024-06-300001935979us-gaap:LandAndBuildingMember2023-12-310001935979us-gaap:LeaseholdImprovementsMember2024-06-300001935979us-gaap:LeaseholdImprovementsMember2023-12-310001935979us-gaap:ComputerEquipmentMember2024-06-300001935979us-gaap:ComputerEquipmentMember2023-12-310001935979us-gaap:OfficeEquipmentMember2024-06-300001935979us-gaap:OfficeEquipmentMember2023-12-310001935979us-gaap:FurnitureAndFixturesMember2024-06-300001935979us-gaap:FurnitureAndFixturesMember2023-12-310001935979bhvn:DepreciablePropertyPlantAndEquipmentMember2024-06-300001935979bhvn:DepreciablePropertyPlantAndEquipmentMember2023-12-310001935979us-gaap:ConstructionInProgressMember2024-06-300001935979us-gaap:ConstructionInProgressMember2023-12-310001935979us-gaap:CommonStockMember2023-12-310001935979us-gaap:AdditionalPaidInCapitalMember2023-12-310001935979us-gaap:RetainedEarningsMember2023-12-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001935979us-gaap:ParentMember2023-12-310001935979us-gaap:RetainedEarningsMember2024-01-012024-03-310001935979us-gaap:ParentMember2024-01-012024-03-310001935979us-gaap:CommonStockMember2024-01-012024-03-310001935979us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001935979us-gaap:CommonStockMember2024-03-310001935979us-gaap:AdditionalPaidInCapitalMember2024-03-310001935979us-gaap:RetainedEarningsMember2024-03-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001935979us-gaap:ParentMember2024-03-310001935979us-gaap:RetainedEarningsMember2024-04-012024-06-300001935979us-gaap:ParentMember2024-04-012024-06-300001935979us-gaap:CommonStockMember2024-04-012024-06-300001935979us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300001935979us-gaap:CommonStockMember2024-06-300001935979us-gaap:AdditionalPaidInCapitalMember2024-06-300001935979us-gaap:RetainedEarningsMember2024-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300001935979us-gaap:ParentMember2024-06-300001935979us-gaap:CommonStockMember2022-12-310001935979us-gaap:AdditionalPaidInCapitalMember2022-12-310001935979us-gaap:RetainedEarningsMember2022-12-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001935979us-gaap:ParentMember2022-12-310001935979us-gaap:RetainedEarningsMember2023-01-012023-03-310001935979us-gaap:ParentMember2023-01-012023-03-310001935979us-gaap:CommonStockMember2023-01-012023-03-310001935979us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310001935979us-gaap:CommonStockMember2023-03-310001935979us-gaap:AdditionalPaidInCapitalMember2023-03-310001935979us-gaap:RetainedEarningsMember2023-03-310001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001935979us-gaap:ParentMember2023-03-310001935979us-gaap:RetainedEarningsMember2023-04-012023-06-300001935979us-gaap:ParentMember2023-04-012023-06-300001935979us-gaap:CommonStockMember2023-04-012023-06-300001935979us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001935979us-gaap:CommonStockMember2023-06-300001935979us-gaap:AdditionalPaidInCapitalMember2023-06-300001935979us-gaap:RetainedEarningsMember2023-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001935979us-gaap:ParentMember2023-06-300001935979bhvn:PurchaseAgreementKnoppAmendmentMember2024-05-012024-05-310001935979bhvn:PurchaseAgreementKnoppAmendmentMember2024-05-010001935979us-gaap:CommonStockMember2024-04-222024-04-220001935979us-gaap:CommonStockMember2024-04-220001935979bhvn:PyramidBiosciencesInc.Member2024-01-072024-01-070001935979bhvn:PyramidBiosciencesInc.Member2024-01-072024-06-300001935979bhvn:PyramidBiosciencesInc.Member2024-01-012024-03-310001935979bhvn:PyramidBiosciencesInc.Member2024-01-012024-06-300001935979us-gaap:CommonStockMember2023-10-310001935979us-gaap:CommonStockMemberbhvn:EquityDistributionAgreementMember2023-10-012024-06-300001935979us-gaap:CommonStockMemberus-gaap:SubsequentEventMemberbhvn:EquityDistributionAgreementMember2024-07-012024-08-090001935979us-gaap:CommonStockMemberus-gaap:SubsequentEventMemberbhvn:EquityDistributionAgreementMember2023-10-012024-08-090001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-03-310001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-12-310001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-04-012024-06-300001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-01-012024-06-300001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2024-03-310001935979us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310001935979us-gaap:AccumulatedTranslationAdjustmentMember2024-04-012024-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2024-01-012024-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2024-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-06-300001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-03-310001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-04-012023-06-300001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-01-012023-06-300001935979us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2023-03-310001935979us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001935979us-gaap:AccumulatedTranslationAdjustmentMember2023-04-012023-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-06-300001935979us-gaap:AccumulatedTranslationAdjustmentMember2023-06-300001935979us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-06-300001935979us-gaap:ResearchAndDevelopmentExpenseMember2024-04-012024-06-300001935979us-gaap:ResearchAndDevelopmentExpenseMember2023-04-012023-06-300001935979us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-06-300001935979us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-06-300001935979us-gaap:GeneralAndAdministrativeExpenseMember2024-04-012024-06-300001935979us-gaap:GeneralAndAdministrativeExpenseMember2023-04-012023-06-300001935979us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-06-300001935979us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-06-300001935979us-gaap:EmployeeStockOptionMember2024-01-012024-06-300001935979us-gaap:RestrictedStockUnitsRSUMember2024-06-300001935979us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-06-300001935979us-gaap:RestrictedStockUnitsRSUMember2024-04-012024-06-300001935979us-gaap:RestrictedStockUnitsRSUMember2023-12-310001935979us-gaap:EmployeeStockOptionMember2024-01-012024-06-300001935979us-gaap:EmployeeStockOptionMember2023-01-012023-06-300001935979us-gaap:WarrantMember2024-01-012024-06-300001935979us-gaap:WarrantMember2023-01-012023-06-300001935979bhvn:RestrictedStockUnitsAndPerformanceShareUnitsMember2024-01-012024-06-300001935979bhvn:RestrictedStockUnitsAndPerformanceShareUnitsMember2023-01-012023-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2019-05-012019-05-310001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2024-01-012024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2023-04-012023-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2024-04-012024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleCollaborativeArrangementMember2023-01-012023-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2021-01-012021-01-310001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2024-04-012024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2024-01-012024-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2023-01-012023-06-300001935979bhvn:YaleUniversityMemberbhvn:YaleMoDEAgreementMember2023-04-012023-06-300001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2015-08-012015-08-310001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2024-06-300001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2023-01-012023-06-300001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2024-04-012024-06-300001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2024-01-012024-06-300001935979bhvn:ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMemberus-gaap:CollaborativeArrangementMember2023-04-012023-06-300001935979bhvn:BMSMemberbhvn:TaldefgrobepAlfaLicenseAgreementMember2024-06-300001935979bhvn:BMSMember2023-04-012023-06-300001935979bhvn:BMSMember2024-04-012024-06-300001935979bhvn:BMSMember2023-01-012023-06-300001935979bhvn:BMSMember2024-01-012024-06-300001935979us-gaap:LicensingAgreementsMemberbhvn:HighlightllPharmaceuticalCoLtdMemberus-gaap:CollaborativeArrangementMember2023-03-310001935979bhvn:HighlightllPharmaceuticalCoLtdMemberus-gaap:CollaborativeArrangementMember2023-10-012023-12-310001935979bhvn:HighlightllPharmaceuticalCoLtdMemberus-gaap:CollaborativeArrangementMember2024-06-300001935979bhvn:HighlightllPharmaceuticalCoLtdMember2023-04-012023-06-300001935979bhvn:HighlightllPharmaceuticalCoLtdMember2023-01-012023-06-300001935979bhvn:HighlightllPharmaceuticalCoLtdMember2024-04-012024-06-300001935979bhvn:HighlightllPharmaceuticalCoLtdMember2024-01-012024-06-300001935979bhvn:OtherAgreementsMember2024-04-012024-06-300001935979bhvn:OtherAgreementsMember2023-04-012023-06-300001935979bhvn:OtherAgreementsMember2024-01-012024-06-300001935979bhvn:OtherAgreementsMember2023-01-012023-06-300001935979bhvn:PurchaseAgreementKnoppAmendmentMember2024-05-012024-05-010001935979bhvn:PurchaseAgreementKnoppAmendment2025AdditionalConsiderationMember2024-05-032024-05-030001935979us-gaap:ForwardContractsMember2024-05-030001935979bhvn:PurchaseAgreementKnoppAmendmentMember2024-05-012024-05-310001935979us-gaap:FairValueInputsLevel3Memberbhvn:DerivativeLiability2024AdditionalConsiderationMember2024-05-030001935979us-gaap:FairValueInputsLevel3Memberbhvn:A2024AdditionalConsiderationTrueUpMember2024-05-012024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractLiability2025AddtionalConsiderationMember2024-05-010001935979us-gaap:FairValueInputsLevel3Memberbhvn:ForwardContractLiability2025AddtionalConsiderationMember2024-05-012024-06-300001935979us-gaap:FairValueInputsLevel3Memberbhvn:A2025AdditionalConsiderationTrueUpMember2024-05-030001935979us-gaap:FairValueInputsLevel3Memberbhvn:A2025AdditionalConsiderationTrueUpMember2024-05-012024-06-300001935979bhvn:DevelopmentalAndRegulatoryMilestonesFirstAssetMemberbhvn:PyramidBiosciencesInc.Memberbhvn:LeadAssetMember2024-06-300001935979bhvn:DevelopmentalAndRegulatoryMilestonesSecondAssetMemberbhvn:PyramidBiosciencesInc.Memberbhvn:SecondAssetMember2024-06-300001935979bhvn:CommercialSalesBasedMilestonesMemberbhvn:PyramidBiosciencesInc.Member2024-06-300001935979bhvn:PittsburghCentreAvenueLeaseAgreementMember2024-03-310001935979bhvn:PittsburghCentreAvenueLeaseAgreementMembersrt:MinimumMember2024-06-300001935979bhvn:PittsburghCentreAvenueLeaseAgreementMembersrt:MaximumMember2024-06-300001935979us-gaap:ResearchAndDevelopmentArrangementMember2024-01-012024-06-300001935979us-gaap:LicensingAgreementsMemberbhvn:ModaPharmaceuticalsLLCMemberbhvn:ModaAgreementMember2021-01-012021-01-010001935979bhvn:ModaPharmaceuticalsLLCMemberbhvn:ModaAgreementMember2021-01-012021-01-010001935979bhvn:ModaAgreementMember2023-08-012023-08-310001935979bhvn:ModaPharmaceuticalsLLCMember2024-01-012024-06-300001935979bhvn:ModaPharmaceuticalsLLCMember2023-01-012023-06-300001935979bhvn:ModaPharmaceuticalsLLCMember2023-04-012023-06-300001935979bhvn:YaleUniversityMemberbhvn:A2023YaleSponsoredResearchAgreementMember2023-05-012023-05-310001935979us-gaap:RelatedPartyMemberbhvn:YaleUniversityMember2024-04-012024-06-300001935979us-gaap:RelatedPartyMemberbhvn:YaleUniversityMember2024-01-012024-06-300001935979us-gaap:RelatedPartyMemberbhvn:YaleUniversityMember2023-04-012023-06-300001935979us-gaap:RelatedPartyMemberbhvn:YaleUniversityMember2023-01-012023-06-300001935979us-gaap:RelatedPartyMemberbhvn:YaleUniversityMember2024-06-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number: 001-41477
Biohaven Ltd.
(Exact name of registrant as specified in its charter) | | | | | | | | |
| British Virgin Islands | | Not applicable |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| | |
| c/o Biohaven Pharmaceuticals, Inc. | | |
215 Church Street, New Haven, Connecticut | | 06510 |
| (Address of principal executive offices) | | (Zip Code) |
(203) 404-0410
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Common Shares, no par value | BHVN | New York Stock Exchange |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | | Accelerated filer | ☐ | |
| Non-accelerated filer | ☐ | | Small reporting company | ☐ | |
| | | | Emerging growth company | ☐ | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of August 6, 2024, the registrant had 94,542,253 common shares, without par value per share, outstanding.
| | | | | | | | |
| | TABLE OF CONTENTS | |
| | Page |
| Part I | Financial Information | |
Item 1: | | |
| | |
| | |
| | |
| | |
Item 2: | | |
Item 3: | | |
Item 4: | | |
| | |
| | |
| Part II | Other Information | |
Item 1: | | |
Item 1A: | | |
Item 2: | | |
| Item 5. | | |
Item 6: | | |
| | |
PART I - FINANCIAL INFORMATION
Item 1. Unaudited Condensed Consolidated Financial Statements
BIOHAVEN LTD.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share amounts)
| | | | | | | | | | | | | | |
| | June 30, 2024 | | December 31, 2023 |
| | (Unaudited) | | |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 239,147 | | | $ | 248,402 | |
| Marketable securities | | 197,801 | | | 133,417 | |
| | | | |
| | | | |
| Prepaid expenses | | 59,532 | | | 35,242 | |
| Income tax receivable | | 7,522 | | | 13,252 | |
| | | | |
| Other current assets | | 7,266 | | | 12,133 | |
| Total current assets | | 511,268 | | | 442,446 | |
| Property and equipment, net | | 18,665 | | | 17,191 | |
| | | | |
| | | | |
| | | | |
| Intangible assets | | 18,400 | | | 18,400 | |
| Goodwill | | 1,390 | | | 1,390 | |
| | | | |
| Other non-current assets | | 32,918 | | | 33,785 | |
| Total assets | | $ | 582,641 | | | $ | 513,212 | |
| Liabilities and Shareholders' Equity | | | | |
| Current liabilities: | | | | |
| | | | |
| Accounts payable | | $ | 17,259 | | | $ | 15,577 | |
| | | | |
| Accrued expenses and other current liabilities | | 57,728 | | | 39,846 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
Forward contract and derivative liabilities | | 81,220 | | | — | |
| Total current liabilities | | 156,207 | | | 55,423 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
Non-current operating lease liabilities | | 26,193 | | | 27,569 | |
| | | | |
| | | | |
Derivative liability, non-current | | 12,180 | | | — | |
| Other non-current liabilities | | 4,321 | | | 2,245 | |
| Total liabilities | | 198,901 | | | 85,237 | |
Commitments and contingencies (Note 11) | | | | |
| | | | |
| | | | |
| Shareholders' Equity: | | | | |
| | | | |
Preferred shares, no par value; 10,000,000 shares authorized, no shares issued and outstanding as of June 30, 2024 and December 31, 2023 | | — | | | — | |
Common shares, no par value; 200,000,000 shares authorized as of June 30, 2024 and December 31, 2023; 92,346,332 and 81,115,723 shares issued and outstanding as of June 30, 2024 and December 31, 2023, respectively | | 1,298,553 | | | 887,528 | |
| Additional paid-in capital | | 83,832 | | | 39,804 | |
| | | | |
| Accumulated deficit | | (998,567) | | | (499,292) | |
Accumulated other comprehensive loss | | (78) | | | (65) | |
| | | | |
| | | | |
| Total shareholders' equity | | 383,740 | | | 427,975 | |
| Total liabilities and shareholders' equity | | $ | 582,641 | | | $ | 513,212 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOHAVEN LTD.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended June 30, | | Six Months Ended June 30, |
| | | 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Operating expenses: | | | | | | | | |
| | | | | | | | |
| Research and development | | $ | 314,819 | | | $ | 79,490 | | | $ | 470,791 | | | $ | 142,951 | |
General and administrative | | 18,953 | | | 14,521 | | | 46,221 | | | 28,842 | |
| Total operating expenses | | 333,772 | | | 94,011 | | | 517,012 | | | 171,793 | |
| Loss from operations | | (333,772) | | | (94,011) | | | (517,012) | | | (171,793) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Other income, net | | 14,178 | | | 5,842 | | | 18,483 | | | 14,071 | |
Loss before provision for income taxes | | (319,594) | | | (88,169) | | | (498,529) | | | (157,722) | |
Provision for income taxes | | 177 | | | 2,177 | | | 746 | | | 3,116 | |
| Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (499,275) | | | $ | (160,838) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Net loss per share — basic and diluted | | $ | (3.64) | | | $ | (1.32) | | | $ | (5.93) | | | $ | (2.36) | |
Weighted average common shares outstanding—basic and diluted | | 87,766,069 | | | 68,248,023 | | | 84,174,099 | | | 68,227,564 | |
| Comprehensive loss: | | | | | | | | |
| Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (499,275) | | | $ | (160,838) | |
Other comprehensive income (loss), net of tax | | 28 | | | (146) | | | (13) | | | (264) | |
| | | | | | | | |
| | | | | | | | |
Comprehensive loss | | $ | (319,743) | | | $ | (90,492) | | | $ | (499,288) | | | $ | (161,102) | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOHAVEN LTD.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
(Unaudited)
| | | | | | | | | | | | | |
| Six Months Ended June 30, | | |
| 2024 | | 2023 | | |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (499,275) | | | $ | (160,838) | | | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 3,400 | | | 3,480 | | | |
Non-cash share-based compensation | 47,109 | | | 8,460 | | | |
Issuance of common shares as payment for acquisition of IPR&D asset | 10,793 | | | — | | | |
Issuance of common shares as payment under license and other agreements | 71,618 | | | — | | | |
Fair value of forward contract and derivative liabilities under license agreement | 102,529 | | | — | | | |
Change in fair value of forward contract and derivative liabilities | (9,129) | | | — | | | |
Issuance of warrant under license agreement | 3,340 | | | — | | | |
Other non-cash items, net | (3,129) | | | (3,682) | | | |
Changes in operating assets and liabilities, net of effects of acquisition: | | | | | |
Prepaid expenses and other current and non-current assets | (13,491) | | | 39,283 | | | |
| Accounts payable | (60) | | | 1,336 | | | |
Accrued expenses and other current and non-current liabilities | 15,860 | | | (10,070) | | | |
| Net cash used in operating activities | (270,435) | | | (122,031) | | | |
| Cash flows from investing activities: | | | | | |
Proceeds from maturities of marketable securities | 182,364 | | | 124,977 | | | |
Proceeds from sales of marketable securities | — | | | 4,920 | | | |
| Purchases of marketable securities | (243,266) | | | (53,372) | | | |
| Purchases of property and equipment | (3,389) | | | (1,330) | | | |
Cash acquired from acquisition of IPR&D asset | 391 | | | — | | | |
| | | | | |
Net cash (used in) provided by investing activities | (63,900) | | | 75,195 | | | |
| Cash flows from financing activities: | | | | | |
Proceeds from issuance of common shares | 318,520 | | | — | | | |
Payment of issuance costs | (800) | | | — | | | |
| Change in restricted cash due to Former Parent | — | | | 5,203 | | | |
Proceeds from equity incentive plan and employee share purchase plan | 4,568 | | | 1,126 | | | |
Other financing activities, net | 2,258 | | | — | | | |
Net cash provided by financing activities | 324,546 | | | 6,329 | | | |
| Effects of exchange rates on cash, cash equivalents, and restricted cash | (13) | | | (147) | | | |
Net decrease in cash, cash equivalents, and restricted cash | (9,802) | | | (40,654) | | | |
| Cash, cash equivalents, and restricted cash at beginning of period | 252,120 | | | 242,604 | | | |
| Cash, cash equivalents, and restricted cash at end of period | $ | 242,318 | | | $ | 201,950 | | | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
1. Nature of the Business and Basis of Presentation
Biohaven Ltd. (“we,” “us," "our," "Biohaven" or the “Company”) was incorporated in Tortola, British Virgin Islands in May 2022. Biohaven is a biopharmaceutical company focused on the discovery, development and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. The Company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple, proprietary drug development platforms. Biohaven's extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; Transient Receptor Potential Melastatin 3 ("TRPM3") antagonism for migraine and neuropathic pain; Tyrosine Kinase 2/Janus Kinase 1 ("TYK2/JAK1") inhibition for neuroinflammatory disorders; glutamate modulation for obsessive compulsive disorder ("OCD") and spinocerebellar ataxia ("SCA"); myostatin inhibition for neuromuscular and metabolic diseases, including spinal muscular atrophy ("SMA") and obesity; and antibody recruiting bispecific molecules ("ARMs") and antibody drug conjugates ("ADCs") for cancer.
The Company is subject to risks and uncertainties common to companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations and the ability to secure additional capital to fund operations. Product candidates currently under development will require significant additional research and development efforts, including preclinical and clinical testing and regulatory approval, prior to commercialization. These efforts may require additional capital, additional personnel and infrastructure, and further regulatory and other capabilities. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
Separation from Biohaven Pharmaceutical Holding Company Ltd.
On October 3, 2022, Biohaven Pharmaceutical Holding Company Ltd. (the “Former Parent”) completed the distribution (the “Distribution”) to holders of its common shares of all of the outstanding common shares of Biohaven Ltd. and the spin-off of Biohaven Ltd.
from the Former Parent (the “Spin-Off”) described in Biohaven’s Information Statement attached as Exhibit 99.1 to Biohaven’s Registration Statement on Form 10, as amended (Reg. No. 001-41477). Collectively, we refer to the Distribution and Spin-Off throughout this Quarterly Report on Form 10-Q as the "Separation." As a result of the Separation, Biohaven Ltd. became an independent, publicly traded company as of October 3, 2022, and commenced regular way trading under the symbol “BHVN”’ on the New York Stock Exchange (the "NYSE") on October 4, 2022. Where we describe historical business activities in this report, we do so as if the Former Parent’s activities related to such assets and liabilities had been performed by the Company.
Basis of Presentation
The accompanying condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and pursuant to the rules and regulations of the SEC. The accompanying condensed consolidated financial statements include the accounts of Biohaven Ltd. and our wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Going Concern
In accordance with Accounting Standards Codification (“ASC”) 205-40, Going Concern, the Company has evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the condensed consolidated financial statements are issued.
Through August 9, 2024, the Company has funded its operations primarily with funding from the Former Parent, proceeds from the sale of its common shares, and the cash contribution received from the Former Parent at the Separation. The Company has incurred recurring losses since its inception and expects to continue to generate operating losses for the foreseeable future.
As of the date of issuance of these condensed consolidated financial statements, the Company expects its existing cash, cash equivalents, and marketable securities will be sufficient to fund operating and financial commitments, and other cash requirements for at least one year after the issuance date of these financial statements.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
1. Nature of the Business and Basis of Presentation (Continued)
To execute its business plans, the Company will require funding to support its continuing operations and pursue its growth strategy. Until such time as the Company can generate significant revenue from product sales or royalties, if ever, it expects to finance its operations through the sale of public or private equity, debt financings or other capital sources, including collaborations with other companies or other strategic transactions. The Company may not be able to obtain financing on acceptable terms, or at all. The terms of any financing may adversely affect the holdings or the rights of the Company’s shareholders. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate some or all of its research and development programs, product portfolio expansion or commercialization efforts, which could adversely affect its business prospects, or the Company may be unable to continue operations.
2. Summary of Significant Accounting Policies
Our significant accounting policies are described in Note 2, "Summary of Significant Accounting Policies" to the consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023 (the "2023 Form 10-K"). Updates to our accounting policies are discussed below in this Note 2.
Unaudited Interim Condensed Consolidated Financial Information
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with GAAP for interim financial information. The accompanying unaudited condensed consolidated financial statements do not include all of the information and footnotes required by GAAP for complete consolidated financial statements. The accompanying year-end condensed consolidated balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America. The unaudited interim condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of June 30, 2024, the results of its operations for the three and six months ended June 30, 2024 and 2023, and its cash flows for the six months ended June 30, 2024 and 2023. The results for the three and six months ended June 30, 2024 are not necessarily indicative of results to be expected for
the year ending December 31, 2024, any other interim periods or any future year or period. The financial information included herein should be read in conjunction with the financial statements and notes in the Company's Annual Report on Form 10-K for the year ended December 31, 2023.
Reclassifications
Certain items in the prior period’s condensed consolidated financial statements have been reclassified to conform to the current year presentation.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of expenses during the reporting periods. Significant estimates and assumptions reflected in these condensed consolidated financial statements include, but are not limited to, the accrual for research and development expenses and valuation of forward contract and derivative liabilities. In addition, management’s assessment of the Company’s ability to continue as a going concern involves the estimation of the amount and timing of future cash inflows and outflows. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates.
Restricted Cash
Restricted cash included in other current assets in the condensed consolidated balance sheets consists primarily of employee contributions to the Company's employee share purchase plan held for future purchases of the Company's outstanding shares.
Restricted cash included in other non-current assets in the condensed consolidated balance sheets represents collateral held by banks for a letter of credit ("LOC") issued in connection with the leased office space in Yardley, Pennsylvania and LOCs issued in connection with the leased office and lab spaces in Cambridge, Massachusetts and Pittsburgh, Pennsylvania. See Note 11, ‘‘Commitments and Contingencies’’ for additional information on the real estate leases.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
2. Summary of Significant Accounting Policies (Continued)
The following represents a reconciliation of cash and cash equivalents in the condensed consolidated balance sheets to total cash, cash equivalents and restricted cash as of June 30, 2024 and June 30, 2023, respectively, in the condensed consolidated statements of cash flows:
| | | | | | | | | | | | | | |
| | As of June 30, 2024 | | As of June 30, 2023 |
| Cash and cash equivalents | | $ | 239,147 | | | $ | 147,612 | |
| Restricted cash held on behalf of Former Parent | | — | | | 40,415 | |
| Restricted cash (included in other current assets) | | 156 | | | 11,574 | |
| Restricted cash (included in other non-current assets) | | 3,015 | | | 2,349 | |
| Total cash, cash equivalents and restricted cash at the end of the period in the condensed consolidated statement of cash flows | | $ | 242,318 | | | $ | 201,950 | |
Forward Contracts and Derivative Liabilities
The Company evaluates certain of its financial and business development transactions to determine the accounting classification of equity linked instruments issued in those transactions. The Company first assesses whether a freestanding equity linked instrument meets liability classification in accordance with ASC 480-10 Distinguishing Liabilities from Equity and ASC 815-40 Derivatives and Hedging - Contracts in Entity's Own Equity. Under ASC 480-10 an instrument is considered liability classified if the instrument is mandatorily redeemable, obligate the issuer to settle an instrument or the underlying shares by paying cash or other assets, or must or may require settlement by issuing variable number of shares.
If an instrument does not meet liability classification under ASC 480-10, the Company assesses the requirements under ASC 815-40, which states that contracts that require or may require net cash settlement are liabilities recorded at fair value. If the instrument does not require liability classification under ASC 815-40, in order to conclude equity classification, the Company assesses whether the instrument is indexed to its common stock and whether the instrument is classified as equity under ASC 815-40 or other applicable GAAP.
Equity linked instruments that are accounted for in accordance with ASC 815-40 are reported as liabilities on the condensed consolidated balance sheets at fair
value. Any change in fair value, as determined at each measurement period, is recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. Changes in fair value are reported on the condensed consolidated statements of cash flows as change in fair value of forward contract and derivative liabilities.
The Company accounted for certain consideration agreed to in connection with the Amendment, dated as of May 1, 2024 (the "Knopp Amendment"), to the Membership Interest Purchase Agreement, dated as of February 24, 2022 (the "Purchase Agreement") entered into with Knopp Biosciences, LLC ("Knopp") as derivative liabilities under ASC 815 (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements").
Warrants
The Company evaluates warrants issued as either equity instruments, liabilities or derivative liabilities in accordance with ASC Topic 480, Distinguishing Liabilities from Equity ("ASC 480") and/or ASC Topic 815, Derivatives and Hedging ("ASC 815"), depending on the specific terms of the warrant agreement.
The Company assessed the warrants issued to Knopp in May 2024 (the "Knopp Warrants") and concluded that they met the criteria for equity classification under ASC 480 and ASC 815. Accordingly, the Knopp Warrants were recorded within additional paid-in capital on the condensed consolidated balance sheet at the time of issuance and are not subject to remeasurement. See Note 6, Shareholders' Equity for further detail on the Knopp Warrants.
Fair Value Measurements
Certain assets and liabilities of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
2. Summary of Significant Accounting Policies (Continued)
hierarchy, of which the first two are considered observable and the last is considered unobservable:
•Level 1—Quoted prices in active markets for identical assets or liabilities.
•Level 2—Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
•Level 3—Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
The carrying values of other current assets, accounts payable, and accrued expenses approximate their fair values due to the short-term nature of these assets and liabilities.
The Company has accounted for certain consideration agreed to in connection with the Knopp Amendment as forward contract liabilities, which are recorded on the condensed consolidated balance sheets at fair value. Any changes in fair value, as determined each measurement period, are recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. The fair value of the forward contract liabilities is determined based on significant inputs not observable in the market, and therefore represents a Level 3 measurement within the fair value hierarchy. The valuations are based on a Monte Carlo simulation of the Company's share price, which requires judgement and assumption on the volatility of
Biohaven's share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk since payable in a variable number of shares. (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements").
Recently Issued Accounting Pronouncements
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting—Improvements to Reportable Segment Disclosures, which improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses. The amendments in ASU No. 2023-07 apply to public entities, including those with a single reportable segment, and are effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact ASU No. 2023-07 will have on its consolidated financial statements
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, to improve the transparency of income tax disclosures by requiring consistent categories and greater disaggregation of information in the rate reconciliation and income taxes paid disaggregated by jurisdiction. The ASU also includes certain other amendments to improve the effectiveness of income tax disclosures. The amendments in ASU 2023-09 are effective for fiscal years beginning after December 15, 2024, with early adoption permitted for annual financial statements that have not yet been issued or made available for issuance. The Company is currently evaluating the impact ASU No. 2023-09 will have on its consolidated financial statements.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
3. Marketable Securities
The amortized cost, gross unrealized holding gains, gross unrealized holding losses and fair value of debt securities available-for-sale by type of security at June 30, 2024 and December 31, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Amortized Cost | | Allowance for Credit Losses | | Net Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| June 30, 2024 | | | | | | | | | | | | |
Debt securities | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
U.S. treasury bills | | $ | 234,748 | | | $ | — | | | $ | 234,748 | | | $ | 2 | | | $ | (5) | | | $ | 234,745 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| December 31, 2023 | | | | | | | | | | | | |
Debt securities | | | | | | | | | | | | |
U.S. corporate bonds | | $ | 46,228 | | | $ | — | | | $ | 46,228 | | | $ | 7 | | | $ | (24) | | | $ | 46,211 | |
Foreign corporate bonds | | 7,180 | | | — | | | 7,180 | | | — | | | (7) | | | 7,173 | |
| | | | | | | | | | | | |
U.S. treasury bills | | 113,908 | | | — | | | 113,908 | | | 27 | | | — | | | 113,935 | |
| | | | | | | | | | | | |
| Total | | $ | 167,316 | | | $ | — | | | $ | 167,316 | | | $ | 34 | | | $ | (31) | | | $ | 167,319 | |
The fair value of debt securities available-for-sale by classification in the condensed consolidated balance sheets was as follows:
| | | | | | | | | | | | | | | | |
| | June 30, 2024 | | December 31, 2023 | | |
| Cash and cash equivalents | | $ | 36,944 | | | $ | 33,902 | | | |
| Marketable securities | | 197,801 | | | 133,417 | | | |
| Total | | $ | 234,745 | | | $ | 167,319 | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
The net amortized cost and fair value of debt securities available-for-sale at June 30, 2024 and December 31, 2023 are shown below by contractual maturity. Actual maturities may differ from contractual maturities because securities may be restructured, called or prepaid, or the Company intends to sell a security prior to maturity.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | June 30, 2024 | | December 31, 2023 |
| | Net Amortized Cost | | Fair Value | | Net Amortized Cost | | Fair Value |
| Due to mature: | | | | | | | | |
| Less than one year | | $ | 234,748 | | | $ | 234,745 | | | $ | 167,316 | | | $ | 167,319 | |
| | | | | | | | |
| | | | | | | | |
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
3. Marketable Securities (Continued)
Summarized below are the debt securities available-for-sale the Company held at June 30, 2024 and December 31, 2023 that were in an unrealized loss position, aggregated by the length of time the investments have been in that position:
| | | | | | | | | | | | | | | | | | | | |
| | Less than 12 months |
| | Number of Securities | | Fair Value | | Unrealized Losses |
| June 30, 2024 | | | | | | |
| Debt securities | | | | | | |
| | | | | | |
| | | | | | |
| U.S. treasury bills | | 11 | | | $ | 133,504 | | | $ | (5) | |
| | | | | | |
| | | | | | |
| | | | | | |
| December 31, 2023 | | | | | | |
| Debt securities | | | | | | |
| U.S. corporate bonds | | 6 | | | $ | 29,537 | | | $ | (24) | |
| Foreign corporate bonds | | 1 | | | 7,173 | | | (7) | |
| | | | | | |
| | | | | | |
| | | | | | |
| Total | | 7 | | | $ | 36,710 | | | $ | (31) | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
The Company did not have any investments in a continuous unrealized loss position for more than twelve months as of June 30, 2024 or December 31, 2023.
The Company reviewed the securities in the table above and concluded that they are performing assets, considering factors such as the credit quality of the investment security based on research performed by external rating agencies and the prospects of realizing the carrying value of the security based on the investment’s current prospects for recovery. As of June 30, 2024, the Company did not intend to sell these securities and did not believe it was more likely than not that it would be required to sell these securities prior to the anticipated recovery of their amortized cost basis.
Net Investment Income
Gross investment income includes income from debt securities available-for-sale, money-market funds, cash and restricted cash. Net investment income included in other income, net in the condensed consolidated statements of operations and comprehensive loss for the three and six months ended June 30, 2024 and 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2024 | | 2023 | | 2024 | | 2023 |
Debt securities (including realized losses) | | $ | 3,000 | | | $ | 2,225 | | | $ | 5,069 | | | $ | 5,634 | |
Other investments | | 2,140 | | | 1,941 | | | 4,402 | | | 2,727 | |
Gross investment income (including realized losses) | | 5,140 | | | 4,166 | | | 9,471 | | | 8,361 | |
Investment expenses | | (40) | | | (68) | | | (70) | | | (138) | |
| Net investment income | | $ | 5,100 | | | $ | 4,098 | | | $ | 9,401 | | | $ | 8,223 | |
We utilize the specific identification method in computing realized gains and losses. The proceeds from the sale of available-for-sale debt securities and the related gross realized capital losses for the three and six months ended June 30, 2024 and 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2024 | | 2023 | | 2024 | | 2023 |
| Proceeds from sales | | $ | — | | | $ | 2,464 | | | $ | — | | | $ | 4,920 | |
| | | | | | | | |
| Gross realized capital losses | | $ | — | | | $ | 17 | | | $ | — | | | $ | 39 | |
BIOHAVEN PHARMACEUTICAL HOLDING COMPANY LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
4. Fair Value of Financial Assets and Liabilities
The preparation of the Company’s condensed consolidated financial statements in accordance with GAAP requires certain assets and liabilities to be reflected at their fair value and others to be reflected on another basis, such as an adjusted historical cost basis. In this note, the Company provides details on the fair value of financial assets and liabilities and how it determines those fair values.
Financial Instruments Measured at Fair Value on the Condensed Consolidated Balance Sheets
Certain assets of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable:
•Level 1 — Quoted prices in active markets for identical assets or liabilities.
•Level 2 — Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
•Level 3 — Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
4. Fair Value of Financial Assets and Liabilities (Continued)
Financial assets measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 and December 31, 2023 and financial liabilities measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 were as follows :
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Fair Value Measurement Using: |
| Balance Sheet Classification | | Type of Instrument | | Level 1 | | Level 2 | | Level 3 | | Total |
| June 30, 2024 | | | | | | | | | | |
| Assets: | | | | | | | | | | |
| Cash and cash equivalents | | Money market funds | | $ | 113,379 | | | $ | — | | | $ | — | | | $ | 113,379 | |
Cash and cash equivalents | | U.S. treasury bills | | — | | | 36,944 | | | — | | | 36,944 | |
| | | | | | | | | | |
| Marketable securities | | U.S. treasury bills | | 29,701 | | | 168,100 | | | — | | | 197,801 | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| Other non-current assets | | Money market funds | | 2,515 | | | — | | | — | | | 2,515 | |
| Total assets | | | | $ | 145,595 | | | $ | 205,044 | | | $ | — | | | $ | 350,639 | |
| | | | | | | | | | |
| Liabilities: | | | | | | | | | | |
Forward contract and derivative liabilities | | Forward contract, current and written put option, current | | $ | — | | | $ | — | | | $ | 81,220 | | | $ | 81,220 | |
| | | | | | | | | | |
Derivative liability, non-current | | Written put option, non-current | | — | | | — | | | 12,180 | | | 12,180 | |
| | | | | | | | | | |
| Total liabilities | | | | $ | — | | | $ | — | | | $ | 93,400 | | | $ | 93,400 | |
| | | | | | | | | | |
| December 31, 2023 | | | | | | | | | | |
| Assets: | | | | | | | | | | |
| Cash and cash equivalents | | Money market funds | | $ | 59,199 | | | $ | — | | | $ | — | | | $ | 59,199 | |
| Cash and cash equivalents | | U.S. treasury bills | | — | | | 27,901 | | | — | | | 27,901 | |
| Cash and cash equivalents | | U.S. corporate bonds | | — | | | 6,001 | | | — | | | 6,001 | |
| Marketable securities | | U.S. treasury bills | | 9,874 | | | 76,160 | | | — | | | 86,034 | |
| Marketable securities | | U.S. corporate bonds | | — | | | 40,210 | | | — | | | 40,210 | |
| Marketable securities | | Foreign corporate bonds | | — | | | 7,173 | | | — | | | 7,173 | |
| Other non-current assets | | Money market funds | | 1,900 | | | — | | | — | | | 1,900 | |
| Total assets | | | | $ | 70,973 | | | $ | 157,445 | | | $ | — | | | $ | 228,418 | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | |
| | | | | | | | | | |
The Company had no financial liabilities measured at fair value on a recurring basis on the condensed consolidated balance sheets at December 31, 2023.
There were no securities transferred into or out of Level 3 during the three and six months ended June 30, 2024 or 2023.
The following is a description, including valuation methodology, of the financial assets measured at fair value on a recurring basis:
Cash Equivalents
Cash equivalents consisted of cash invested in short-term money market funds and debt securities with an original maturity of 90 days or less at the date of purchase. The carrying value of cash equivalents approximates fair value as maturities are less than three months. When quoted prices are available in an active market, cash equivalents are classified in Level 1 of the fair value hierarchy. Fair values of cash equivalent instruments that do not trade on a regular basis in active markets are classified as Level 2.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
4. Fair Value of Financial Assets and Liabilities (Continued)
Marketable Securities and Other Non-Current Assets
Quoted prices for identical assets in active markets are considered Level 1 and consist of on-the-run U.S. Treasuries and money market funds. The fair values of the Company’s Level 2 debt securities are obtained from quoted market prices of debt securities with similar characteristics, quoted prices from identical assets in inactive markets, or discounted cash flows to estimate fair value.
Forward Contract and Derivative Liabilities
In connection with the Knopp Amendment, Knopp has the option to request a one-time cash true-up payment from the Company in December 2024, as defined in the agreement (the "2024 Additional Consideration True-up"). The Company also agreed to issue to Knopp additional common shares of the Company with an approximate value of $75,000 within 60 days of the first anniversary of execution of the Knopp Amendment (the “2025 Additional Consideration”). In addition, Knopp has the option to request a one-time cash true-up payment from the Company in December 2025, as defined in the agreement (the "2025 Additional Consideration True-up"). See Note 10, "License, Acquisitions and Other Agreements," for further details.
The following table provides a roll forward of the fair value of the Company's forward contract and derivative liabilities related to the Knopp Amendment for which fair value is determined by Level 3 inputs for the three months ended June 30, 2024:
| | | | | | | | | | |
| | Carrying Value | | |
Fair value at May 1, 2024 | | $ | 93,290 | | | |
Change in fair value of derivative in other income (expense), net | | 110 | | | |
Fair value at June 30, 2024 | | $ | 93,400 | | | |
The fair value of the forward contract and derivative liabilities recognized in connection with the Knopp Amendment were determined based on significant inputs not observable in the market, and therefore represents a Level 3 measurement within the fair value hierarchy. The valuation is based on a Monte Carlo simulation of Biohaven's share price, which requires judgement and assumption on the volatility of Biohaven's share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk since payable in a variable number of shares for the 2025 Additional Consideration or potentially cash for the 2024 Additional Consideration True-up and 2025 Additional Consideration True-up. A summary of the unobservable inputs (Level 3 inputs) used in measuring the Company’s forward contract and derivative liabilities related to the Knopp Amendment as of June 30, 2024 and May 1, 2024 are as follows, presented on a weighted-average basis:
| | | | | | | | | | | | | | |
| | As of June 30, 2024 | | As of May 1, 2024 |
| | | | |
Time to payment and potential payment (years) | | 0.91 | | 1.08 |
Volatility (annual) | | 70.0 | % | | 80.0 | % |
Discount rate | | 15.6 | % | | 14.8 | % |
Our expectations of the volatility of Biohaven's share price at the reporting date could be materially different than our actual future volatility, and if so, would mean the estimated fair value could be significantly higher or lower than the fair value determined. The Company expects the fair value of the forward contract to be approximately $75,000 on the first anniversary of the Knopp Amendment. An increase in the derivative liabilities related to the 2024 Additional Consideration True-up and 2025 Additional Consideration True-up between the reporting date and settlement date of the derivatives would have a material adverse effect on the Company's financial performance.
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
5. Balance Sheet Components
Property and Equipment, Net
Property and equipment, net consisted of the following:
| | | | | | | | | | | | | | | | |
| | As of June 30, 2024 | | As of December 31, 2023 | | |
| Building and land | | $ | 14,078 | | | $ | 11,728 | | | |
Leasehold improvements | | 824 | | | 802 | | | |
| Computer hardware and software | | 875 | | | 875 | | | |
| Office and lab equipment | | 11,027 | | | 9,961 | | | |
| Furniture and fixtures | | 1,793 | | | 1,550 | | | |
| | $ | 28,597 | | | $ | 24,916 | | | |
| Accumulated depreciation | | (10,201) | | | (8,283) | | | |
| | 18,396 | | | 16,633 | | | |
| Equipment not yet in service | | 269 | | | 558 | | | |
| Property and equipment, net | | $ | 18,665 | | | $ | 17,191 | | | |
Depreciation expense was $977 and $1,918 for the three and six months ended June 30, 2024, respectively, and $800 and $1,564 for the three and six months ended June 30, 2023, respectively.
Equipment not yet in service primarily consisted of lab equipment that had not been placed into service as of June 30, 2024 and December 31, 2023.
Other Non-current Assets
Other non-current assets consisted of the following:
| | | | | | | | | | | | | | | |
| | As of June 30, 2024 | | As of December 31, 2023 | |
| | | | | |
| | | | | |
| | | | | |
| Operating lease right-of-use assets | | $ | 29,903 | | | $ | 31,385 | | |
| Other | | 3,015 | | | 2,400 | | |
| Other non-current assets | | $ | 32,918 | | | $ | 33,785 | | |
Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following:
| | | | | | | | | | | | | | |
| | As of June 30, 2024 | | As of December 31, 2023 |
| | | | |
| Accrued employee compensation and benefits | | $ | 10,845 | | | $ | 837 | |
| Accrued clinical trial costs | | 36,446 | | | 29,501 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
Operating lease liabilities - current portion | | 3,550 | | | 3,308 | |
| Other accrued expenses and other current liabilities | | 6,887 | | | 6,200 | |
| Accrued expenses and other current liabilities | | $ | 57,728 | | | $ | 39,846 | |
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
6. Shareholders' Equity
Changes in shareholders’ equity for the three and six months ended June 30, 2024 and June 30, 2023 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Shares | | | | | | | | | | |
| | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Loss | | Total Shareholders' Equity |
| Balances as of December 31, 2023 | | 81,115,723 | | | $ | 887,528 | | | | | $ | 39,804 | | | $ | (499,292) | | | $ | (65) | | | $ | 427,975 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Net loss | | — | | | — | | | | | — | | | (179,504) | | | — | | | (179,504) | |
Issuance of common shares as payment for acquisition of IPR&D asset | | 242,958 | | | 10,347 | | | | | — | | | — | | | — | | | 10,347 | |
Issuance of common shares as payment under license and other agreements | | 97,233 | | | 5,637 | | | | | — | | | — | | | — | | | 5,637 | |
| Issuance of common shares under 2022 Equity Incentive Plan | | 351,307 | | | 7,452 | | | | | (5,296) | | | — | | | — | | | 2,156 | |
| Non-cash share-based compensation expense | | — | | | — | | | | | 34,877 | | | — | | | — | | | 34,877 | |
| Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (41) | | | (41) | |
| Balances as of March 31, 2024 | | 81,807,221 | | | 910,964 | | | | | 69,385 | | | (678,796) | | | (106) | | | 301,447 | |
| Net loss | | — | | | — | | | | | — | | | (319,771) | | | — | | | (319,771) | |
| Issuance of common shares, net of offering costs | | 8,544,951 | | | 317,720 | | | | | — | | | — | | | — | | | 317,720 | |
| Issuance of common shares as payment for acquisition of IPR&D asset | | 10,452 | | | 446 | | | | | — | | | — | | | — | | | 446 | |
| Issuance of common shares as payment under license and other agreements | | 1,872,874 | | | 65,981 | | | | | — | | | — | | | — | | | 65,981 | |
| Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 110,834 | | | 3,442 | | | | | (1,125) | | | — | | | — | | | 2,317 | |
| Issuance of warrant as payment under license agreement | | — | | | — | | | | | 3,340 | | | — | | | — | | | 3,340 | |
| Non-cash share-based compensation expense | | — | | | — | | | | | 12,232 | | | — | | | — | | | 12,232 | |
| Other comprehensive income | | — | | | — | | | | | — | | | — | | | 28 | | | 28 | |
| Balances as of June 30, 2024 | | 92,346,332 | | | $ | 1,298,553 | | | | | $ | 83,832 | | | $ | (998,567) | | | $ | (78) | | | $ | 383,740 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
6. Shareholders' Equity (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Shares | | | | | | | | | | |
| | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income | | Total Shareholders' Equity |
Balances as of December 31, 2022 | | 68,190,479 | | | $ | 615,742 | | | | | $ | 13,869 | | | $ | (91,124) | | | $ | 284 | | | $ | 538,771 | |
| Net loss | | — | | | — | | | | | — | | | (70,492) | | | — | | | (70,492) | |
| Issuance of common shares under 2022 Equity Incentive Plan | | 22,000 | | | 504 | | | | | (172) | | | — | | | — | | | 332 | |
Non-cash share-based compensation expense | | — | | | — | | | | | 3,765 | | | — | | | — | | | 3,765 | |
| Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (118) | | | (118) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Balances as of March 31, 2023 | | 68,212,479 | | | 616,246 | | | | | 17,462 | | | (161,616) | | | 166 | | | 472,258 | |
| Net loss | | — | | | — | | | | | — | | | (90,346) | | | — | | | (90,346) | |
| Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 104,474 | | | 1,264 | | | | | (470) | | | — | | | — | | | 794 | |
| Non-cash share-based compensation expense | | — | | | — | | | | | 4,695 | | | — | | | — | | | 4,695 | |
| Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (146) | | | (146) | |
Balance as of June 30, 2023 | | 68,316,953 | | | $ | 617,510 | | | | | $ | 21,687 | | | $ | (251,962) | | | $ | 20 | | | $ | 387,255 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Knopp Amendment
In May 2024, the Company entered into the Knopp Amendment under which the parties thereto agreed to revise the success-based payment and royalty payment obligations under the Membership Purchase Agreement. As consideration, the Company issued 1,872,874 Biohaven Shares to Knopp, valued at approximately $65,981 in May 2024.
As further consideration for the revisions to the success-based payment and royalty payment obligations in the Knopp Amendment, the Company issued to Knopp a warrant to purchase 294,195 of the Company's common shares with a purchase price of $67.98, subject to certain specified development milestones and the Company achieving a specified market capitalization.
The warrant was recorded at its initial fair value of $3,340 within additional paid-in capital on the condensed consolidated balance sheet as of June 30,2024 and is not subject to remeasurement. See Note 10 for further detail on the Knopp Amendment.
April 2024 Public Offering
On April 22, 2024, the Company closed an underwritten public offering of 6,451,220 of its common shares, which included the exercise in full of the underwriters' option to purchase additional shares, at a price of $41.00 per share. The net proceeds raised in the offering, after deducting underwriting discounts and expenses of the offering payable by Biohaven, were approximately $247,830. The Company intends to use
the net proceeds received from the offering for general corporate purposes.
Pyramid Acquisition
In January 2024, the Company acquired Pyramid pursuant to the Pyramid Agreement. In consideration for the Pyramid acquisition, Biohaven made an upfront payment of 255,794 Company common shares, valued at approximately $10,894. As of June 30, 2024, 253,410 of these common shares have been issued by the Company.
During the first quarter of 2024, the Company recorded $5,689 of R&D expense in the condensed consolidated statement of operations and comprehensive loss for a developmental milestone which became due under the Pyramid Agreement, to be paid in 98,129 Company common shares. As of June 30, 2024, 97,233 of these common shares have been issued by the Company. Refer to Note 10, "License, Acquisitions and Other Agreements" for further discussion of the Pyramid acquisition.
Equity Distribution Agreement
In October 2023, the Company entered into an equity distribution agreement pursuant to which the Company may offer and sell common shares having an aggregate offering price of up to $150,000 from time to time through or to the sales agent, acting as its agent or principal (the "Equity Distribution Agreement"). Sales of the Company's common shares, if any, will be made in sales deemed to be “at-the-market offerings”. The sales agent is not required to sell any specific amount of
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
6. Shareholders' Equity (Continued)
securities but will act as the Company's sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between the sales agent and the Company. The Company currently plans to use the net proceeds from any at-the-market offerings of its common shares for general corporate purposes.
As of June 30, 2024, the Company sold and issued 2,093,731 common shares under the Equity Distribution Agreement for net proceeds of approximately $69,890. Subsequent to June 30, 2024, the Company issued and sold an additional 2,154,857 common shares for net proceeds of $76,360. As of August 9, 2024, the date of this report, the Company issued and sold a total of 4,248,588 common shares under the Equity Distribution Agreement for total net proceeds of $146,250, and no shares remain available to be issued.
7. Accumulated Other Comprehensive (Loss) Income
Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2024:
| | | | | | | | | | | | | | |
| | Three Months Ended June 30, 2024 | | Six Months Ended June 30, 2024 |
| | | | |
| Net unrealized investment gains (losses): | | | | |
| Beginning of period balance | | $ | (32) | | | $ | 3 | |
| | | | |
| | | | |
Other comprehensive income (loss)(1)(2) | | 29 | | | (6) | |
| End of period balance | | (3) | | | (3) | |
| | | | |
| Foreign currency translation adjustments: | | | | |
| Beginning of period balance | | (74) | | | (68) | |
Other comprehensive loss(1) | | (1) | | | (7) | |
| | | | |
| End of period balance | | (75) | | | (75) | |
| | | | |
Total beginning of period accumulated other comprehensive loss | | (106) | | | (65) | |
Total other comprehensive income (loss) | | 28 | | | (13) | |
Total end of period accumulated other comprehensive loss | | $ | (78) | | | $ | (78) | |
| | | | |
(1) There was no tax on other comprehensive loss and no amounts reclassified from accumulated other comprehensive (loss) income during the period.
BIOHAVEN PHARMACEUTICAL HOLDING COMPANY LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
7. Accumulated Other Comprehensive (Loss) Income (Continued)
Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2023:
| | | | | | | | | | | | | | |
| | Three Months Ended June 30, 2023 | | Six Months Ended June 30, 2023 |
| | | | |
| Net unrealized investment gains (losses): | | | | |
| Beginning of period balance | | $ | (278) | | | $ | (145) | |
Other comprehensive loss before reclassifications(1) | | (1) | | | (156) | |
Amounts reclassified from accumulated other comprehensive loss(1)(2) | | 17 | | | 39 | |
Other comprehensive income (loss)(1) | | 16 | | | (117) | |
| End of period balance | | (262) | | | (262) | |
| | | | |
| Foreign currency translation adjustments: | | | | |
| Beginning of period balance | | 444 | | | 429 | |
Other comprehensive loss(1) | | (162) | | | (147) | |
| | | | |
| End of period balance | | 282 | | | 282 | |
| | | | |
Total beginning of period accumulated other comprehensive income | | 166 | | | 284 | |
Total other comprehensive loss | | (146) | | | (264) | |
Total end of period accumulated other comprehensive income | | $ | 20 | | | $ | 20 | |
| | | | |
(1) There was no tax on other comprehensive income (loss) and immaterial tax on amounts reclassified from accumulated other comprehensive income (loss) during the period.
(2) Amounts reclassified from accumulated other comprehensive income (loss) for specifically identified debt securities are included in other income (expense), net on the condensed consolidated statement of operations and comprehensive loss.
8. Non-Cash Share-Based Compensation
Non-Cash Share-based Compensation Expense
The Company measures non-cash share-based compensation at the grant date based on the fair value of the award and recognizes non-cash shared-based compensation as expense over the requisite service period of the award (generally three years) using the straight-line method. Non-cash share-based compensation expense, consisting of expense for share options, restricted share units ("RSUs"), performance share options, and the Employee Share Purchase Plan ("ESPP"), was classified in the condensed consolidated statements of operations and comprehensive loss as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2024 | | 2023 | | 2024 | | 2023 |
Research and development expenses | | $ | 7,071 | | | $ | 2,457 | | | $ | 28,362 | | | $ | 4,698 | |
General and administrative expenses | | 5,161 | | | 2,238 | | | 18,747 | | | 3,762 | |
| Total non-cash share-based compensation expense | | $ | 12,232 | | | $ | 4,695 | | | $ | 47,109 | | | $ | 8,460 | |
Share Options
All share option grants are awarded at fair value on the date of grant. The fair value of share options is estimated using the Black-Scholes option pricing model. Stock options generally expire 10 years after the grant date.
The aggregate intrinsic value of share options is calculated as the difference between the exercise price of the share options and the fair value of the Company's common shares for those share options that had exercise prices lower than the fair value of the Company's common shares at June 30, 2024.
As of June 30, 2024, total unrecognized compensation cost related to the unvested share options was $90,206, which is expected to be recognized over a weighted average period of 2.33 years, which does not consider the impact of a change in control. The weighted average grant date fair value per share of share options granted under the Company's share option plan during the six months ended June 30, 2024 and 2023 was $30.76 and $10.16, respectively. The Company expects
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
8. Non-Cash Share-Based Compensation (Continued)
approximately 7,978,678 of the unvested stock options to vest over the requisite service period.
The following table is a summary of the Company's share option activity for the six months ended June 30, 2024:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Number of Shares | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term | | Aggregate Intrinsic Value |
| | | | | | | (in years) | | |
Outstanding as of December 31, 2023 | | 11,379,429 | | $ | 11.48 | | | | | |
| Granted | | 2,500,161 | | $ | 42.05 | | | | | |
| Exercised | | (295,890) | | $ | 9.00 | | | | | |
| Forfeited | | (38,684) | | $ | 20.38 | | | | | |
Outstanding as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | |
Options exercisable as of June 30, 2024 | | 5,566,338 | | $ | 13.44 | | | 8.54 | | $ | 122,803 | |
Vested and expected to vest as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | |
Restricted Share Units
The Company’s RSUs are considered nonvested share awards and require no payment from the employee. For each RSU, employees receive one common share at the end of the vesting period. The employee can elect to receive the one common share net of taxes or pay for taxes separately and receive the entire share. Compensation cost is recorded based on the market price of the Company’s common shares on the grant date and is recognized on a straight-line basis over the requisite service period.
As of June 30, 2024, there was $9,405 of total unrecognized compensation cost related to Company RSUs that are expected to vest. These costs are expected to be recognized over a weighted-average period of 2.53 years, which does not consider the impact of a change in control. The total fair value of RSUs vested during the three and six months ended June 30, 2024 was $3,770.
The following table is a summary of the RSU activity for the six months ended June 30, 2024:
| | | | | | | | | | | | | | |
| | Number of shares | | Weighted Average Grant Date Fair Value |
Unvested as of December 31, 2023 | | — | | | $ | — | |
Granted | | 356,011 | | | $ | 42.34 | |
Forfeited | | (3,280) | | | $ | 41.93 | |
Vested | | (89,059) | | | $ | 42.34 | |
Unvested as of June 30, 2024 | | 263,672 | | | $ | 42.34 | |
BIOHAVEN PHARMACEUTICAL HOLDING COMPANY LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
9. Net Loss Per Share
Basic and diluted net loss per share attributable to common shareholders of Biohaven was calculated as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended June 30, | | Six Months Ended June 30, |
| | 2024 | | 2023 | | 2024 | | 2023 |
| Numerator: | | | | | | | | |
| | | | | | | | |
| Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (499,275) | | | $ | (160,838) | |
| Denominator: | | | | | | | | |
Weighted average common shares outstanding—basic and diluted | | 87,766,069 | | | 68,248,023 | | | 84,174,099 | | | 68,227,564 | |
| Net loss per share — basic and diluted | | $ | (3.64) | | | $ | (1.32) | | | $ | (5.93) | | | $ | (2.36) | |
The Company's potential dilutive securities include share options which have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted average number of common shares outstanding used to calculate both basic and diluted net loss per share attributable to common shareholders of the Company is the same. The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
| | | | | | | | | | | | | | |
| | | As of June 30, |
| | | 2024 | | 2023 |
| Options to purchase common shares | | 13,545,016 | | | 9,639,557 | |
| Warrants to purchase common shares | | 294,195 | | | — | |
Restricted share units | | 263,672 | | | — | |
| Total | | 14,102,883 | | | 9,639,557 | |
10. License, Acquisitions and Other Agreements
The Company has entered into various licensing, developmental and acquisition agreements which provide the Company with rights to certain know-how, technology and patent rights. The agreements generally include upfront fees, milestone payments upon achievement of certain developmental, regulatory and commercial and sales milestones, as well as sales-
based royalties, with percentages that vary by agreement.
License and Other Agreements
As of June 30, 2024, the Company has potential future developmental, regulatory and commercial milestone payments under its license and other agreements of up to approximately $140,650, $641,975, and $2,150,450, respectively. See below for a detailed discussion of these agreements. The Company has not recorded these potential contingent consideration payments as liabilities in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024.
Yale Agreements
In September 2013, the Company entered into an exclusive license agreement (the "Yale Agreement") with Yale University to obtain a license to certain patent rights for the commercial development, manufacture, distribution, use and sale of products and processes resulting from the development of those patent rights, related to the use of riluzole in treating various neurological conditions, such as general anxiety disorder, post-traumatic stress disorder and depression.
The Yale Agreement was amended and restated in May 2019. As of June 30, 2024, under the amended Yale Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $2,000 and annual royalty payments of a low single-digit percentage based on net sales of riluzole-based products from the licensed patents or from products based on troriluzole. Under the amended and restated agreement, the royalty rates are reduced as compared to the original agreement. In addition, under the amended and restated agreement, the Company may develop products based on riluzole or troriluzole. The amended and restated agreement retains a minimum annual royalty of up to $1,000 per year, beginning after the first sale of product under the agreement. If the Company grants any sublicense rights under the Yale Agreement, it must pay Yale University a low single-digit percentage of sublicense income that it receives.
For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the Yale Agreement.
In January 2021, the Company entered into a worldwide, exclusive license agreement with Yale
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
10. License, Acquisitions and Other Agreements (Continued)
University for the development and commercialization of a novel Molecular Degrader of Extracellular Protein ("MoDE") platform (the "Yale MoDE Agreement"). The platform pertains to the clearance of disease-causing protein and other biomolecules by targeting them for lysosomal degradation using multi-functional molecules. The Yale MoDE Agreement includes an obligation to pay a minimum annual royalty of up to $1,000 per year, and low single digit royalties on the net sales of licensed products. If the Company grants any sublicense rights under the Yale MoDE Agreement, it must pay Yale University a low single-digit percentage of sublicense income that it receives. As of June 30, 2024, under the Yale MoDE Agreement, the Company has remaining contingent development and commercial milestone payments of up to $650 and $2,950, respectively. The Yale MoDE Agreement terminates on the later of twenty years from the effective date, twenty years from the filing date of the first investigational new drug application for a licensed product or the last to expire of a licensed patent.
The Company did not record any material milestone or royalty payments under the Yale MoDE Agreement for the three months ended June 30, 2024. For the six months ended June 30, 2024, the Company recorded research and development expense of $150 related to the achievement of a developmental milestone under the Yale MoDE Agreement. For the three and six months ended June 30, 2023, the Company did not record any material milestone or royalty payments under the Yale MoDE Agreement.
ALS Biopharma Agreement
In August 2015, the Company entered into an agreement (the "ALS Biopharma Agreement") with ALS Biopharma and Fox Chase Chemical Diversity Center Inc. ("FCCDC"), pursuant to which ALS Biopharma and FCCDC assigned the Company their worldwide patent rights to a family of over 300 prodrugs of glutamate modulating agents, including troriluzole, as well as other innovative technologies. Under the ALS Biopharma Agreement, the Company is obligated to use commercially reasonable efforts to commercialize and develop markets for the patent products. As of June 30, 2024, under the ALS Biopharma Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $4,000, as well as royalty payments of a low single-digit percentage based on net sales of products licensed under the ALS Biopharma Agreement, payable on a quarterly basis.
The ALS Biopharma Agreement terminates on a country-by-country basis as the last patent rights expire
in each such country. If the Company abandons its development, research, licensing or sale of all products covered by one or more claims of any patent or patent application assigned under the ALS Biopharma Agreement, or if the Company ceases operations, it has agreed to reassign the applicable patent rights back to ALS Biopharma.
For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the ALS Biopharma Agreement.
Taldefgrobep Alfa License Agreement
In February 2022, following the transfer of intellectual property, the Company announced that it entered into a worldwide license agreement with BMS for the development and commercialization rights to taldefgrobep alfa (also known as BMS-986089), a novel, Phase 3-ready anti-myostatin adnectin (the "Taldefgrobep Alfa License Agreement").
As of June 30, 2024, under the Taldefgrobep Alfa License Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $200,000, as well as tiered, sales-based royalty percentages from the high teens to the low twenties. There were no upfront or contingent payments to BMS related to the Taldefgrobep Alfa License Agreement.
For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the Taldefgrobep Alfa License Agreement.
Agreement with Hangzhou Highlightll Pharmaceutical Co. Ltd.
In March 2023, the Company and Hangzhou Highlightll Pharmaceutical Co. Ltd. ("Highlightll") entered into an exclusive, worldwide (excluding People’s Republic of China and its territories and possessions) license agreement (the "Highlightll Agreement") pursuant to which Biohaven obtained the right to research, develop, manufacture and commercialize Highlightll’s brain penetrant dual TYK2/JAK1 inhibitor program. In connection with the Highlightll Agreement, the Company was obligated to pay Highlightll a cash payment of $10,000 and 721,136 common shares (collectively, "the Highlightll Upfront Payments"), upon the completion of certain post-closing activities. In December 2023, the Company entered into a second amendment to the Highlightll Agreement, which granted the Company an exclusive option and right of first refusal to any Selective TYK2 Inhibitor being developed
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
10. License, Acquisitions and Other Agreements (Continued)
by or on behalf of Highlightll or its affiliates and provided for the payment of the Highlightll Upfront Payments. As a result, the Company made a $10,000 cash payment and issued 721,136 shares, valued at $21,814 to Highlightll during the fourth quarter of 2023, which was recorded as R&D expense during the fourth quarter of 2023.
As of June 30, 2024, under the Highlightll Agreement, the Company has remaining contingent development, regulatory approval, and commercial milestone payments of up to $75,000, $37,500, and $837,500, respectively. Additionally, the Company has agreed to make tiered royalty payments as a percentage of net sales starting at mid single digits and peaking at low teens digits. During the royalty term, if the Company offers to include China clinical sites in its Phase 3 study sufficient for submission to Chinese National Medical Products Administration and Highlightll, at its sole discretion, agrees, then Highlightll will pay royalties in the low tens digits to the Company on China sales upon approval.
The Highlightll Agreement terminates on a country-by-country basis upon expiration of the royalty term and can also be terminated if certain events occur, e.g., material breach or insolvency.
For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments related to the Highlightll Agreement.
Other Agreements
In addition to the agreements detailed above, the Company has entered into various other license agreements and development programs. The Company records milestones and other payments, including funding for research arrangements, which become due under these agreements to research and development expense in the condensed consolidated statements of operations and comprehensive loss. Amounts recorded for the period were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended June 30, | Six Months Ended June 30, |
| | | 2024 | | 2023 | 2024 | | 2023 |
Milestone payments | | $ | 375 | | | $ | — | | $ | 1,875 | | | $ | — | |
| | | | | | | |
For the three and six months ended June 30, 2024 and 2023, the Company did not make any upfront payments under these agreements.
Acquisitions
Kv7 Platform Acquisition
In April 2022, the Company closed the acquisition from Knopp of Channel Biosciences, LLC (“Channel”), a wholly owned subsidiary of Knopp owning the assets of Knopp’s Kv7 channel targeting platform (the “Kv7 Platform Acquisition”), pursuant to the Purchase Agreement, dated February 24, 2022.
Under the Purchase Agreement, the Company agreed to make success-based payments based on developmental and regulatory milestones through approvals in the United States, Europe, the Middle East and Asia ("EMEA") and Japan for the lead asset, BHV-7000 (formerly known as KB-3061), developmental and regulatory milestones for the Kv7 pipeline development in other indications and additional country approvals, and commercial sales-based milestones of BHV-7000. Additionally, the Company agreed to make scaled royalty payments in cash for BHV-7000 and the pipeline programs, with percentages starting at high single digits and peaking at low teens for BHV-7000 and starting at mid-single digits and peaking at low tens digits for the pipeline programs.
In May 2024, the Company entered into the Knopp Amendment under which the parties thereto agreed to replace the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for BHV-7000 and the pipeline programs. The parties also agreed to reduce the success-based payments payable under the Purchase Agreement. The Company retains the ability to pay these contingent milestone payments in cash or in the Company's common shares at Biohaven's election, subject to the same increases if the Company elects to pay in the Company's common shares. As of June 30, 2024, under the Purchase Agreement, as amended, the Company had remaining success-based payments comprised of (i) to up to $185,000 based on regulatory approvals in the United States and EMEA for BHV-7000 and (ii) up to an additional $60,000 based on regulatory approval in the United States for the other Kv7 pipeline programs.
In consideration of the revisions to the success-based payment and royalty payment obligations, the Company agreed to issue to Knopp 1,872,874 Company common shares, valued at approximately $75,000, through a private placement within 60 days of the date of execution of the Knopp Amendment (the “2024 Additional Consideration”) and additional Company common shares with an approximate value of $75,000 within 60 days of the first anniversary of execution of the
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
10. License, Acquisitions and Other Agreements (Continued)
Knopp Amendment (the “2025 Additional Consideration”). The Company has also given Knopp the option to request a one-time cash true-up payment from the Company in December 2024 in the event that Knopp continues to hold the Company's common shares representing the 2024 Additional Consideration and the value of such shares has declined (the "2024 Knopp True-Up"), and a one-time cash true-up payment from the Company in December 2025 in the event that Knopp continues to hold the Company's common shares representing the 2025 Additional Consideration and the value of such shares has declined (the "2025 Knopp True-Up"), in each case, subject to certain conditions.
The Company concluded that the agreement to issue the 2024 Additional Consideration at a future date represented a fixed forward contract under ASC 815 and classified the commitment as a forward contract liability on its condensed consolidated balance sheet on the execution date of the Knopp Amendment. The Company initially measured the forward contract associated with the 2024 Additional Consideration at a fair value of $75,220, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. In May 2024, the Company issued the 2024 Additional Consideration at an approximate value of $65,981 and recognized a $9,239 gain upon settlement of the forward contract in other income (expense), net in its condensed consolidated statement of operations and comprehensive loss during the three months ended June 30, 2024. The gain on settlement of the 2024 Additional Consideration is due to the decline in fair value of the 2024 Additional Consideration from the execution date to the issuance date due to a decline in Biohaven's share price. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" and Note 6, "Shareholders' Equity" for further discussion.
The 2024 Additional Consideration True-up represents a net cash settled written put option measured at fair value on a recurring basis. The Company has concluded that the 2024 Additional Consideration True-up represents a net cash settled written put option on the Company’s shares and is a freestanding derivative liability under ASC 815. Accordingly, the Company classified the 2024 Additional Consideration True-up as a current derivative liability on its condensed consolidated balance sheet. The Company initially recorded the 2024 Additional Consideration True-up at a fair value of $15,540, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. The Company subsequently remeasures the fair value of the derivative
liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized expense of $590 for the three months ended June 30, 2024, related to the 2024 Additional Consideration True-up. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion.
The Company has concluded that the agreement to issue the 2025 Additional Consideration at a future date represents a forward contract settleable in a variable number of shares under ASC 480, and classified the commitment as a current forward contract liability on its condensed consolidated balance sheet. The Company initially measured the 2025 Additional Consideration at a fair value of $63,940, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. The Company subsequently remeasures the fair value of the forward contract liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized expense of $1,150 for the three months ended June 30, 2024, related to the 2025 Additional Consideration. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion.
The Company has concluded that the 2025 Additional Consideration True-up represents a net cash settled written put option on the Company’s shares and is a freestanding derivative liability under ASC 815. Accordingly, the Company classified the 2025 Additional Consideration True-up as a non-current derivative liability on its condensed consolidated balance sheet. The Company initially recorded the 2025 Additional Consideration True-up at a fair value of $13,810, which was recorded as R&D expense during the three months ended June 30, 2024. The Company subsequently remeasures the fair value of the derivative liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized a gain of $1,630 for the three months ended June 30, 2024, related to the 2025 Additional Consideration True-up. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion.
As further consideration for the revisions to the success-based payment and royalty payment obligations in the Knopp Amendment, the Company issued to Knopp a warrant (the “Warrant”) to purchase 294,195 Company common shares at a purchase price per share of $67.98,
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
10. License, Acquisitions and Other Agreements (Continued)
subject to certain specified development milestones and the Company achieving a specified market capitalization. Refer to Note 6, "Shareholders' Equity" for further discussion.
The Company has not recorded any of the remaining contingent consideration payments to Knopp as a liability in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024.
Pyramid Acquisition
In January 2024, the Company acquired Pyramid Biosciences, Inc. (“Pyramid”), pursuant to an Agreement and Plan of Merger, dated January 7, 2024 ("the Pyramid Agreement"). In consideration for the Pyramid acquisition, Biohaven made an upfront payment of 255,794 Company common shares, valued at approximately $10,894.
The Company accounted for this purchase as an asset acquisition as substantially all of the fair value of the gross assets acquired was concentrated in a single identifiable asset, IPR&D. The IPR&D asset has no alternative future use and relates primarily to BHV-1510. There was no material value assigned to any other assets or liabilities acquired in the acquisition. As such, the upfront payment discussed above was recorded as a charge to R&D expense in the accompanying condensed consolidated statements of operations and comprehensive loss during the three months ended March 31, 2024.
As of June 30, 2024, under the Pyramid Agreement, the Company has remaining success-based payments comprised of (i) up to $5,000 based on developmental and regulatory milestones for the lead asset, BHV-1510 (formerly known as PBI-410), (ii) up to an additional $30,000 based on developmental and regulatory milestones for a second asset (formerly known as PBI-200) and (iii) up to $40,000 for commercial sales-based milestones of BHV-1510. Contingent developmental and regulatory milestone payments may be paid in cash or Biohaven common shares at the election of Biohaven and commercial sales-based milestones are to be made in cash.
The Company has not recorded any of the remaining contingent consideration payments as a liability in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024.
During the first quarter of 2024, the Company recorded $5,689 of R&D expense in the condensed consolidated statement of operations and comprehensive loss for a developmental milestone which became due under the Pyramid Agreement, to be paid in 98,129 common shares of the Company. See Note 6, "Shareholders' Equity" for discussion of common shares issued to as part of the Pyramid Agreement.
11. Commitments and Contingencies
Lease Agreements
The Company leases certain office and laboratory space. Other than the Pittsburgh Centre Avenue Lease described below, there have been no material changes to the lease obligations from those disclosed in Note 11, "Commitments and Contingencies" to the consolidated financial statements included in the 2023 Form 10-K.
Pittsburgh Centre Avenue Lease Agreement
In March 2024, the Company entered into a lease agreement in Pittsburgh, Pennsylvania for lab space (the "Pittsburgh Centre Avenue Lease"), which will be used to support the research and development of the Company's ion channel platform and replace the Company's current operating lease in Pittsburgh. The lease is expected to commence in mid 2025 after substantial completion of building improvements, and has a term of 122 months, with an option to extend for one additional period of 60 months. The Company expects to record the Pittsburgh Centre Avenue Lease as an operating lease. The Company has annual commitments relating to the Pittsburgh Centre Avenue Lease ranging from $1,859 to $2,373, excluding any additional tenant improvement allowance that would increase the base rent.
Research Commitments
The Company has agreements with several contract manufacturing organizations ("CMOs") and contract research organizations ("CROs") to provide products and services in connection with the Company’s preclinical studies and clinical trials. As of June 30, 2024, the Company had no remaining maximum research commitments in excess of one year.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnification of varying scope and terms to vendors, lessors, business partners and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by
BIOHAVEN LTD.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
11. Commitments and Contingencies (Continued)
third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. The Company’s amended and restated memorandum and articles of association also provide for indemnification of directors and officers in specific circumstances. To date, the Company has not incurred any material costs as a result of such indemnification provisions. The Company does not believe that the outcome of any claims under indemnification arrangements will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its condensed consolidated financial statements as of June 30, 2024 or December 31, 2023.
License, Acquisition and Other Agreements
The Company has entered into licensing, developmental and acquisition agreements with various parties under which it is obligated to make contingent and non-contingent payments (see Note 10, "License, Acquisitions and Other Agreements").
Other Agreements
Moda Agreement
On January 1, 2021, the Company entered into a consulting services agreement (the "Moda Agreement") with Moda Pharmaceuticals LLC ("Moda") to further the scientific advancement of technology, drug discovery platforms (including the technology licensed under the Yale MoDE Agreement), product candidates and related intellectual property owned or controlled by the Company.
Under the Moda Agreement, the Company paid Moda an upfront cash payment of $2,700 and 37,836 shares of the Former Parent valued at approximately $3,243. In addition, Moda will be eligible to receive additional development and regulatory milestone payments of up to $81,612 and commercial milestone payments of up to $30,171. The Moda Agreement has a term of four years and may be terminated earlier by the Company or Moda under certain circumstances including, for example, the Company's discontinuation of research on the MoDE platform or default. In August 2023, the Company entered into an amendment to the Moda Agreement with Moda. Under the amendment, Moda will be eligible to receive development and
regulatory milestone payments up to $25,200 and commercial milestone payments up to $23,000, in addition to the milestones noted above.
The Company did not record any material milestone payments related to the Moda Agreement for the three months ended June 30, 2024. For the six months ended June 30, 2024, the Company recorded research and development expense of $850 related to developmental milestones under the Moda Agreement. For the three and six months ended June 30, 2023, the Company did not record any material milestone payments related to the Moda Agreement.
Legal Proceedings
From time to time, in the ordinary course of business, the Company is subject to litigation and regulatory examinations as well as information gathering requests, inquiries and investigations. As of June 30, 2024, there were no matters which would have a material impact on the Company’s financial results.
12. Income Taxes
The following table provides a comparative summary of the Company's income tax provision and effective income tax rate for the three and six months ended June 30, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | Six Months Ended June 30, |
| | 2024 | | 2023 | 2024 | | 2023 |
Income tax provision | | $ | 177 | | | $ | 2,177 | | $ | 746 | | | $ | 3,116 | |
| Effective income tax rate | | 0.1 | % | | 2.5 | % | 0.1 | % | | 2.0 | % |
The decrease in income tax provisions for the three and six months ended June 30, 2024 as compared to the same periods in 2023 was primarily attributable to the Company adopting the guidance contained in a Notice of Proposed Rule Making issued during the third quarter of 2023 by the United States Internal Revenue Service ("the Notice"). The Notice allows the Company to immediately deduct certain R&D expenditures which were incurred in the US and reimbursed by the Company’s foreign parent. Previously, these expenditures were required to be capitalized under the Tax Cuts and Jobs Act, which was effective for tax years beginning on or after January 1, 2022.
13. Related Party Transactions
Relationship with the Former Parent
Upon the effectiveness of the Separation on October 3, 2022, the Former Parent ceased to be a related party of the Company.
On October 3, 2022, the Company entered into agreements with the Former Parent in connection with the Separation, including a Transition Services Agreement. For a full discussion of agreements entered into with the Former Parent, refer to Note 14, "Related Party Transactions" to the consolidated financial statements included in the 2023 Form 10-K. The Company did not record any material income for transition services provided to the Former Parent during the three and six months ended June 30, 2024. For the three and six months ended June 30, 2023, the Company recorded $1,674 and $5,559, respectively, in other income reflecting transition services provided to the Former Parent.
Related Party Agreements
License Agreement with Yale University
On September 30, 2013, the Company entered into the Yale Agreement with Yale University (see Note 10). The Company’s Chief Executive Officer is one of the inventors of the patents that the Company has licensed from Yale University and, as such, is entitled to a specified share of the glutamate product-related royalty revenues that may be received by Yale University under the Yale Agreement.
In January 2021, the Company entered into the Yale MoDE Agreement with Yale University (see Note 10 for details). Under the license agreement, the Company acquired exclusive, worldwide rights to Yale University's intellectual property directed to its MoDE platform. Under the Yale MoDE Agreement, the Company entered into the Yale MoDE SRA (see Note 10 for details), which included funding of up to $4,000 over the life of the agreement. In May 2023, the Company entered into an additional sponsored research agreement with Yale University (the "2023 Yale SRA"), which includes funding of up to $612 over the life of the agreement.
For the three and six months ended June 30, 2024, the Company recorded $582 and $1,027, respectively, in R&D expense, including certain administrative expenses, related to the Yale MoDE Agreement, the Yale Agreement, and the 2023 Yale SRA (collectively, the "Yale Agreements"). For the three and six months ended June 30, 2023, the Company recorded $947 and $1,699, respectively, in research and development expense,
including certain administrative expenses, related to the Yale Agreements. As of June 30, 2024, the Company did not owe any amounts to Yale University.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K for the year ended December 31, 2023 (the "2023 Form 10-K") filed with the Securities and Exchange Commission (the “SEC”). Some of the statements contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business, constitute forward looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). We have based these forward-looking statements on our current expectations and projections about future events. The following information and any forward-looking statements should be considered in light of factors discussed elsewhere in this Quarterly Report on Form 10-Q and our other filings with the SEC.
Our actual results and timing of certain events may differ materially from the results discussed, projected, anticipated, or indicated in any forward-looking statements. We caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, among other things, may differ materially from the forward-looking statements contained in this Quarterly Report on Form 10-Q. Statements made herein are as of the date of the filing of this Form 10-Q with the SEC and should not be relied upon as of any subsequent date. Even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this Quarterly Report on Form 10-Q, they may not be predictive of results or developments in future periods. We disclaim any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to reflect any change in our expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
We caution readers not to place undue reliance on any forward-looking statements made by us, which speak only as of the date they are made.
Overview
We are a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. We are advancing our innovative portfolio of therapeutics, leveraging our proven drug development experience and multiple proprietary drug development platforms. Our extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; Transient Receptor Potential Melastatin 3 ("TRPM3") antagonism for migraine and neuropathic pain; Tyrosine Kinase 2/Janus Kinase 1 ("TYK2/JAK1") inhibition for neuroinflammatory disorders; glutamate modulation for obsessive-compulsive disorder (“OCD”) and spinocerebellar ataxia ("SCA"); myostatin inhibition for neuromuscular and metabolic diseases, including spinal muscular atrophy ("SMA") and obesity; and antibody recruiting bispecific molecules ("ARMs") and antibody drug conjugates ("ADCs") for cancer.
Separation from Biohaven Pharmaceutical Holding Company Ltd.
On October 3, 2022, Biohaven Pharmaceutical Holding Company Ltd. (the “Former Parent”) completed the distribution (the “Distribution”) to holders of its common shares of all of the outstanding common shares of Biohaven Ltd. and the spin-off of Biohaven Ltd. from the Former Parent (the “Spin-Off”) described in Biohaven’s Information Statement attached as Exhibit 99.1 to Biohaven’s Registration Statement on Form 10, as amended (Reg. No. 001-41477). Collectively, we refer to the Distribution and Spin-Off throughout this Quarterly Report on Form 10-Q as the "Separation." As a result of the Separation, Biohaven Ltd. became an independent, publicly traded company as of October 3, 2022, and commenced regular way trading under the symbol “BHVN”’ on the New York Stock Exchange (the "NYSE") on October 4, 2022. Where we describe historical business activities in this report, we do so as if the Former Parent’s activities related to such assets and liabilities had been performed by the Company.
Clinical-Stage Milestones
Our clinical-stage milestones include the following: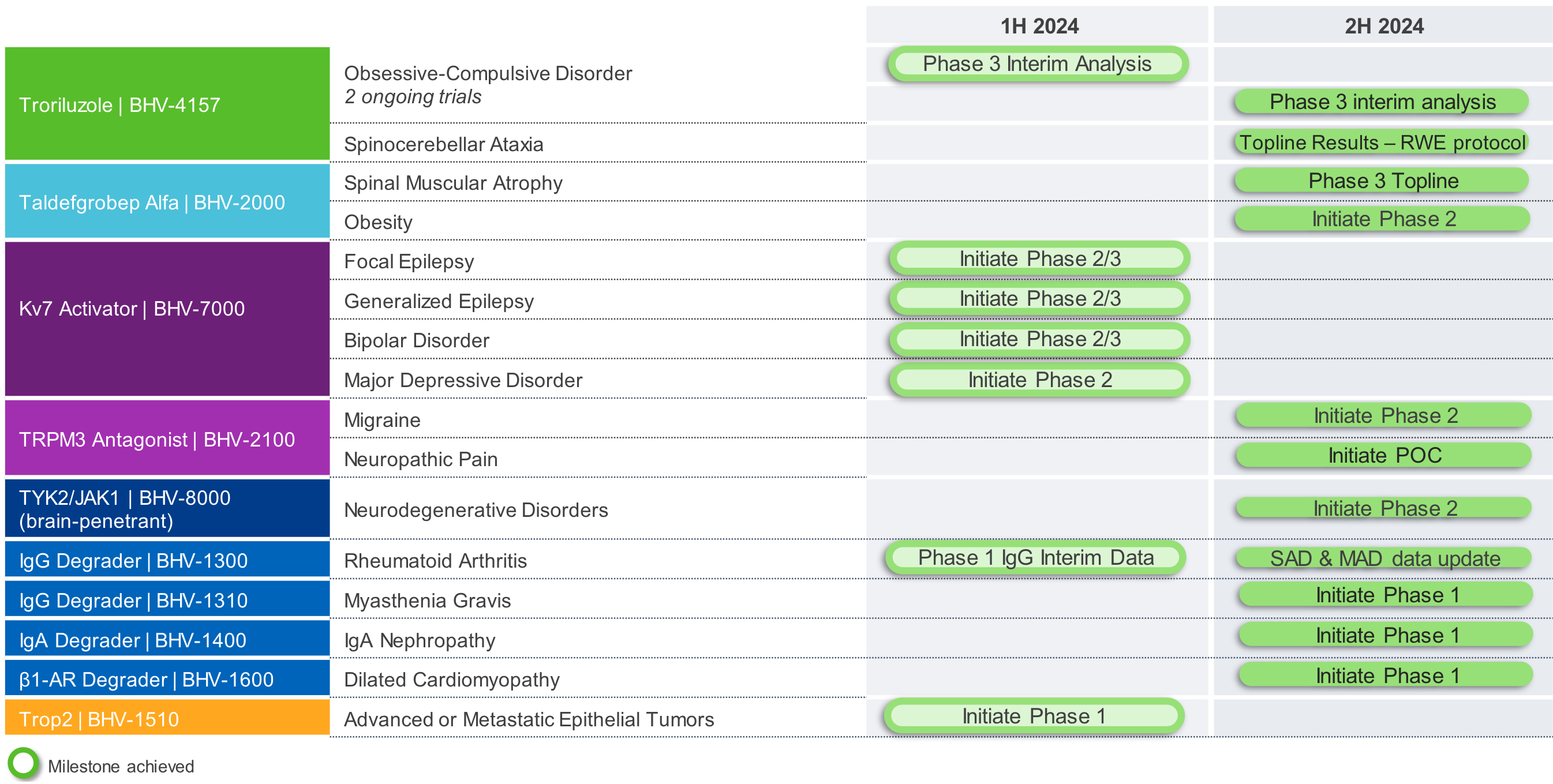
Glutamate Modulation Platform
The most advanced product candidate from our glutamate receptor antagonist platform is troriluzole (previously referred to as trigriluzole and BHV-4157), which is currently in two Phase 3 trials in OCD and, for which we submitted a new drug application (“NDA”) in Spinocerebellar Ataxia Type 3 (“SCA3”) to the U.S. FDA and marketing authorisation application ("MAA") to the European Medicines Agency ("EMA"). Troriluzole is also being evaluated by the Global Coalition for Adaptive Research ("GCAR") as part of Glioblastoma Adaptive Global Innovative Learning Environment - NCT03970447 ("GBM AGILE"), a revolutionary patient-centered, adaptive platform trial for registration that tests multiple therapies for patients with newly-diagnosed and recurrent glioblastoma ("GBM"). Other product candidates include BHV-5500, which is an antagonist of the glutamate N-methyl-D-aspartate (“NMDA”) receptor and its oral prodrug BHV-5000.
Troriluzole
Spinocerebellar Ataxia
SCAs are a group of ultra-rare, dominantly inherited neurodegenerative disorders predominantly characterized by atrophy of the cerebellum, brainstem, and spinal cord. The disease course of SCA is one of relentless progression over years and inevitably leads to clinical deterioration of motor function, gait imbalance with frequent falling, severe speech impairment, swallowing difficulties, and premature death. SCAs are thought to be pathogenetically related but disease course and brain region involvement are known to vary
between the different genotypes. SCA3, also known as Machado-Joseph disease, is the most common genotype, with a prevalence of up to 6,000 patients in North America and up to 4,600 in the European Union (“EU”) and Japan, and accounts for approximately 30% to 50% of SCAs worldwide. Currently, there are no approved symptomatic or neuroprotective treatments for SCA.
In May 2022, the Company announced top-line results from the Phase 3 clinical trial (Study BHV4157-206) evaluating the efficacy and safety of its investigational therapy, troriluzole, in patients with SCA. The primary endpoint, change from baseline to week 48 on the modified functional Scale for the Assessment and Rating of Ataxia ("f-SARA"), did not reach statistical significance in the overall SCA population as there was less than expected disease progression in the placebo arm over the course of the study. Preliminary post hoc analysis of efficacy measures by genotype suggested a treatment effect in patients with the SCA3 genotype (p=0.045, LSM difference from placebo). A risk reduction in falls was also observed in the SCA3 population, as well as across all SCA genotypes. Troriluzole was well tolerated with an adverse event profile similar to placebo.
Based on the findings of further analyses performed and the debilitating nature of SCA, in May 2023 we announced that we submitted a New Drug Application ("NDA") to the FDA for troriluzole for the treatment of SCA3. In July 2023, the FDA informed us that it would not review the recently submitted NDA application for troriluzole given that the study's primary endpoint was not met and thus, would not permit a
substantive review. In followup to the regulatory decision on the NDA application, we held followup meetings with the FDA regarding the SCA data.
We continue to have constructive dialogue with the FDA regarding our SCA development program and potential future data analyses to address regulatory concerns in the previously issued refuse-to-file decision on the NDA application for SCA3. As a result of regulatory interactions, Biohaven designed a new protocol to assess the effectiveness of troriluzole after 3 years of treatment in SCA. This new protocol, BHV4157-206-RWE (NCT06529146), uses real world evidence ("RWE") of effectiveness using real-world data ("RWD") sources to examine the treatment effects of troriluzole in SCA after up to 3 years of therapy compared to external control subjects collected from the US SCA Natural History cohort ("CRC-SCA"). The data analysis will incorporate new patient data that was previously unavailable and not analyzed in the prior FDA submission. The primary outcome measure for BHV4157-206-RWE is the change from baseline on the f-SARA. The protocol was designed to be consistent with FDA’s Guidance for Industry, "Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision Making for Drug and Biological Products." The protocol and SAP were submitted and reviewed by FDA prior to database lock of newly available 3-year data. Topline data from this protocol is expected in the second half of 2024 and, if positive, could form the basis of a new drug application to the FDA. We will provide further updates on the SCA development program as warranted by any continued positive progress from the outcome of future regulatory interactions on this topic.
In October, 2023, the European Medicines Agency ("EMA") informed us that our Marketing Authorization Application ("MAA") for troriluzole (Dazluma) in the treatment of SCA has been validated and is now under review by EMA's Committee for Medicinal Products for Human Use ("CHMP").
We remain committed to working closely with the health authorities to bring troriluzole to people with SCA, given no therapy is currently approved for this ultra-rare genetic disorder.
Obsessive Compulsive Disorder
We commenced a Phase 2/3 double-blind, randomized, controlled trial to assess the efficacy of troriluzole in adults with OCD in December 2017. The Phase 2/3 study results were announced in June 2020. Troriluzole 200 mg administered once daily as adjunctive therapy in OCD patients with inadequate response to standard of care treatment showed consistent numerical improvement over placebo on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) at all study timepoints (weeks 4 to 12) but did not meet the primary outcome measure at week 12. Troriluzole treated subjects (n = 111) had a mean Y-BOCS improvement of -3.4 points from baseline versus -2.9 for placebo-treated (n = 115) subjects [difference -0.5 and p-value = 0.451] at week 4, -5.1 points (n = 96) versus -3.6 for placebo-treated (n = 108) subjects [difference
-1.5 and p-value = 0.041] at week 8, and -5.9 points (n = 99) versus -4.9 for placebo-treated (n = 102) subjects [difference -1.0 and p-value = 0.220] at week 12. Troriluzole’s safety profile was generally consistent with past clinical trial experience with its active metabolite, riluzole. Treatment emergent adverse events (“TEAE”s) were mostly reported to be mild in intensity. TEAEs that occurred in at least 5% of patients in the troriluzole group, and more frequently in the troriluzole group than in the placebo group, were headache, dizziness, fatigue, somnolence, nausea and nasopharyngitis.
Given the strong signal in the Phase 2/3 proof of concept study and after receiving feedback from the FDA in an End of Phase 2 meeting, in December 2020 we initiated enrollment in a Phase 3 program. The Phase 3 program will have an estimated total enrollment of up to 700 participants in each trial with a primary endpoint of change from baseline on the Y-BOCS total score at week 4, 8 and 10. The two Phase 3 randomized, double-blind, placebo-controlled trials that make-up our Phase 3 program for OCD are currently ongoing.
In January 2024, we announced plans to conduct a pre-planned interim analysis ("IA") to evaluate efficacy in the first of our two Phase 3 studies in OCD. The IA was planned to be conducted by an independent Data Monitoring Committee after approximately 70% of subjects in the primary analysis population reached the primary endpoint. The Data Monitoring Committee convened in the second quarter of 2024 to review the IA and informed the Company that the study may continue. As such, we continue enrolling patients in the first Phase 3 study in OCD and expect that this study will be fully enrolled in the first quarter of 2025. We expect to report topline data from the first Phase 3 study in OCD in the first half of 2025.
There is a similarly designed pre-planned IA for the second Phase 3 study in OCD, with topline results from this IA anticipated in the second half of 2024.
Glioblastoma
In December 2021, GCAR selected troriluzole for evaluation in GBM AGILE. GBM AGILE is a revolutionary patient-centered, adaptive platform trial for registration that tests multiple therapies for patients with newly-diagnosed and recurrent GBM, the most fatal form of brain cancer. Troriluzole will be evaluated in all patient subgroups of the trial which include newly-diagnosed methylated MGMT, newly-diagnosed unmethylated MGMT, and recurrent GBM. Troriluzole was selected for inclusion in GBM AGILE based on compelling evidence showing deregulation of glutamate in GBM. The therapeutic potential of troriluzole in GBM and other oncology indications is supported by several recent clinical and translational research studies conducted with troriluzole and its active moiety.
In July 2022, the Company and GCAR announced that enrollment has commenced in GBM AGILE for the
evaluation of troriluzole. Enrollment in the study is ongoing.
Myostatin Platform
Taldefgrobep Alfa (BHV-2000)
In February 2022, we announced a worldwide license agreement with BMS for the development and commercialization rights to taldefgrobep alfa (also known as BMS-986089 and now referred to as BHV-2000), a novel, Phase 3-ready anti-myostatin adnectin. Myostatin is a natural protein that limits skeletal muscle growth, an important process in healthy muscular development that can lead to improvements of lean mass and loss of adipose tissue. In patients with neuromuscular diseases, active myostatin can critically limit the growth needed to achieve developmental and functional milestones. Myostatin inhibition is a promising therapeutic strategy for enhancing muscle mass and strength in a range of pediatric and adult neuromuscular conditions. In addition, preclinical and early clinical data suggest that blocking myostatin and downstream signaling through its receptors on skeletal muscle may produce physical and metabolic changes that are important to individuals living with overweight and obesity, including reducing body fat and improving insulin sensitivity while increasing lean muscle mass. Taldefgrobep’s novel mode of action and unique impact on body composition suggest it could be used as monotherapy or in combination with other anti-obesity medications.
Spinal Muscular Atrophy
In July 2022, we commenced enrollment in a Phase 3 clinical trial of BHV-2000 assessing the efficacy and safety of taldefgrobep alfa in Spinal Muscular Atrophy ("SMA"). SMA is a rare, progressively debilitating motor neuron disease in which development
and growth of muscle mass are compromised, resulting in progressive weakness and muscle atrophy, reduced motor function, impaired quality of life and often death. The Phase 3 placebo-controlled, double-blind trial is designed to evaluate the efficacy and safety of taldefgrobep as an adjunctive therapy for participants who are already taking a stable dose of nusinersen or risdiplam or have a history of treatment with onasemnogene abeparvovec-xioi (Zolgensma), compared to placebo. The study is neither restricted nor limited to patients based on ambulatory status or classification of SMA and is designed to randomize approximately 180 patients in this randomized, double-blind, placebo-controlled global trial. Baseline characteristics of the population enrolled in the ongoing Phase 3 study in SMA were reported and confirmed to be well matched to the target clinical population. The primary endpoint of the study, the 32 Item Motor Function Measurement, is a reliable and validated endpoint for measuring clinically meaningful benefit in SMA. We expect to report topline data from our Phase 3 study in the second half of 2024.
In February 2023, we received Fast Track designation from the FDA for taldefgrobep alfa for the treatment of SMA. In December 2022, we received orphan drug designation from the FDA for taldefgrobep in the treatment of SMA. In July 2023, we received orphan drug designation from the European Commission for taldefgrobep alfa in the treatment of SMA.
In April 2024, we announced that the FDA granted "rare pediatric disease" designation for taldefgrobep alfa. The designation provides for the potential for taldefgrobep to receive a priority review voucher (“PRV”) if ultimately approved for the indication of SMA.
Metabolic Disorders
Obesity is a disease of excess and/or abnormal deposits of adipose tissue and a current global public health crisis. By 2030, it is expected that nearly one billion people will be living with obesity, including 50% of the adult and 25% of the adolescent US population. The primary driver of obesity-related morbidity and mortality is metabolically active visceral adipose tissue and associated deposits of adipose tissue in and around organs such as the heart, liver, kidneys, and muscle.
Preclinical and clinical data have demonstrated the potential for anti-myostatin therapies to produce physical and metabolic changes that are highly relevant to individuals living with overweight and obesity, including reducing total body fat and visceral adiposity, and improving insulin sensitivity and bone mineral density, while increasing lean muscle mass.
In October 2023, we announced preclinical data demonstrating the ability of taldefgrobep alfa to significantly reduce fat mass while increasing lean mass in an obese mouse model. In a mouse model of diet-induced obesity, untreated mice exhibited an increase in fat mass of 31%, while the mice treated with taldefgrobep alfa demonstrated increases in lean mass of 25% from baseline (p≤.0.001) and lost 11% of their baseline fat (p≤.0.001) compared to vehicle (placebo) treated mice. Insulin and leptin levels were consistently lower in mice treated with taldefgrobep alfa compared to the untreated mice. There was no difference in food intake over time across the taldefgrobep alfa and untreated mice, counter to what has been observed with incretin mimetics (e.g., semaglutide) which are consistently associated with a reduction in energy intake.
In May 2024, we announced preclinical data from a diet induced obesity mouse model, which showed treatment with taldefgrobep alfa together with a glucagon-like peptide-1 ("GLP-1") agonist produced greater reductions in body weight and fat mass, and a larger increase in lean muscle mass, compared to treatment with GLP-1 alone (see figure below).
We plan to initiate a Phase 2 clinical trial of taldefgrobep in the management of metabolic disease in the second half of 2024. The study will evaluate the ability of taldefgrobep to maintain lean mass muscle as an adjunctive to standard of care GLP-1 therapy in adults living with overweight and obesity. We are evaluating and have not yet finalized potential clinical trial designs, including size and primary and secondary endpoints.
Ion Channel Platform
Kv7
BHV-7000
In April 2022, we closed the acquisition from Knopp Biosciences LLC (“Knopp”) of Channel Biosciences, LLC, a wholly owned subsidiary of Knopp owning the assets of Knopp’s Kv7 channel targeting platform, pursuant to a Membership Interest Purchase Agreement, dated February 24, 2022 (the "Purchase Agreement"). The acquisition of the Kv7 channel targeting platform added the latest advances in ion-channel modulation to our growing neuroscience portfolio. BHV-7000 (formerly known as KB-3061), the lead asset from the Kv7 platform is an activator of Kv7.2/Kv7.3, a key ion channel involved in neuronal signaling and in regulating the hyperexcitable state in epilepsy.
In the second quarter of 2022, our Clinical Trial Application for BHV-7000 was approved by Health Canada, and we subsequently began Phase 1 clinical development. First-in-human single ascending dose ("SAD") and multiple ascending dose ("MAD") studies have now been completed. BHV-7000 was well-tolerated at all dose levels in both studies with no SAEs and no dose-limiting toxicities.
In 2023, we initiated a Phase 1 open-label electroencephalogram ("EEG") study designed to evaluate the effects of BHV-7000 on changes from baseline in EEG spectral power after administration of single doses of BHV-7000 (10, 25, or 50 mg) to healthy adult volunteers. BHV-7000 was well-tolerated at all doses studied and EEG data showed dose-dependent increases in brain spectral power, with minimal power increase in the delta frequency band and the highest spectral power increases in the alpha, beta, and gamma frequency bands. The minimal impact of BHV-7000 on slower frequencies (i.e., delta) is consistent with the low incidence of central nervous system ("CNS") adverse events, in particular somnolence, seen in the BHV-7000 Phase 1 SAD/MAD studies, and the study results confirm the CNS activity of BHV-7000 at projected therapeutic concentrations.
Based on the results from the EEG study and the safety profile in SAD/MAD trials, along with PK data from a new once-daily extended-release (“ER”) formulation, Biohaven plans on exploring three oral dose levels of once-daily BHV-7000 (25 mg, 50 mg, and 75 mg) in the Phase 2/3 clinical trials in epilepsy and mood disorders. This dosing approach with a Kv7 activator will allow for assessment of distinct target concentrations over a wide range, above and below EC50 drug concentrations efficacious in nonclinical models, not previously feasible with drugs in this class.
Epilepsy
Epilepsy is the initial disease we are targeting with activators from our Kv7 platform. Epilepsy affects approximately 3.5 million Americans, or more than 1.2% of adults and 0.6% of children in the U.S., and more than 50 million patients worldwide, according to the World Health Organization (“WHO”). It is the fourth most common neurological disorder, and many patients struggle to achieve freedom from seizures, with more than one third of patients requiring two or more medications to manage their epilepsy. While the use of anti-seizure medications is often accompanied by dose-limiting side effects, our clinical candidate BHV-7000 is specifically designed to target subtypes of Kv7 potassium channels without engagement of GABAA receptors. The lack of GABAA-R activity potentially gives BHV-7000 a wide therapeutic window which we expect to result in an improved side effect profile, limiting the somnolence and fatigue often seen in patients receiving anti-seizure medications. By adding BHV-7000 to our pipeline, we aim to bring this potassium channel modulator as a potential solution to patients with epilepsy who remain uncontrolled on their current regimens.
In January 2024, we completed our End-of-Phase 2 meeting with the FDA to advance to Phase 3 trials and announced that more than 110 global clinical sites have been selected in the first of two focal epilepsy trials. Enrollment in our Phase 2/3 program commenced in the first quarter of 2024. The two pivotal studies evaluating the efficacy of BHV-7000 in refractory focal epilepsy are planned as randomized, double-blind, placebo-controlled, 8- and 12-week trials with a primary endpoint of change from baseline in 28-day average seizure frequency in adults with focal epilepsy. One of the focal epilepsy studies will evaluate 25 mg and 50 mg doses of BHV-7000 and the second study will evaluate 50 mg and 75 mg doses of BHV-7000 (see figure below).
In addition to the focal epilepsy program, we initiated a Phase 2/3 study of BHV-7000 in idiopathic generalized epilepsy ("IGE") in the second quarter of 2024. The pivotal study evaluating the efficacy of BHV-7000 with IGE is planned
as a randomized, double-blind, placebo-controlled 24-week time-to-event trial with a primary endpoint of time to second generalized seizure in adults and adolescents with IGE (see figure below).
Mood Disorders
Approximately 1 in 5 adults in the US are living with neuropsychiatric illnesses that are, in turn, associated with inadequate treatment, poor quality of life, disability, and considerable direct and indirect costs. There is significant unmet need for novel and effective therapeutic options that are not limited by long latency periods to clinical effects, low response rates, and significant risks and side effects. Increasing evidence from animal models and clinical trials now suggests that Kv7.2/7.3 targeting drugs offer the potential to treat a spectrum of these neuropsychiatric diseases including, but not limited to, mood disorders, such as major depressive disorder ("MDD") and bipolar disorder.
Major Depressive Disorder
We initiated a Phase 2 clinical trial with BHV-7000 for the treatment of MDD in the second quarter of 2024. The study is a 6 week, randomized, double-blind, placebo-controlled trial in approximately 300 subjects, with a primary endpoint of measurement on the Montgomery-Asberg Depression Rating Scale ("MADRS"). See figure below for trial design.
Bipolar disorder
We also initiated a Phase 2/3 clinical trial with BHV-7000 for the treatment of bipolar disorder in the second quarter of 2024. The study is a 3 week, randomized, double-blind, placebo-controlled trial in approximately 256 subjects, with a primary endpoint of measurement on the Young Mania Rating Scale ("YMRS"). See figure below for trial design.
KCNQ2 Developmental Epileptic Encephalopathy
We are currently exploring BHV-7000 as a potential treatment for KCNQ2 developmental epileptic encephalopathy ("KCNQ2-DEE"), a rare pediatric epileptic encephalopathy first described in 2012 resulting from dominant-negative mutations in the KCNQ2 gene. BHV-7000 has been granted Rare Pediatric Disease Designation by the United States Food and Drug Administration (the “FDA”) for the treatment of KCNQ2-DEE.
Neuropathic Pain
We are currently evaluating the activity of BHV-7000 and other compounds from our proprietary series of selective Kv7.2/7.3 activators in multiple preclinical models of neuropathic pain.
Migraine
We are currently exploring BHV-7000 as a potential treatment for migraine. Kv7.2/7.3 openers have shown significant activity in cortical spreading depression models of migraine.
TRPM3 Ion Channel Antagonists
In January 2022, we entered into an Exclusive License and Research Collaboration Agreement with Katholieke Universiteit Leuven ("KU Leuven") to develop and commercialize TRPM3 antagonists to address the growing proportion of people worldwide living with chronic pain disorders (the "KU Leuven Agreement"). The TRPM3 antagonist platform was discovered at the Centre for Drug Design and Discovery and the Laboratory of Ion Channel Research at KU Leuven. Under the KU Leuven Agreement, we receive exclusive global rights to develop, manufacture and commercialize KU Leuven's portfolio of small-molecule TRPM3 antagonists. The portfolio includes the lead candidate, henceforth known as BHV-2100.
BHV-2100
BHV-2100 is an orally-bioavailable small molecule antagonist of TRPM3. TRPM3 is expressed in the relevant human tissue types for pain and migraine, and both preclinical models and human genetics implicate TRPM3 in pain and migraine.
In May 2024, we reported positive pharmacokinetic and safety data from the completed Phase 1 study with BHV-2100. The results demonstrated rapid absorption with therapeutic concentrations achieved by 20 minutes. The favorable tolerability profile at single doses up to 500 mg exceeds the anticipated therapeutic dose and is well above the EC90 concentration. Based on these findings, we plan to advance BHV-2100 into a Phase 2 study in the acute treatment of migraine and a proof-of-concept study in pain in the second half of 2024.
Migraine
We expect to initiate a BHV-2100 Phase 2 study in acute migraine in the second half of 2024. We are evaluating and have not yet finalized clinical trial design, including trial size, and primary and secondary endpoints for the anticipated clinical trial.
Neuropathic Pain
BHV-2100 is also being developed as a potential non-opioid treatment for neuropathic pain. We are evaluating the ability of BHV-2100 to reduce pain behaviors across several preclinical models of neuropathic pain, including chemotherapy induced
neuropathy, diabetic neuropathy, and nerve injury. The Company expects to conduct a proof of concept study for neuropathic pain in the second half of 2024.
Additional research on TRPM3-mediated disorders
Under the KU Leuven Agreement, Biohaven is supporting further basic and translational research at KU Leuven on the role of TRPM3 in pain and other disorders. In addition to BHV-2100, we are optimizing other lead compounds for TRPM3-mediated disorders of the peripheral and central nervous systems.
Inflammation and Immunology Platform
TYK2/JAK1
Agreement with Hangzhou Highlightll Pharmaceutical Co. Ltd.
In March 2023, we entered into an exclusive, worldwide (excluding People’s Republic of China and its territories and possessions) license agreement with Hangzhou Highlightll Pharmaceutical Co. Ltd. ("Highlightll"), pursuant to which we obtained the right to research, develop, manufacture and commercialize Highlightll’s brain penetrant dual TYK2/JAK1 inhibitor program (the "Highlightll Agreement").
BHV-8000
Dysregulation of the immune system has been implicated in several neurodegenerative and neuroinflammatory disorders including Parkinson's disease, multiple sclerosis, Alzheimer's disease, amyotrophic lateral sclerosis and autoimmune encephalitis. Over-active immune cells and microglia driving chronic neuroinflammation result in release of cytokines with activation of leukocytes that is thought to contribute to neuronal injury, death, gliosis, and demyelination. The TYK2 and JAK1 signal transduction pathways mediate highly complementary immune and inflammatory signaling events. Targeted, small-molecule therapies that inhibit TYK2 or JAK kinases have separately demonstrated robust efficacy in autoimmune, dermatologic and gastrointestinal disorders. TYK2 is a validated immune target as evidenced by a recent peripheral program that gained FDA approval, and there are multiple additional peripheral non-CNS programs in clinical development. Brain penetrant inhibitors of TYK2/JAK1 have the potential to bring this validated immune target to brain disorders.
There are currently no brain penetrant, selective, dual TYK2/JAK1 inhibitors approved for brain disorders. In May 2023, we began dosing with BHV-8000 (previously TLL-041), in a Phase 1 study in normal healthy volunteers. In May 2024, we reported positive results from the Phase 1 single and multiple ascending dose study with BHV-8000 in healthy subjects, including evidence of target engagement along with a safe and well tolerated profile. The Phase 1 study also confirmed cerebrospinal fluid ("CSF") exposures of BHV-8000 and
evidence of biomarker target engagement within the CNS. We also announced the successful completion of two FDA meetings with favorable feedback enabling registrational programs for Parkinson's disease and for the prevention of ARIA, a novel indication.
We anticipate beginning Phase 2/3 clinical trials with BHV-8000 in the second half of 2024 targeting neuroinflammatory conditions, potentially including Amyloid-Related Imaging Abnormalities ("ARIA") in Alzheimer’s disease, Alzheimer's disease, Parkinson's disease and multiple sclerosis. We are evaluating and have not yet finalized potential clinical trial designs, including size and primary and secondary endpoints.
MoDE Degraders
Bispecific Molecular Degraders of Extracellular Proteins
Molecular Degraders of Extracellular Proteins (“MoDEs”) are bispecific molecules that target pathologic circulating proteins and direct them to the liver for degradation by the endolysosomal pathway. Our MoDE platform is being explored for use in a wide range of therapeutic areas, including indications in immune-mediated diseases, cancer and other diseases. We are planning for MoDEs to be administered as intravenous or subcutaneous formulations. We expect to initiate a total of 4 Investigational New Drug Applications or the foreign equivalent ("IND") for the degrader program in 2024.
BHV-1300
BHV-1300 is an IgG degrader which we are initially developing for the treatment of rheumatoid arthritis ("RA"). RA is a chronic autoimmune disease estimated to affect 1 to 2% of the global population. RA primarily affects the joints, causing pain, swelling, stiffness, and loss of function.
We evaluated the effect of single and multiple doses of BHV-1300 in cynomolgus monkeys. In September 2023, we reported data from confirmatory studies that showed a 75-80% reduction of IgG levels two days after a single dose and over 90% of IgG lowering after three doses.
Maximal lowering across FcRn inhibitors is 60-80% within approximately 7 to 21 days after initiation of single or multiple doses, respectively, in cynomolgus. In contrast, a single dose of BHV-1300 lowers IgG by approximately 75 to 80% after approximately 2 days, and after three rapid doses to greater than 90% lowering. The length of significant exposure to BHV-1300 is approximately one day within the dosage interval compared to continuous exposure required of the FcRn inhibitors. Mechanism related liabilities of FcRn inhibitors seen in animals and man, including hypoalbuminemia and hypercholesterolemia, are not expected and do not occur with BHV-1300 in cynomolgus. See figures below comparing the speed and depth of lowering to FcRn inhibitors.
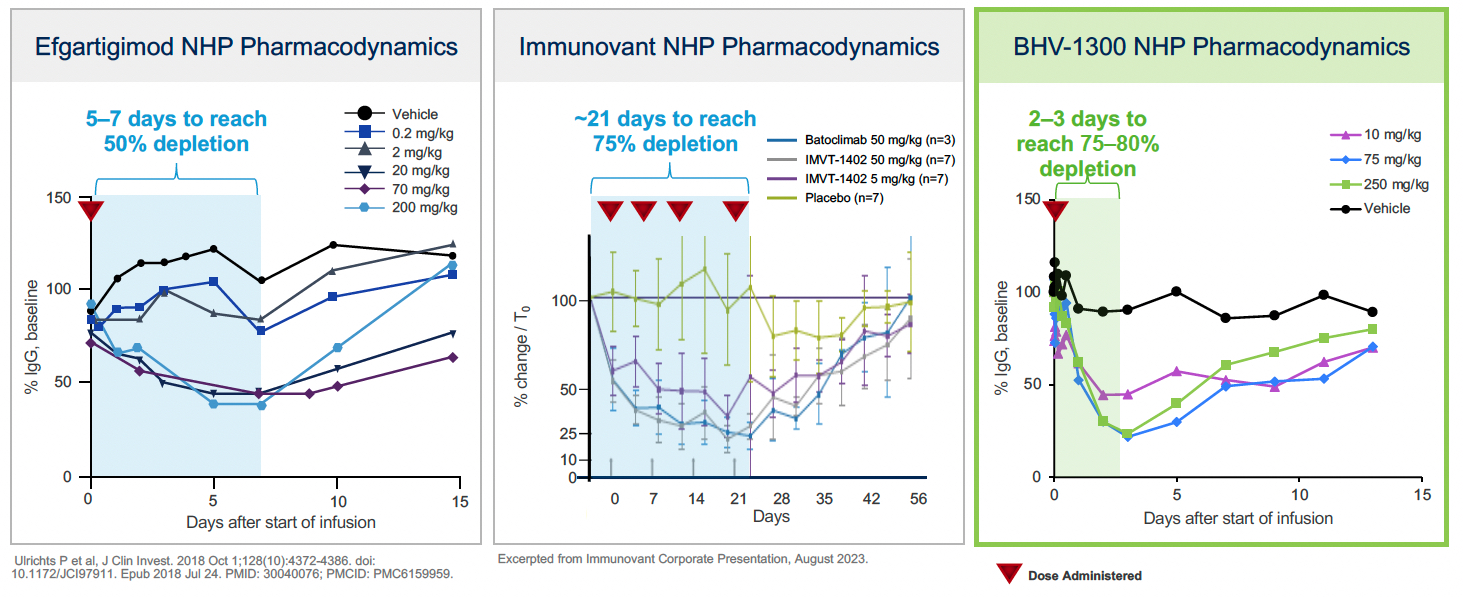
In January 2024, we reported preclinical pharmacodynamic single dose data with BHV-1300 which demonstrated the Biohaven IgG degrader technology allows for co-administration with Fc-containing biologics. The PK of Humira® was unaltered after being dosed 12 hours after BHV-1300 administration (see figure below).
| | |
|
| * Adapted from BLA 761154, IND 116471, Study no. r-fkb327-01 |
The Phase 1 study examining BHV-1300 was initiated in the first quarter of 2024 and remains ongoing. In May 2024, we reported preliminary results from our Phase 1 study of BHV-1300. These results in healthy subjects reported that BHV-1300 rapidly and selectively lowers IgG in a dose-dependent manner in
the first 4 cohorts completed to date (see figure below). Preliminary IgG lowering data is consistent with modeling, with dose- and time-dependent IgG lowering observed even in initial low-dose cohorts. Some subjects experienced IgG reductions as low as 50 to 70% of baseline. BHV-1300 demonstrated reduction of IgG without significantly impacting LFTs, albumin, LDL cholesterol or other serum labs. BHV-1300 has been safe and well tolerated to date, with no serious or severe adverse events. Most AEs were mild, deemed unrelated to study drug and resolved spontaneously. As expected from the selectivity of the molecule for IgG, when compared to placebo, there were no meaningful reductions in average IgA, IgM or IgE levels during the week after dosing. No adverse trends have been observed in vital signs or ECGs. Modeling suggests additional cohorts in the Phase 1 study will achieve greater than 70% lowering of IgG utilizing doses compatible with subcutaneous administration.
The Phase 1 study has also compared intravenous and subcutaneous administration of BHV-1300. Subcutaneous administration of BHV-1300 demonstrated an average of approximately 44% higher than expected exposure compared to the dose-equivalent intravenous formulation without injection site reactions. This new human data confirms the feasibility
of the Company's plan for convenient dosing of BHV-1300 with a patient self-administered subcutaneous auto-injector. The Company expects to provide further updates on the BHV-1300 development program by the end of 2024.
BHV-1310
BHV-1310 is a next generation bispecific IgG degrader with the same specificity as BHV-1300 for IgG1, IgG2 and IgG4 which is initially being developed for the treatment of rare disorders including conditions like generalized myasthenia gravis ("gMG") and potentially other acute or chronic conditions, or chronic conditions with acute exacerbations or flares.
MG is a neuromuscular disorder that is estimated to affect approximately 36,000 to 60,000 people in the United States. Patients with gMG develop antibodies that attack critical signaling receptor proteins at the junction between nerve and muscle cells, inhibiting communication between nerves and muscle and resulting in weakness of the skeletal muscles.
In January 2024, we demonstrated optimization of degrader technology with BHV-1310 which allows for deeper reductions in IgG after single dose (see figure below). The deep and rapid reductions observed suggest that BHV-1310 could have potential application in acute settings. We expect to initiate Phase 1 studies of BHV-1310 in the second half of 2024. We are evaluating and have not yet finalized potential clinical trial designs, including size and primary and secondary endpoints.
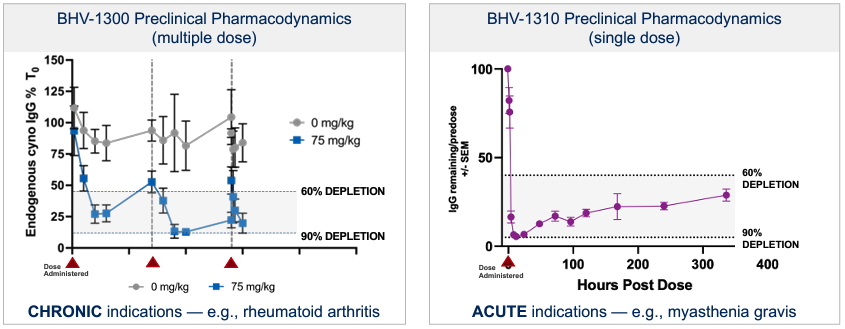
BHV-1400
BHV-1400 is a selective MoDE which is being developed to target Gd-IgA1 for the treatment of IgA Nephropathy. Specific removal of pathogenic Gd-IgA1 and associated circulating immune complexes with preservation of normal IgA potentially permits disease remission without incurring an infection risk. We shared preliminary data demonstrating the chimeric antibody-ASGPR ligand conjugate specifically mediated endocytosis of Gd-IgA1, as opposed to normal IgA1 and IgA2, in an endocytosis assay with ASGPR-expressing cell lines, and that MoDE degraders successfully internalize and degrade these immune-complexes. We
expect to initiate Phase 1 studies of BHV-1400 in the second half of 2024. We are evaluating and have not yet finalized potential clinical trial designs, including size and primary and secondary endpoints.
BHV-1600
BHV-1600 is a selective MoDE designed to remove circulating agonistic antibodies of all isotypes and subclasses directed against myocardial beta-1 adrenergic receptor ("β-1 AR") through hepatic ASGPR binding and hepatocellular degradation. This molecule was created using a peptide that mimics the antigenic epitope common to most patients with autoantibodies directed to β-1 AR. This peptide mimics the native
sequence such that circulating antibodies are efficiently trapped and subsequently removed by hepatic endocytosis through the ASGPR receptor.
We are developing BHV-1600 for the treatment of dilated cardiomyopathy. Dilated cardiomyopathy is a condition where the cardiac muscle contracts less effectively, the chambers of the heart are enlarged and thinning of cardiac walls results. This can lead to cardiac valvular incompetency, arrhythmias, thrombosis, and heart failure. We expect to initiate Phase 1 studies of BHV-1600 in the second half of 2024. We are evaluating and have not yet finalized potential clinical trial designs, including size and primary and secondary endpoints.
Additional Degraders
We are currently developing additional MoDE degraders which will advance to INDs in 2025. These include potential treatments for Type 1 diabetes with our degrader targeting anti-insulin and anti-proinsulin autoantibodies, kidney disease with our degrader targeting phospholipase A2 receptor ("PLA2R") antibodies for idiopathic membranous nephropathy, an IgG4 specific degrader to target IgG4-mediated rare diseases, and gene therapy administration optimization with our degrader to target neutralizing antibodies to Adeno-Associated Virus Serotype 9 ("AAV9").
Oncology Platform
CD-38
BHV-1100
In the fourth quarter of 2021, we initiated a Phase 1a/1b trial in multiple myeloma patients using its antibody recruiting molecule BHV-1100 in combination with autologous cytokine induced memory-like natural killer cells and immune globulin to target and kill multiple myeloma cells expressing the cell surface protein CD38. BHV-1100 is the lead clinical asset from Biohaven’s Antibody Recruiting Molecule ("ARM™") Platform developed from a strategic alliance with PeptiDream Inc. ("PeptiDream") (TYO: 4587). This open-label single center Phase 1a/1b study will assess the safety and tolerability as well as exploratory efficacy endpoints in newly diagnosed multiple myeloma patients who have tested positive for minimal residual disease (“MRD+”) in first or second remission prior to autologous stem cell transplant (“ASCT”). We plan to enroll 30 newly diagnosed multiple myeloma patients. The primary outcome measures are dose limiting toxicities following combination product administration (time frame: 100 days post-combination product administration) and incidence and severity of side effects related to the combination product (time frame: 90 to 100 days post-combination product administration).
Antibody Drug Conjugates
BHV-1510
In January 2024, we acquired BHV-1510 through our acquisition of Pyramid Biosciences, Inc. ("Pyramid").
BHV-1510 is a next-generation Trophoblast Cell Surface Antigen 2 ("TROP-2") directed ADC employing an optimized next-generation construct with novel linker-payload and enzymatic, site-specific conjugation, targeting TROP2-expressing carcinomas. Carcinoma refers to a malignant neoplasm of epithelial origin. Carcinomas account for 80 to 90 percent of all cancer cases and several examples have been successfully treated with ADCs. Abundant TROP-2 expression has been described for many carcinoma subtypes.
In preclinical TROP-2 expressing tumor models, BHV-1510 has shown improved antitumor activity versus other TROP-2 directed ADCs, in addition to improved plasma stability, more potent in vitro cytotoxicity, superior bystander effect, and greater immunogenic cell death with the novel TopoIx payload. Improved and differentiated safety has been seen in cynomolgus monkey GLP toxicology studies, suggesting a wide therapeutic index. BHV-1510 has similar favorable characteristics to our proprietary MATE conjugation technology, which should allow highly stable site-specific conjugation, resulting in a favorable PK, toxicity and manufacturability profile.
The IND for BHV-1510 was approved by the FDA in January 2024. The First-in Human, Phase 1/2 trial evaluating BHV-1510 in patients with advanced solid tumors commenced in the second quarter of 2024. This trial consists of two parts; Phase 1 dose escalation and Phase 2 dose expansion, in patients with advanced incurable cancer that have progressed on or are intolerant to standard therapy. The primary objective of Phase 1 is safety, to identify a recommended dose for expansion ("RDE") or maximum tolerated dose. Phase 1 dose escalation will be implemented based on a Bayesian optimal interval design, with the lowest dose initiated as a single patient cohort. Patients are expected to be dosed in escalating cohorts, with dosing regimens administered intravenously every three weeks. The Phase 2 dose expansion part of the study will consist of non-randomized efficacy finding expansion cohorts, defined by specific tumor types that will be treated at the RDE to estimate the anti-tumor activity of BHV-1510. Up to approximately 170 subjects are planned to be evaluated.
Preclinically, BHV-1510 has shown enhanced cellular cytotoxicity, bystander killing, and immunogenic cell death resulting in improved efficacy as monotherapy, and synergistic efficacy in combination with anti-PD-1 therapy. In IND-enabling studies, BHV-1510 also showed a broader therapeutic margin relative to more advanced Trop-2 ADCs, including a lack of lung toxicity, that may translate to an improved clinical efficacy and safety profile. In May 2024, we announced that we entered into a clinical supply agreement with Regeneron Pharmaceuticals, Inc. ("Regeneron") under which we will sponsor and fund the planned combination clinical trial and Regeneron will provide Libtayo®. Libtayo® is a fully-human monoclonal antibody targeting the immune checkpoint receptor programmed cell death protein-1 ("PD-1").
BHV-1500
BHV-1500 is a next-generation CD30-directed ADC employing a Biohaven proprietary site-specific conjugation (MATE reagent), targeting CD30-expressing tumors such as Hodgkin's and other lymphoma and the MMAE payload. Hodgkin's disease and other CD30-expressing lymphoma are characterized by the uncontrolled growth of malignant lymphocytes or lymphoblasts. Adcetris has demonstrated effectiveness in the treatment of Hodgkin's Lymphoma.
In preclinical CD30 expressing murine tumor models, BHV-1500 has shown improved antitumor activity versus Adcetris (brentuximab vedotin), and substantially improved safety, plasma stability and pharmacokinetics in monkeys. We expect to submit an IND for BHV-1500 in 2025.
Recent Developments
Amendment to Knopp Purchase Agreement
In May 2024, we entered into an amendment to the Purchase Agreement with Knopp (the “Knopp Amendment”). Under the Knopp Amendment, the parties thereto agreed to replace the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for BHV-7000 and the pipeline programs. The parties also agreed to reduce the success-based payments payable under the Purchase Agreement by removing all commercial sales-based milestones, which were up to $562.5 million, and reducing the developmental and regulatory milestones, which were up to $575 million, to up to $210 million based on regulatory approvals in the United States and EMEA for BHV-7000 ($25 million of which has already been paid) and up to an additional $60 million based on regulatory approval in the United States for the other Kv7 pipeline programs. We retain the ability to pay these contingent milestone payments in cash or in Biohaven Shares at our election, subject to the same increases if we elect to pay in Biohaven Shares.
In consideration of the revisions to the success-based payment and royalty payment obligations, we agreed to issue to Knopp 1,872,874 Biohaven Shares, valued at approximately $75 million, through a private placement within 60 days of the date of execution of the Knopp Amendment (the “2024 Additional Consideration”) and additional Biohaven Shares with an approximate value of $75 million within 60 days of the first anniversary of execution of the Knopp Amendment (the “2025 Additional Consideration”). We have also given Knopp the option to request a one-time cash true-up payment from us in December 2024 in the event that Knopp continues to hold the Biohaven Shares representing the 2024 Additional Consideration and the value of such shares has declined, and a one-time cash true-up payment from us in December 2025 in the event that Knopp continues to hold the Biohaven Shares representing the 2025 Additional Consideration and the value of such shares has declined, in each case, subject
to certain conditions. In May 2024, we issued the 2024 Additional Consideration at an approximate value of $66.0 million.
As further consideration for the revisions to the success-based payment and royalty payment obligations in the Knopp Amendment, we issued to Knopp a warrant (the “Warrant”) to purchase 294,195 Biohaven Shares at a purchase price per share of $67.98, subject to certain specified development milestones and the Company achieving a specified market capitalization.
For further discussion of the Knopp Amendment, refer to Note 4, "Fair Value of Financial Assets and Liabilities", Note 6, "Shareholders' Equity, " and Note 10, " License, Acquisitions and Other Agreements" to the accompanying condensed consolidated financial statements included in this Form 10-Q.
Components of Our Results of Operations
Revenue
To date, we have not generated any revenue from product sales and we do not expect to generate any revenue from the sale of products in the near future. If our development efforts for our product candidates are successful and result in regulatory approval or additional license agreements with third parties, then we may generate revenue in the future from product sales.
Operating Expenses
Research and Development Expenses
Research and development ("R&D") expenses consist primarily of costs incurred in connection with the development of our product candidates. We expense research and development costs as incurred. These expenses include:
•expenses incurred under agreements with contract research organizations ("CROs") or contract manufacturing organizations (“CMOs”), as well as investigative sites and consultants that conduct our clinical trials, preclinical studies and other scientific development services;
•manufacturing scale-up expenses and the cost of acquiring and manufacturing preclinical and clinical trial materials and commercial materials, including manufacturing validation batches;
•employee-related expenses, including salaries, benefits, travel and non-cash share-based compensation expense for employees engaged in research and development functions;
•costs related to compliance with regulatory requirements;
•development milestone payments incurred prior to regulatory approval of the product candidate;
•rent and operating expenses incurred for leased lab facilities and equipment; and
•payments made in cash, equity securities or other forms of consideration under third-party licensing or other agreements prior to regulatory approval of the product candidate.
We recognize external development costs based on an evaluation of the progress to completion of specific tasks using estimates from our clinical personnel and information provided to us by our service providers.
Our external direct research and development expenses are tracked on a program-by-program basis for our product candidates and consist primarily of external costs, such as fees paid to outside consultants, CROs, CMOs, and central laboratories in connection with our preclinical development, process development, manufacturing and clinical development activities. Our direct research and development expenses by program also include fees and certain development milestones incurred under license agreements. We do not allocate employee costs, or other indirect costs, to specific programs because these costs are deployed across multiple programs and, as such, are not separately classified. We use internal resources primarily to oversee the research and development as well as for managing our preclinical development, process development, manufacturing and clinical development activities.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will remain significant over the next several years as we increase personnel costs, conduct late-stage clinical trials, and prepare regulatory filings for our product candidates. We also expect to incur additional expenses related to milestones payable to third parties with whom we have entered into license agreements to acquire the rights to our product candidates.
The successful development and commercialization of our product candidates is highly uncertain. At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the preclinical and clinical development of any of our product candidates or when, if ever, material net cash inflows may commence from any of our product candidates. This uncertainty is due to the numerous risks and uncertainties associated with product development and commercialization, including the uncertainty of:
•the scope, progress, outcome and costs of our preclinical development activities, clinical trials and other research and development activities;
•establishment of an appropriate safety profile with IND-enabling studies;
•successful patient enrollment in, and the initiation and completion of, clinical trials;
•the timing, receipt and terms of any marketing approvals from applicable regulatory authorities;
•establishment of commercial manufacturing capabilities or making arrangements with third-party manufacturers;
•development and timely delivery of commercial-grade drug formulations that can be used in our clinical trials and for commercial launch;
•acquisition, maintenance, defense and enforcement of patent claims and other intellectual property rights;
•significant and changing government regulation;
•initiation of commercial sales of our product candidates, if and when approved, whether alone or in collaboration with others; and
•maintenance of a continued acceptable safety profile of the product candidates following approval.
General and Administrative Expenses
General and administrative ("G&A") expenses consist primarily of personnel costs, including salaries, benefits and travel expenses for our executive, finance, business, corporate development and other administrative functions; and non-cash share-based compensation expense. General and administrative expenses also include facilities and other related expenses, including rent, depreciation, maintenance of facilities, insurance and supplies; and for public relations, audit, tax and legal services, including legal expenses to pursue patent protection of our intellectual property.
We anticipate that our general and administrative expenses, including payroll and related expenses, will remain significant in the future as we continue to support our research and development activities and prepare for potential commercialization of our product candidates, if successfully developed and approved. We also anticipate increased expenses associated with general operations, including costs related to accounting and legal services, director and officer insurance premiums, facilities and other corporate infrastructure, office-related costs, such as information technology costs, and certain costs to establish ourself as a standalone public company, as well as ongoing additional costs associated with operating as an independent, publicly traded company.
Other Income, Net
Other income, net primarily consists of changes in the fair value of our forward contract and derivative liabilities and net investment income.
The fair value of the forward contracts and derivative liabilities recognized in connection with the Knopp Amendment are determined using a Monte Carlo simulation of the Company's stock price over the respective duration and terms of each instrument being valued. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" to the accompanying condensed consolidated financial statements included in this Form 10-Q for detail on valuation inputs and methodology. The fair value of these liabilities are recorded on the condensed consolidated balance sheets with changes in fair value recorded in other income, net in the condensed consolidated statements of operations and comprehensive loss.
Net investment income is comprised of interest income and net accretion and amortization on investments in addition to realized gains and losses. Refer to Note 3, "Marketable Securities," to the accompanying condensed consolidated financial statements included in this Form 10-Q for further detail on our investments.
Provision for Income Taxes
The income tax expense in the condensed consolidated financial statements was calculated on a separate return method and presented as if the Company’s operations were separate taxpayers in the respective jurisdictions up to and including the Separation. Cash tax payments, income taxes receivable and deferred taxes, net of valuation allowance, are reflective of our actual tax balances prior and subsequent to the Separation.
As a company incorporated in the BVI, we are principally subject to taxation in the BVI. Under the
current laws of the BVI, the Company and all dividends, interest, rents, royalties, compensation and other amounts paid by the Company to persons who are not resident in the BVI and any capital gains realized with respect to any shares, debt obligations, or other securities of the Company by persons who are not resident in the BVI are exempt from all provisions of the Income Tax Ordinance in the BVI.
We have historically outsourced all of the research and clinical development for our programs under a master services agreement with Biohaven Pharmaceuticals, Inc. ("BPI"). As a result of providing services under this agreement, BPI was profitable during the three and six months ended June 30, 2024 and 2023. A similar arrangement is also in place for our subsidiary, Biohaven Biosciences Ireland Limited ("BBIL") also operates under a similar arrangement. Both Companies are subject to taxation in the US and Ireland, respectively. As such, in each reporting period, the tax provision includes the effects of the results of operations of BPI and BBIL.
At June 30, 2024 and December 31, 2023, we continued to maintain a full valuation allowance against our net deferred tax assets, comprised primarily of research and development tax credit carryforwards and net operating loss carryforwards, based on management’s assessment that it is more likely than not that the deferred tax assets will not be realized.
Our income tax provisions primarily represent Federal and state taxes related to the profitable operations of our subsidiaries in the United States and Ireland.
Results of Operations
Comparison of the Three Months Ended June 30, 2024 and 2023
The following tables summarize our results of operations for the three months ended June 30, 2024 and 2023:
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | |
| | | 2024 | | 2023 | | Change |
| In thousands | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Operating expenses: | | | | | | |
| | | | | | |
| Research and development | | $ | 314,819 | | | $ | 79,490 | | | $ | 235,329 | |
| General and administrative | | 18,953 | | | 14,521 | | | 4,432 | |
| Total operating expenses | | 333,772 | | | 94,011 | | | 239,761 | |
| Loss from operations | | (333,772) | | | (94,011) | | | (239,761) | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Other income, net | | 14,178 | | | 5,842 | | | 8,336 | |
Loss before provision for income taxes | | (319,594) | | | (88,169) | | | (231,425) | |
Provision for income taxes | | 177 | | | 2,177 | | | (2,000) | |
| Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (229,425) | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Research and Development Expenses
| | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended June 30, | | |
| | | 2024 | | 2023* | | Change |
| In thousands | | |
| Direct research and development expenses by program: | | | | | | |
BHV-4157 (Troriluzole) | | $ | 17,076 | | | $ | 19,141 | | | $ | (2,065) | |
BHV-2000 (Taldefgrobep Alfa) | | 17,748 | | | 11,078 | | | 6,670 | |
BHV-7000 & BHV-7010 (Kv7) | | 199,250 | | | 9,904 | | | 189,346 | |
BHV-2100 (TRPM3 Antagonist) | | 4,272 | | | 1,311 | | | 2,961 | |
BHV-8000 (TYK2/JAK1) | | 2,675 | | | 1,115 | | | 1,560 | |
BHV-1300 (IgG Degrader) | | 7,358 | | | 4,220 | | | 3,138 | |
BHV-1310 (IgG Degrader) | | 3,938 | | | — | | | 3,938 | |
BHV-1400 (IgA Degrader) | | 7,612 | | | — | | | 7,612 | |
| | | | | | |
BHV-1510 (Trop2) | | 3,043 | | | — | | | 3,043 | |
| Other programs | | 1,473 | | | 3,175 | | | (1,702) | |
| Unallocated research and development costs: | | | | | | |
| Personnel related (including non-cash share-based compensation) | | 24,960 | | | 19,280 | | | 5,680 | |
| | | | | | |
| Preclinical research programs | | 18,091 | | | 6,514 | | | 11,577 | |
| Other | | 7,323 | | | 3,752 | | | 3,571 | |
| Total research and development expenses | | $ | 314,819 | | | $ | 79,490 | | | $ | 235,329 | |
*Certain prior year amounts have been reclassified to conform to current year presentation
R&D expenses, including non-cash share-based compensation costs, were $314.8 million for the three months ended June 30, 2024, compared to $79.5 million for the three months ended June 30, 2023. The increase of $235.3 million was due, in part, to a one-time non-cash expense of $171.9 million paid to Knopp for a milestone and royalty buyback related to the BHV-7000 and broader Kv7 platform that was recognized during the three months ended June 30, 2024 (the buyback reduced our potential future milestone payments by $867.5 million, and replaced the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for the Kv7 programs). The increase in R&D expenses was also due to advancing our 6 clinical platforms including 5 Phase 3 starts for BHV-7000, follow-on Kv7 assets, preclinical research programs, and increases in direct program spend for additional and advancing multiple clinical development programs. See further discussion of the Knopp Amendment included in Note 10, "License, Acquisitions and Other Agreements" to the condensed consolidated financial statements included in this Form 10-Q. The increase in expense was also related to advancing our 6 clinical platforms including 5 phase 3 starts for BHV-7000, follow-on Kv7 assets, preclinical research programs, and increases in direct program spend for additional and advancing multiple clinical development programs during the three months ended June 30, 2024, as compared to the same period in the prior year.
Non-cash share-based compensation expense was $7.1 million for the three months ended June 30, 2024, an increase of $4.6 million as compared to the same period in 2023. Non-cash share-based compensation expense was higher in 2024 primarily due to our annual equity incentive awards granted in the fourth quarter of 2023 and the first quarter of 2024.
General and Administrative Expenses
General and administrative expenses were $19.0 million for the three months ended June 30, 2024, compared to $14.5 million for the three months ended June 30, 2023. The increase of $4.4 million was primarily due to increased non-cash share-based compensation expense. Non-cash share-based compensation expense was $5.2 million for the three months ended June 30, 2024, an increase of $2.9 million as compared to the same period in 2023. Non-cash share-based compensation expense was higher in 2024 primarily due to our annual equity incentive awards granted in the fourth quarter of 2023 and the first quarter of 2024.
Other Income, Net
Other income, net was a net income of $14.2 million for the three months ended June 30, 2024, compared to $5.8 million for the three months ended June 30, 2023. The increase was primarily due to a $9.2 million gain recorded upon settlement of our forward contract liability for the 2024 Additional Consideration issued under the Knopp Amendment, and increased investment income. This was partially offset by a decrease in other
income recognized during the three months ended June 30, 2024 as compared to the same period in 2023 related to the Transition Services Agreement entered into with the Former Parent. See Note 4, "Fair Value of Financial Assets and Liabilities" to the accompanying condensed consolidated financial statements included in this Form 10-Q for discussion of the forward contract and derivative liabilities recorded in connection with the Knopp Amendment.
Provision for Income Taxes
We recorded an income tax provision of $0.2 million for the three months ended June 30, 2024, compared to
a provision for income taxes of $2.2 million for the three months ended June 30, 2023. The decrease in income tax expense for the three months ended June 30, 2024 as compared to 2023 was primarily attributable to our adoption of the guidance contained in a Notice of Proposed Rule Making issued during the third quarter of 2023 by the United States Internal Revenue Service ("the Notice"). See further discussion of the Notice in Note 12, "Income Taxes" to the condensed consolidated financial statements included in this Form 10-Q.
Comparison of the Six Months Ended June 30, 2024 and 2023
The following tables summarize our results of operations for the six months ended June 30, 2024 and 2023: | | | | | | | | | | | | | | | | | | | | |
| | Six Months Ended June 30, | | |
| | 2024 | | 2023 | | Change |
| In thousands | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Operating expenses: | | | | | | |
| | | | | | |
| Research and development | | $ | 470,791 | | | $ | 142,951 | | | $ | 327,840 | |
| General and administrative | | 46,221 | | | 28,842 | | | 17,379 | |
| Total operating expenses | | 517,012 | | | 171,793 | | | 345,219 | |
| Loss from operations | | (517,012) | | | (171,793) | | | (345,219) | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Other income, net | | 18,483 | | | 14,071 | | | 4,412 | |
Loss before provision for income taxes | | (498,529) | | | (157,722) | | | (340,807) | |
Provision for income taxes | | 746 | | | 3,116 | | | (2,370) | |
Net loss | | $ | (499,275) | | | $ | (160,838) | | | $ | (338,437) | |
| | | | | | |
| | | | | | |
| | | | | | |
Research and Development Expenses | | | | | | | | | | | | | | | | | | | | |
| | Six Months Ended June 30, | | |
| | 2024 | | 2023* | | Change |
| In thousands | | |
| Direct research and development expenses by program: | | | | | | |
BHV-4157 (Troriluzole) | | $ | 34,163 | | | $ | 37,543 | | | $ | (3,380) | |
BHV-2000 (Taldefgrobep Alfa) | | 28,876 | | | 17,933 | | | 10,943 | |
BHV-7000 & BHV-7010 (Kv7) | | 224,053 | | | 17,773 | | | 206,280 | |
BHV-2100 (TRPM3 Antagonist) | | 8,892 | | | 2,026 | | | 6,866 | |
BHV-8000 (TYK2/JAK1) | | 6,924 | | | 1,161 | | | 5,763 | |
BHV-1300 (IgG Degrader) | | 16,583 | | | 4,475 | | | 12,108 | |
BHV-1310 (IgG Degrader) | | 7,199 | | | — | | | 7,199 | |
BHV-1400 (IgA Degrader) | | 11,704 | | | — | | | 11,704 | |
BHV-1510 (Trop2) | | 22,629 | | | — | | | 22,629 | |
| Other programs | | 1,522 | | | 3,780 | | | (2,258) | |
| Unallocated research and development costs: | | | | | | |
| Personnel related (including non-cash share-based compensation) | | 65,565 | | | 36,959 | | | 28,606 | |
| Preclinical research programs | | 30,717 | | | 13,803 | | | 16,914 | |
| Other | | 11,964 | | | 7,498 | | | 4,466 | |
| Total research and development expenses | | $ | 470,791 | | | $ | 142,951 | | | $ | 327,840 | |
*Certain prior year amounts have been reclassified to conform to current year presentation
R&D expenses, including non-cash share-based compensation costs, were $470.8 million for the six months ended June 30, 2024, compared to $143.0 million for the six months ended June 30, 2023. The increase of $327.8 million was primarily due to non-cash expense of $171.9 million paid to Knopp for a milestone and royalty buyback related to the BHV-7000 and broader Kv7 platform that was recognized during the three months ended June 30, 2024 (the buyback reduced our potential future milestone payments by $867.5 million, and replaced the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for the Kv7 programs). See further discussion of the Knopp Amendment included in Note 10, "License, Acquisitions and Other Agreements" to the condensed consolidated financial statements included in this Form 10-Q. The increase in expense was also related to advancing our 6 clinical platforms including 5 phase 3 starts for BHV-7000, follow-on Kv7 assets, preclinical research programs, and increases in direct program spend for additional and advancing multiple clinical development programs in 2024, as compared to the same period in the prior year. The $22.6 million increase in expense for BHV-1510 was primarily due to the Pyramid Acquisition, which resulted in $10.9 million (non-cash) of expense recorded to R&D during the six months ended June 30, 2024, a $1.5 million milestone payment which became due during the first quarter of 2024, and a $5.7 million non-cash milestone payment which became due during the first quarter of 2024.
Non-cash share-based compensation expense was $28.4 million for the six months ended June 30, 2024, an increase of $23.7 million as compared to the same period in 2023. Non-cash share-based compensation expense was higher in the 2024 primarily due to our annual equity incentive awards granted in the fourth quarter of 2023 and first quarter of 2024.
General and Administrative Expenses
G&A expenses, including non-cash share-based compensation costs, were $46.2 million for the six months ended June 30, 2024, compared to $28.8 million for the six months ended June 30, 2023. The increase of $17.4 million was primarily due to increased non-cash share-based compensation expense. Non-cash share-based compensation expense was $18.7 million for the six months ended June 30, 2024, an increase of $15.0 million as compared to the same period in 2023. Non-cash share-based compensation expense was higher in 2024 primarily due to our annual equity incentive awards being granted in the fourth quarter of 2023 and the first quarter of 2024.
Other Income , Net
Other income, net was a net income of $18.5 million for the six months ended June 30, 2024, compared to $14.1 million for the six months ended June 30, 2023. The increase was primarily due to a $9.2 million gain recorded upon settlement of our forward contract liability for the 2024 Additional Consideration issued
under the Knopp Amendment, and increased investment income. This was partially offset by a decrease of $5.6 million in other income recognized during the six months ended June 30, 2024 as compared to the same period in 2023 related to the Transition Services Agreement entered into with the Former Parent. See Note 4, "Fair Value of Financial Assets and Liabilities" to the accompanying condensed consolidated financial statements included in this Form 10-Q for discussion of the forward contract and derivative liabilities recorded in connection with the Knopp Amendment.
Provision for Income Taxes
We recorded an income tax provision of $0.7 million for the six months ended June 30, 2024, compared to an income tax provision of $3.1 million for the six months ended June 30, 2023. The decrease in income tax expense for the six months ended June 30, 2024 as compared to 2023 was primarily attributable to our adoption of the Notice. See further discussion of the Notice in Note 12, "Income Taxes" to the condensed consolidated financial statements included in this Form 10-Q.
Liquidity and Capital Resources
Since our inception, we have not generated any revenue and have incurred significant operating losses and negative cash flows from operations. We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for our product candidates. We expect to continue to incur significant expenses for at least the next several years as we advance our product candidates from discovery through preclinical development and clinical trials and seek regulatory approval and pursue commercialization of any approved product candidate. In addition, if we obtain marketing approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. In addition, we may incur expenses in connection with the in-license or acquisition of additional product candidates
Historically, we have funded our operations primarily with the cash contribution received from the Former Parent at the Separation and proceeds from the sale of our common shares. We have incurred recurring losses since our inception and expect to continue to generate operating losses for the foreseeable future.
As of June 30, 2024, we had cash and cash equivalents of $239.1 million and marketable securities of $197.8 million. Cash in excess of immediate requirements is invested in marketable securities and money market funds with a view to liquidity and capital preservation. We continuously assess our working capital needs, capital expenditure requirements, and future investments or acquisitions.
Cash Flows
The following table summarizes our cash flows for each of the periods presented: | | | | | | | | | | | | | | | | | | | | | | |
| | Six Months Ended June 30, | | | | |
| | | 2024 | | 2023 | | Change | | |
| In thousands | | | | | | |
| Net cash used in operating activities | | $ | (270,435) | | | $ | (122,031) | | | $ | (148,404) | | | |
Net cash (used in) provided by investing activities | | (63,900) | | | 75,195 | | | (139,095) | | | |
Net cash provided by financing activities | | 324,546 | | | 6,329 | | | 318,217 | | | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | | (13) | | | (147) | | | 134 | | | |
Net (decrease) in cash, cash equivalents and restricted cash | | $ | (9,802) | | | $ | (40,654) | | | $ | 30,852 | | | |
Operating Activities
Net cash used in operating activities was $270.4 million for the six months ended June 30, 2024 and $122.0 million for the six months ended June 30, 2023. The $148.4 million increase in net cash used in operating activities for the six months ended June 30, 2024 was primarily due to an increase in R&D spending to advance our 6 clinical platforms including 5 phase 3 starts for BHV-7000, follow-on Kv7 assets, preclinical research programs, and increases in direct program spend for additional and advancing multiple clinical development programs in 2024, as compared to the same period in the prior year. The R&D spend during the period includes $2.5 million in milestone payments for BHV-1510 and BHV-1300.
Investing Activities
Net cash used in investing activities was $63.9 million for the six months ended June 30, 2024, compared to net cash provided by investing activities of $75.2 million for the six months ended June 30, 2023. The $139.1 million increase in net cash used in investing activities for the six months ended June 30, 2024 was primarily driven by an increase in purchases of marketable securities with cash in excess of immediate requirements, partially offset by an increase in maturities of marketable securities (see Note 3, "Marketable Securities," to the Condensed Consolidated Financial Statements for additional details), as compared to the same period in the prior year.
Financing Activities
Net cash provided by financing activities was $324.5 million for the six months ended June 30, 2024 compared to $6.3 million for the six months ended June 30, 2023. The $318.2 million increase in net cash provided by financing activities for the six months ended
June 30, 2024 was primarily driven by an increase in proceeds from the issuance of common shares from our April 2023 public equity offering and Equity Distribution Agreement, both during the second quarter of 2024, as compared to the same period in the prior year.
April 2024 Public Offering
On April 22, 2024, we closed an underwritten public offering of 6,451,220 of its common shares, which included the exercise in full of the underwriters' option to purchase additional shares, at a price to the public of $41.00 per share. The net proceeds raised in the offering, after deducting underwriting discounts and expenses of the offering payable by us, were approximately $247.8 million. We intend to use the net proceeds received from the offering for general corporate purposes.
Equity Distribution Agreement
In October 2023, we entered into an equity distribution agreement pursuant to which we may offer and sell common shares having an aggregate offering price of up to $150.0 million from time to time through or to the sales agent, acting as our agent or principal (the "Equity Distribution Agreement"). Sales of our common shares, if any, will be made in sales deemed to be “at-the-market offerings”. The sales agent is not required to sell any specific amount of securities but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between the sales agent and us. We currently plan to use the net proceeds from any at-the-market offerings of our common shares for general corporate purposes.
As of June 30, 2024, we have sold and issued 2,093,731 common shares under the Equity Distribution Agreement for net proceeds of approximately $69,890. Subsequent to June 30, 2024, we have issued and sold an additional 2,154,857 common shares for net proceeds of $76,360. As of August 9, 2024, the date of this report, the we have issued and sold a total of 4,248,588 common shares under the Equity Distribution Agreement for total net proceeds of $146,250, and no shares remain available to be issued.
Knopp Amendment
In May 2024, we entered into the Knopp Amendment which reduced our potential future milestone payments by $867.5 million, and replaced the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for our Kv7 programs. As consideration, we agreed to issue to Knopp the 2024 Additional Consideration and 2025 Additional Consideration, both non-cash common share payments, as well as agreed to one-time cash-true ups for both the 2024 Additional Consideration and 2025 Additional Consideration. See Recent Developments elsewhere in this Current Report on Form 10-Q for additional information on the Knopp Amendment.
On May 30, we issued 1,872,874 common shares valued at $66.0 million to Knopp to settle the forward contract liability related to the 2024 Additional Consideration and recognized a non-cash gain of $9.2 million on settlement. We expect to issue the common shares representing the 2025 Additional Consideration on or before June 30, 2025, in accordance with the Knopp Amendment.
In the event that Knopp continues to hold the shares of the Company's common shares representing the 2024 Additional Consideration and 2025 Additional Consideration on December 1, 2024 and 2025, respectively, and the Market Price declines relative to the respective Reference Price, both as defined in the agreement, we would be responsible for a cash payment equal to the difference.
Knopp Warrant
As further consideration under the Knopp Amendment, we issued to Knopp a warrant to purchase 294,195 of the Company's common shares with a purchase price of $67.98, subject to certain specified development milestones and the Company achieving a specified market capitalization.
As of June 30, 2024, the vesting conditions have not been met and the warrant is still outstanding.
Funding Requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we advance and expand preclinical activities, clinical trials and potential commercialization of our product candidates. Our costs will also increase as we:
•continue to advance and expand the development of our discovery programs and clinical-stage assets;
•continue to initiate and progress other supporting studies required for regulatory approval of our product candidates, including long-term safety studies, drug-drug interaction studies, preclinical toxicology and carcinogenicity studies;
•initiate preclinical studies and clinical trials for any additional indications for our current product candidates and any future product candidates that we may pursue;
•continue to build our portfolio of product candidates through the acquisition or in-license of additional product candidates or technologies;
•continue to develop, maintain, expand and protect our intellectual property portfolio;
•pursue regulatory approvals for our current and future product candidates that successfully complete clinical trials;
•establish and support our sales, marketing and distribution infrastructure to commercialize any future product candidates for which we may obtain marketing approval; and
•hire additional clinical, medical, commercial, and development personnel.
We expect that our cash, cash equivalents and marketable securities, as of the date of this Quarterly Report on Form 10-Q, will be sufficient to fund operating and financial commitments and other cash requirements for more than one year. We expect we will need to raise additional capital until we are profitable. If no additional capital is raised through either public or private equity financings, debt financings, strategic relationships, alliances and licensing agreements, or a combination thereof, we may delay, limit or reduce discretionary spending in areas related to research and development activities and other general and administrative expenses in order to fund our operating costs and working capital needs.
We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. We expect that we will require additional capital to pursue in-licenses or acquisitions of other product candidates. If we receive regulatory approval for our product candidates, we expect to incur commercialization expenses related to product manufacturing, sales, marketing and distribution, depending on where we choose to commercialize or whether we commercialize jointly or on our own.
Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical product candidates, we are unable to estimate the exact amount of our working capital requirements. Our future funding requirements will depend on and could increase significantly as a result of many factors, including:
•the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials;
•the costs, timing and outcome of regulatory review of our product candidates;
•the costs and timing of hiring new employees to support our continued growth;
•the costs of preparing, filing, and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims;
•the extent to which we acquire or in-license other product candidates and technologies;
•the timing, receipt and amount of sales of, or milestone payments related to or royalties on,
our current or future product candidates, if any; and
•other capital expenditures, working capital requirements, and other general corporate activities.
Until such time, if ever, that we can generate product revenue sufficient to achieve profitability, we expect to finance our cash needs through a combination of public and private equity offerings, debt financings, other third-party funding, strategic alliances, licensing arrangements or marketing and distribution arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing shareholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through other third-party funding, strategic alliances, licensing arrangements or marketing and distribution arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us.
Contractual Obligations and Commitments
Except as discussed in Note 11, "Commitments and Contingencies" to our Condensed Consolidated Financial Statements included in Item 1, “Unaudited Condensed Consolidated Financial Statements,” of this Quarterly Report on Form 10-Q, there have been no material changes to our contractual obligations and commitments as included in our audited consolidated financial statements included in the 2023 Form 10-K.
Critical Accounting Policies and Significant Judgments and Estimates
We have prepared our condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States ("GAAP"). Our preparation of our condensed consolidated financial statements requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, expenses, and related disclosures at the date of the condensed consolidated financial statements. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially
from these estimates under different assumptions or conditions.
Excluding the discussion below, during the six months ended June 30, 2024, there were no material changes to our critical accounting policies as reported in our annual consolidated financial statements included in the 2023 Form 10-K.
Valuation of Forward Contract and Derivative Liabilities
We have accounted for certain consideration agreed to in connection with the Knopp Amendment as forward contracts and derivative liabilities. The fair value of these liabilities have been determined based on Monte Carlo simulations of Biohaven's share price, which requires judgment and assumptions on the volatility of our share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk as they are payable in either cash or a variable number of shares.
Our expectations of the volatility of Biohaven's share price at the reporting date could be materially different than our actual future volatility, and if so, would mean the estimated fair value could be significantly higher or lower than the fair value determined.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations, if applicable, is disclosed in Note 2 to our condensed consolidated financial statements appearing at the beginning of this Quarterly Report on Form 10-Q.
Item 3. Quantitative and Qualitative Disclosures about Market Risks
Foreign Currency Translation
Our operations include activities in countries outside the U.S. As a result, our financial results are impacted by factors such as changes in foreign currency exchange rates or weak economic conditions in the foreign markets where we operate. Our monetary exposures on our balance sheet are currently immaterial to our financial position as of June 30, 2024.
We do not engage in any hedging activities against changes in foreign currency exchange rates.
Interest Rate Risk
As of June 30, 2024, we invest our excess cash balances in marketable securities of highly rated financial institutions and investment-grade debt instruments. We seek to diversify our investments and limit the amount of investment concentrations for individual institutions, maturities and investment types. Most of our interest-bearing securities are subject to interest rate risk and could decline in value if interest rates fluctuate. Based on the type of securities we hold,
we do not believe a change in interest rates would have a material impact on our financial statements. If interest rates were to increase or decrease by 1.00%, the fair value of our investment portfolio would (decrease) increase by approximately $(0.4) million and $0.4 million, respectively.
We do not engage in any hedging activities against changes in interest rates.
Equity Price Risk
We hold financial liabilities in the form of derivative liabilities related to the potential one-time cash true-up payments under the Knopp Amendment that are measured at fair value and recorded through net income (loss) in our condensed consolidated statement of operations and comprehensive loss. A primary driver of the fair value of these derivative liabilities is our 20-day volume weighted average share price ("VWAP") relative to the Reference Price, as defined in the agreement. A decrease in our 20-day VWAP relative to the Reference Price would result in an increase in the fair value of the liabilities.
Further information regarding the fair value of these financial liabilities that are measured at fair value on a recurring basis, and the unobservable inputs used in the valuation of these financial liabilities, is presented in Note 4, "Fair Value of Financial Assets and Liabilities" and Note 10, "License, Acquisitions and Other Agreements" to the accompanying condensed consolidated financial statements included in this Form 10-Q.
Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist of cash, cash equivalents, and short-term debt securities. The Company maintains a portion of its cash deposits in government insured institutions in excess of government insured limits. The Company deposits its cash in financial institutions that it believes have high credit quality and has not experienced any losses on such accounts. The Company's cash management policy permits investments in U.S. federal government and federal agency securities, corporate bonds or commercial paper, supranational and sovereign obligations, certain qualifying money market mutual funds, certain repurchase agreements, and places restrictions on credit ratings, maturities, and concentration by type and issuer. The Company is exposed to credit risk in the event of a default by the financial institutions holding its cash in excess of government insured limits and in the event of default by corporations and governments in which it holds investments in cash equivalents and short-term debt securities, to the extent recorded on the condensed consolidated balance sheet.
We have not experienced any credit losses or recorded any allowance for credit losses related to our cash, cash equivalents, and short-term debt securities.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, refers to controls and procedures that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that such information is accumulated and communicated to a company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
In designing and evaluating our disclosure controls and procedures, management recognizes that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a control system, misstatements due to error or fraud may occur and not be detected.
Based on the evaluation of our disclosure controls and procedures as of June 30, 2024, our Chief Executive Officer and Chief Financial Officer have concluded that, as of June 30, 2024, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Controls over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended June 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, we may be subject to litigation and claims arising in the ordinary course of business. We are not currently a party to any material legal proceedings, and we are not aware of any pending or threatened legal proceeding against us that we believe could have a material adverse effect on our business, operating results, cash flows or financial condition.
Item 1A. Risk Factors
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. Our risk factors have not changed materially from those described in "Part I, Item 1A. Risk Factors" of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on February 29, 2024.
Item 2. Unregistered Sales of Equity Securities, Use of Proceeds, and Issuer Purchases of Equity Securities
Pyramid Agreement
In January 2024, we acquired Pyramid Biosciences, Inc. ("Pyramid"), pursuant to an Agreement and Plan of Merger, dated January 7, 2024 (the "Pyramid Agreement"). In consideration for the Pyramid acquisition we made an upfront payment of 255,794 of our common shares. As of June 30, 2024, 253,410 of these common shares have been issued by the Company, including 10,452 shares that were issued in the second quarter of 2024. We also agreed to make additional success-based payments upon the achievement of certain regulatory milestones, which we may elect to pay in cash or our common shares. In January 2024, a payment became due to Pyramid related to achievement of a developmental milestone under the Pyramid Agreement, which we elected to pay in 98,129 of our common shares. As of June 30, 2024, 97,233 of these common shares have been issued by the Company. The shares related to both of these payments were not registered under the Securities Act upon issuance, and a portion of the shares were subsequently registered under our Registration Statement on Form S-3.
Pyramid represented that, among other things, it is an institutional accredited investor as defined in Rule 501(a) of Regulation D of the Securities Act. The foregoing shares shall be issued in reliance on the private offering exemption provided by Section 4(a)(2) of the Securities Act. See Note 10, "License, Acquisitions and Other Agreements," to the Condensed Consolidated Financial Statements appearing elsewhere in this report for additional details on this transaction.
Amendment to Knopp Purchase Agreement
In May 2024, we entered into an amendment (the “Amendment”) to our existing Membership Interest Purchase Agreement, dated as of February 24, 2022, with Knopp Biosciences LLC (“Knopp”) in order to make certain changes to the royalty payment obligations and success-based payments. See Item 5, “Other Information” for additional information related to the Amendment.
In connection with the Amendment, we issued to Knopp 1,872,874 of our common shares, valued at approximately $75 million, through a private placement within 60 days of the date of execution of the Amendment (the “2024 Additional Consideration”). We also agreed to issue additional shares of our common shares, valued at approximately $75 million, within 60 days of the first anniversary of execution of the Amendment (the “2025 Additional Consideration”). In addition, we issued to Knopp a warrant (the "Warrant") to purchase 294,195 of our common shares at a purchase price per share of $67.98, subject to certain specified development milestones and achieving a specified market capitalization.
The foregoing issuance and sale of our common shares in connection with the execution of the Amendment and the Warrant have not been registered under the Securities Act of 1933 (the “Securities Act”) or any state securities laws. We have relied on the exemption from the registration requirements of the Securities Act under Section 4(a)(2) thereof, for a transaction by an issuer not involving any public offering.
Item 5. Other Information
Rule 10b5-1 Trading Plans
During the quarter ended June 30, 2024, none of our directors or officers adopted or terminated a "Rule 10b5-1 trading arrangement" or "non-Rule 10b5-1 trading arrangement," as each term is defined in Item 408 of Regulation S-K.
Item 6. Exhibits | | | | | | | | |
Exhibit No. | | Description |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
2.1# | | |
4.1# | | |
| 31.1 | | |
| 31.2 | | |
| 32.1‡ | | |
| 101 | | The following materials from the Registrant's Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2024 are formatted in iXBRL (Inline eXtensible Business Reporting Language): (i) the Condensed Consolidated Balance Sheets, (ii) the Condensed Consolidated Statements of Operations and Comprehensive Loss, (iii) the Condensed Consolidated Statements of Cash Flows and (iv) the Notes to Condensed Consolidated Financial Statements, tagged as blocks of text and including detailed tags. |
| 104 | | Cover Page Interactive Data File (formatted in iXBRL in Exhibit 101). |
___________________________________________________
# Portions of this exhibit (indicated by asterisks) have been omitted as such information is (i) not material and (ii) would likely cause competitive harm to the Registrant if publicly disclosed.
‡ These certifications are being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| | BIOHAVEN LTD. |
| Dated: August 9, 2024 | |
| | By: | /s/ Vlad Coric, M.D. |
| | | Vlad Coric, M.D. |
| | | Chief Executive Officer |
| | | (On behalf of the Registrant and as the Principal Executive Officer) |
| | | |
| | By: | /s/ Matthew Buten |
| | | Matthew Buten |
| | | Chief Financial Officer |
| | | (Principal Financial Officer) |
Exhibit 2.1
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [***], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) THE TYPE THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL
Execution Copy
AMENDMENT TO MEMBERSHIP INTEREST PURCHASE AGREEMENT
THIS AMENDMENT TO MEMBERSHIP INTEREST PURCHASE AGREEMENT (“Amendment”) is entered into as of May 1, 2024 (the “Amendment Effective Date”), by and among Biohaven Therapeutics Ltd. (“Buyer”), Biohaven Ltd., a British Virgin Islands company (“Biohaven”), Knopp Biosciences LLC, a Delaware limited liability company (“Seller”), and Biohaven Pharmaceuticals, Inc. (the “BPI” and, together with Buyer and Biohaven, the “Buyer Parties”). Each of Buyer, Biohaven, BPI, and Seller is individually a “Party” and collectively, they are the “Parties”. Capitalized terms used but not otherwise defined herein have the meanings ascribed to them in the Agreement.
WITNESSETH
WHEREAS, Buyer, Channel Biosciences, LLC, a Delaware limited liability company (the “Company”), Biohaven Pharmaceutical Holding Company Ltd. (“Holding”) and Seller entered into that certain Membership Interest Purchase Agreement, dated as of February 24, 2022 (the “Agreement”), pursuant to which Buyer purchased the issued and outstanding membership interests of Company from Seller and Holding guaranteed certain obligations of Buyer under the Agreement; and
WHEREAS, Biohaven is the successor to Holding and is responsible for Holding’s obligations thereunder;
WHEREAS, on April 4, 2022, the Company merged with and into BPI, with BPI surviving the merger as the surviving corporation;
WHEREAS, the Parties wish to modify and amend the Agreement in accordance with the terms and conditions set forth herein,
NOW, THEREFORE, for valuable consideration, the receipt and sufficiency of which is hereby acknowledged, and intending to be legally bound, the Parties agree as follows:
1.Recitals. The recitals set forth above are incorporated into and made a part of this Amendment.
2.Definitions.
(a)New Definitions. The following definitions are added to the Agreement:
“10-Day VWAP” means, as of any date, the volume-weighted average sales price per Parent Share taken to four decimal places on the NYSE over the 10-consecutive trading day period preceding such date, as calculated by Bloomberg Financial LP under the function “VWAP” (or, if not available, in another authoritative source reasonably selected by Buyer).
“2024 Payment Date” means the day that the 2024 Additional Consideration is issued, which shall be no later than 60 days following the Amendment Effective Date.
“2024 Reference Price” means the 10-Day VWAP as of Amendment Effective Date, which is $40.0454.
“2025 Payment Date” means the day that the 2025 Additional Consideration is issued, which shall be no later than 60 days following the first anniversary of the Amendment Effective Date.
“2025 Reference Price” means the 10-Day VWAP as of the first anniversary of the Amendment Effective Date.
“Amendment Effective Date” means May 1, 2024.
“Payment Date” means the 2024 Payment Date and the 2025 Payment Date.
“Warrant” means the warrant issued to Seller by Parent pursuant to the Amendment to the Membership Interest Purchase Agreement, by and among Buyer, Parent, Seller, and Biohaven Pharmaceuticals, Inc., dated as of the Amendment Effective Date.
“Warrant Exercise Date” means the date that the Warrant is exercised pursuant to its terms.
“Warrant Shares” means the shares of Common Stock, without par value, of Parent issued pursuant to the Warrant.
(b)Amended Definitions. The following definitions in the Agreement are modified as follows:
“Net Sales”. The clause “, without duplication,” is hereby added after the word “billed” in the first sentence of the definition of “Net Sales” in the Agreement. The words “its Affiliates and its and their respective licensees” in the first sentence of the definition of “Net Sales” in the Agreement are hereby replaced with the words “Related Parties”.
“Parent” is amended and restated to mean “Biohaven Ltd., a BVI business company formed under the laws of the territory of the British Virgin Islands.”
“Registrable Shares” is amended and restated to mean “the Closing Consideration Shares, the Additional Consideration Shares and the Earn-Out Shares held by Seller, in each case, that have been held for at least 60 days following the date of issuance, including, without limitation, any Parent Shares paid, issued or distributed in respect of any such Parent Shares by way of stock dividend, stock split or distribution, or in connection with a combination of recapitalization, reorganization, merger or consolidation, or otherwise, but excluding Parent Shares acquired before or after the Closing Date other than Earn-Out Shares and Additional Consideration Shares; provided, however, that such Parent Shares will not be “Registrable Shares” (a) after such Parent Shares have been sold pursuant to an effective registration statement or in compliance with Rule 144 or other exemptions from registration or (b) when
the remainder of such Parent Shares held by Seller could, in the opinion of counsel satisfactory to Buyer, be sold by Seller in a single transaction without the volume and manner of sale limitations under Rule 144 unless Seller has taken action, without the consent or agreement of Buyer, subsequent to the date hereof to cause such Parent Shares not to be eligible for such sale under Rule 144.”
Related Party. The words “to which it grants” in the definition of “Related Party” in the Agreement are hereby replaced with the words “which receive”.
“Transaction Consideration” is amended and restated to mean “the Closing Cash Consideration plus the Closing Equity Consideration plus the Additional Consideration plus the Contingent Payments.”
(c)Deleted Definition. The following definition is deleted from the Agreement:
“Registration Statement” has the meaning set forth in Section 5.23.
3.Additional Consideration. A new Section 2.3.1 is added to the Agreement as follows:
“2.3.1 Additional Consideration. On the 2024 Payment Date, Parent shall issue to Seller the number of Parent Shares equal to $75,000,000 divided by the 2024 Reference Price (the “2024 Additional Consideration”) and on the 2025 Payment Date, Parent shall issue to Seller the number of Parent Shares equal to $75,000,000 divided by the 2025 Reference Price (the “2025 Additional Consideration”) (the 2024 Additional Consideration and 2025 Additional Consideration are referred to collectively as the “Additional Consideration”; the Parent Shares issued as the 2024 Additional Consideration and 2025 Additional Consideration are referred to herein respectively as the “2024 Additional Consideration Shares” and the “2025 Additional Consideration Shares”, respectively, and collectively with the Warrant Shares, as the “Additional Consideration Shares”). Notwithstanding the foregoing, if either the 2024 Additional Consideration Shares or the 2025 Additional Consideration Shares are not listed on the NYSE or any other national stock exchange on the applicable date of issuance, Seller may, in Seller’s discretion, in each instance, elect to receive from Buyer an amount in cash of $75,000,000 in lieu of the 2024 Additional Consideration Shares or the 2025 Additional Consideration Shares, as applicable, in which event such amount shall be paid by Buyer to Seller in immediately available funds on the 2024 Payment Date, in the case of a payment in lieu of the 2024 Additional Consideration Shares, or on the 2025 Payment Date, in the case of a payment in lieu of the 2025 Additional Consideration Shares.”
4.Regulatory Milestone Payments. Section 2.4(a) of the Agreement is hereby amended and restated in full as follows:
“(a) Regulatory Milestone Payments. As additional consideration for the Transactions, upon the satisfaction of the criteria described in each subsection of this Section 2.4(a) (each, a “Regulatory Milestone”), Buyer shall, subject to the terms of this Section 2.4(a), pay or cause to be paid to Seller in cash the amount specified in such subsection (each such payment, a “Regulatory Milestone Payment”). Except as expressly specified below, each Regulatory Milestone Payment shall be payable only once [***]:
(i) [***];
(ii) [Intentionally omitted];
(iii) [Intentionally omitted];
(iv) [Intentionally omitted];
(v) [***];
(vi) [Intentionally omitted];
(vii) [***];
(viii) [Intentionally omitted];
(ix) [***]; and
(x) [Intentionally omitted].”
5.Sales Milestones. Section 2.4(b) of the Agreement is amended and restated in full as follows:
“(b) [Intentionally omitted]”
6.Net Sales Payments. Section 2.4(c) of the Agreement is amended and restated in full as follows:
“(c) Net Sales Payments. As additional consideration for the Transactions, on a Kv7 Product-by-Kv7 Product and country-by-country basis, with respect to the Net Sales of a particular Kv7 Product in a country until the latest of: (a) [***] after the First Commercial Sale of such Kv7 Product in such country, (b) the expiration of the last to expire of a Valid Claim of a Transferred Patent (or any Related Patent thereof) or a Kv7 Discovery Platform Patent (or any Related Patent thereof), in each case, that would be infringed by the manufacture, use, sale, importation or offer for sale in such country of such Kv7 Product and (c) [***] (the “Net Sales Term”), Buyer shall, subject to the terms of this Section 2.4, on a quarterly basis, pay or cause to be paid to Seller in cash an amount equal to [***] of the Net Sales of all Kv7 Products during such quarter (each such payment, a “Net Sales Payment” and together with the Regulatory Milestone Payments, the “Contingent Payments”).”
7.Payment of Contingent Consideration. The first two sentences of Section 2.4(e)(i) of the Agreement are amended and restated as follows:
“Buyer shall deliver, or cause to be delivered, written notice to Seller of the achievement of any Regulatory Milestone no later than [***] after the occurrence thereof, and, subject to Section 2.4(h), within 30 days of the delivery of such notice, Buyer shall deliver, or cause to be delivered, the applicable Regulatory Milestone Payment to Seller, provided that, if the Regulatory Milestone in Section 2.4(a)(v) is achieved (the “Achievement”), the Regulatory Milestone Payment with respect to the Regulatory
Milestone in Section 2.4(a)(v) shall be paid no later than the earlier of (A) [***] after the date of the First Commercial Sale of a Kv7 Product containing KB-3061 in any country; (B) upon the consummation of a Change of Control (as such term is defined in the Warrant) after the Achievement with [***], other than any Change of Control that results in Buyer retaining any right to manufacture, market or sell any Kv7 Product containing KB-3061 and continuing to operate as a wholly owned subsidiary of a publicly traded company listed on a national stock exchange under the name of “Biohaven” or a variation thereof; or (C) upon the consummation of a Sale Transaction after the Achievement that involves the sale or transfer (but not license) of all or substantially all of Buyer’s and its Affiliates’ right, title and interest in and to KB-3061 [***]. In no event shall any Regulatory Milestone Payment be paid more than once other than with respect to the Regulatory Milestone set forth in Section 2.4(a)(ix), [***].”
8.Additional Consideration True-Up Payments. A new Section 2.5.1 is hereby added to the Agreement as follows:
“2.5.1 Additional Consideration True-Up Payments.
(a)(i) If Seller continues to own any 2024 Additional Consideration Shares on December 1, 2024, it may deliver written notice to Buyer on December 1, 2024 or within five Business Days thereafter (the “2024 True-Up Request Period”) requesting a one-time payment from Buyer (the “2024 True-Up Payment”) in an amount equal to (i) the number of 2024 Additional Consideration Shares held by Seller at the end of the day on December 1, 2024, multiplied by (ii)(A) the 2024 Reference Price, minus (B) the sum of (I) the Market Price of the Parent Shares as of December 1, 2024, plus (II) the aggregate amount of cash dividends declared in respect of each Parent Share with a record date between the Amendment Effective Date and December 1, 2024; provided, that notwithstanding the foregoing, the 2024 True-Up Payment amount shall be reduced by the product of (X) the amount (if any) by which the volume-weighted average sales price of all 2024 Additional Consideration Shares sold prior to December 1, 2024 exceeded the 2024 Reference Price, multiplied by (Y) the number of 2024 Additional Consideration Shares sold on or prior to December 1, 2024. If such notice is received during the 2024 True-Up Request Period, Buyer shall promptly, and in any event within ten Business Days, pay, or cause to be paid, the 2024 True-Up Payment to Seller in cash in accordance with the instructions set forth in the Funds Flow Memorandum (as may be updated by Seller in a written notice followed by oral confirmation); provided, that the 2024 True-Up Payment shall only be available to Seller if at no time between the Amendment Effective Date and the payment of the 2024 True-Up Payment has Seller or any of its Subsidiaries maintained any short position in the Parent Shares or entered into any other derivative or other agreement, arrangement or understanding that hedges or transfers, in whole or in part, directly or indirectly, any of the economic consequences of ownership of Parent Shares (the “2024 No-Hedging Condition”). The 2024 True-Up Payment, if any, shall be treated as adjustments to the consideration paid pursuant to the Transactions for Tax purposes.
(ii) The Notice delivered by Seller in clause (a)(i) shall provide a reasonably detailed reporting of, and support for, all sales of 2024 Additional Consideration Shares prior to December 1, 2024, including the price of such sales, and a certification of any executive officer of Seller that the 2024 No-Hedging Condition has not been violated. Seller acknowledges and agrees that it shall not be entitled to receive the 2024 True-Up Payment if the condition in the proviso in the foregoing sentence is violated.
(iii) In the event that Parent changes the number of Parent Shares or securities convertible or exchangeable into or exercisable for Parent Shares issued and outstanding prior to December 1, 2024 as a result of a reclassification, stock split (including a reverse stock split), stock dividend or distribution, recapitalization, merger, issuer tender or exchange offer or other similar transaction, the 2024 True-Up Payment shall be equitably adjusted.
(b)(i) If Seller continues to own any 2025 Additional Consideration Shares on December 1, 2025, it may deliver written notice to Buyer on December 1, 2025 or within five Business Days thereafter (the “2025 True-Up Request Period”) requesting a one-time payment from Buyer (the “2025 True-Up Payment”) in an amount equal to (i) the number of 2025 Additional Consideration Shares held by Seller at the end of the day on December 1, 2025, multiplied by (ii)(A) the 2025 Reference Price, minus (B) the sum of (I) the Market Price of the Parent Shares as of December 1, 2025, plus (II) the aggregate amount of cash dividends declared in respect of each Parent Share with a record date between the first anniversary of the Amendment Effective Date and December 1, 2025; provided, that notwithstanding the foregoing, the 2025 True-Up Payment amount shall be reduced by the product of (X) the amount (if any) by which the volume-weighted average sales price of all 2025 Additional Consideration Shares sold prior to December 1, 2025 exceeded the 2025 Reference Price, multiplied by (Y) the number of 2025 Additional Consideration Shares sold on or prior to December 1, 2025. If such notice is received during the 2025 True-Up Request Period, Buyer shall promptly, and in any event within ten Business Days, pay, or cause to be paid, the 2025 True-Up Payment to Seller in cash in accordance with the instructions set forth in the Funds Flow Memorandum (as may be updated by Seller in a written notice followed by oral confirmation); provided, that the 2025 True-Up Payment shall only be available to Seller if at no time between the Amendment Effective Date and the payment of the 2025 True-Up Payment has Seller or any of its Subsidiaries maintained any short position in the Parent Shares or entered into any other derivative or other agreement, arrangement or understanding that hedges or transfers, in whole or in part, directly or indirectly, any of the economic consequences of ownership of Parent Shares (the “2025 No-Hedging Condition”). The 2025 True-Up Payment, if any, shall be treated as adjustments to the consideration paid pursuant to the Transactions for Tax purposes.
(ii) The Notice delivered by Seller in clause (a)(i) shall provide a reasonably detailed reporting of, and support for, all sales of 2025 Additional Consideration Shares prior to December 1, 2025, including the price of such sales, and a certification of any executive officer of Seller that the 2025 No-Hedging Condition has not been violated. Seller acknowledges and agrees that it shall not be entitled to receive the 2025 True-Up Payment if the condition in the proviso in the foregoing sentence is violated.
(iii) In the event that Parent changes the number of Parent Shares or securities convertible or exchangeable into or exercisable for Parent Shares issued and outstanding prior to December 1, 2025 as a result of a reclassification, stock split (including a reverse stock split), stock dividend or distribution, recapitalization, merger, issuer tender or exchange offer or other similar transaction, the 2025 True-Up Payment shall be equitably adjusted.”
9.Registration Rights. Section 5.23 of the Agreement is amended and restated in full as follows:
“5.23 Registration Rights.
(a)So long as Parent’s existing registration statement under the Securities Act on Form S-3 ASR (Registration No. 333-274822) or a replacement thereto (the “Registration Statement”) remains in effect, Parent shall file (at Parent’s sole cost and expense) a prospectus supplement to register (a) the resale of the 2024 Additional Consideration Shares and the Warrant Shares that are Registrable Shares eligible for registration on the Registration Statement within 30 days following a written request by Seller (which may be provided by Seller as early as the 31st day following the Amendment Effective Date) at any time after the date that is 60 days following the Amendment Effective Date, (b) the resale of the 2025 Additional Consideration Shares that are Registrable Shares eligible for registration on the Registration Statement within 30 days following a written request by Seller (which may be provided by Seller as early as the 2025 Payment Date) at any time after the date that is 30 days following the 2025 Payment Date or (c) the resale of Earn-Out Shares that are Registrable Shares eligible for registration on the Registration Statement within 30 days following a written request by Seller (which may be provided by Seller as early as the date of the issuance of such Earn-Out Shares) at any time after the date that is 30 days following the date of the issuance of such Earn-Out Shares; provided, in each case, that if Seller determines in its reasonable good faith judgment that the filing of such prospectus supplement would (i) materially interfere with a significant acquisition, corporate organization, financing, securities offering or other similar transaction involving Parent or any of its Affiliates; (ii) require premature disclosure of material information that Parent has a bona fide business purpose for preserving as confidential; or (iii) render Parent unable to comply with requirements under the Securities Act or the Exchange Act, Buyer may delay the filing of such prospectus supplement until such time as the foregoing conditions no longer exist. Buyer shall use reasonable best efforts to maintain the effectiveness of such Registration Statement for a period ending on the date the Seller no longer holds more than 10,000 Registrable Shares.
(b)For not more than sixty (60) consecutive days or for a total of not more than one hundred twenty (120) days in any twelve (12) month period, Parent may suspend the use of any prospectus supplement included in any Registration Statement contemplated by this Section in the event that Parent determines in good faith that such suspension is necessary to (A) delay the disclosure of material non-public information concerning Parent, the disclosure of which at the time is not, in the good faith opinion of Parent, in the best interests of Parent or (B) amend or supplement the affected Registration Statement or the related prospectus or prospectus supplement so that such Registration Statement, prospectus or prospectus supplement shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the prospectus and prospectus supplement in light of the circumstances under which they were made, not misleading (an “Allowed Delay”); provided, that Parent shall promptly (i) notify Seller in writing of the commencement of an Allowed Delay, but shall not disclose to Seller any material non-public information giving rise to an Allowed Delay, (ii) advise Seller in writing to cease all sales under such Registration Statement until the end of the Allowed Delay and (iii) use commercially reasonable efforts to terminate an Allowed Delay as promptly as practicable.”
10.Stock Exchange Listing. Section 5.24(b) of the Agreement is amended and restated in full as follows:
“(b) to cause the Earn-Out Shares and the Additional Consideration Shares to be approved for listing on the NYSE, subject to official notice of issuance, prior to the date of issuance.”
11.Warrant Issuance. Simultaneously with the execution of this Amendment, Biohaven shall issue to Seller a warrant, in the form attached hereto as Exhibit A, to purchase shares of Common Stock, without par value, of Biohaven on the terms and subject to the conditions set forth therein.
12.Seller Representations and Warranties. Seller hereby represents to the Buyer Parties as of the Amendment Effective Date as follows:
(a)Authority; Approval. Seller has all requisite corporate or similar power and authority and has taken all corporate or similar action necessary in order to execute, deliver and perform its obligations under this Amendment and each of the Transaction Documents to which it is a party. This Amendment has been duly executed and delivered by Seller and, when executed and delivered by the Buyer Parties, will constitute a valid and binding agreement of Seller enforceable against Seller in accordance with its terms, subject to the Bankruptcy and Equity Exception. The Board of Managers of Seller has approved this Amendment. No other company or equity holder proceedings are necessary to authorize this Amendment or to consummate the Transactions.
(b)Governmental Filings; No Violations.
(i) Other than the filings, notices, reports, consents, registrations, approvals, permits, expirations of waiting periods or authorizations which may be required (i) under the HSR Act, the Exchange Act and the Securities Act or state securities, takeover and “blue sky” Laws or (ii) to be made with the NYSE (collectively, the “Seller Approvals”), no expirations of waiting periods under applicable Laws are required and no notices, reports or other filings are required to be made by Seller with, nor are any consents, registrations, approvals, permits or authorizations required to be obtained by Seller from, any Governmental Entity in connection with the execution, delivery and performance of this Amendment by Seller or the consummation of the transactions contemplated by this Amendment.
(ii) The execution, delivery and performance of this Amendment by Seller does not conflict with, or result in any breach or violation of or default (with or without notice, lapse of time, or both) under, or give rise to any right of termination, loss of rights, adverse modification of provisions, cancellation or acceleration of any obligation under, or result in the creation of a Lien on any of the assets of Seller under any provision of (i) the Organizational Documents of Seller, (ii) any Contract binding upon Seller or (iii) assuming (solely with respect to performance of this Agreement and the Transaction Documents and consummation of the Transactions) receipt of the Seller Approvals, any Laws to which Seller is subject, except, in the case of clauses (ii) and (iii) above, for any breach, violation, default, termination, loss, adverse modification, cancellation, acceleration or creation that would not, individually or in the aggregate, reasonably be expected to be prevent, materially delay or materially impair the transactions contemplated by this Amendment.
(c)Purchase Entirely for Own Account. Seller acknowledges that the Additional Consideration Shares shall be acquired for investment for Seller’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof, and Seller has no present intention of selling, granting any participation or otherwise distributing such Parent Shares. Seller can bear the economic risk of an investment in such Parent Shares indefinitely and a total loss with respect to
such investment. Seller does not have any contract, undertaking, agreement, arrangement or understanding with any Person to sell, transfer or grant participation to a Person any of such Parent Shares.
(d)Investment Experience and Accredited Investor Status. Seller is an “accredited investor” (as defined in Regulation D under the Securities Act). Seller has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Additional Consideration Shares.
(e)Acquiring Person. Neither Seller nor any of its Affiliates beneficially owns (as determined pursuant to Rule 13d-3 under the Exchange Act without regard for the number of days in which a Person has the right to acquire such beneficial ownership, and without regard to Seller’s rights under this Agreement), any securities of Parent, except for securities that may be beneficially owned by either (i) employee benefit plans of Seller or any of its Affiliates or (ii) any executive officer or director of Seller.
(f)No “Bad Actor” Disqualification. Seller has not taken any of the actions set forth in, and is not subject to, the disqualification provisions of Rule 506(d)(1) of the Securities Act. Seller’s responses in the questionnaire delivered to Buyer by Seller related to qualification under Rule 506(d)(1) are true and correct.
(g)Restricted Securities. Seller understands that the Additional Consideration Shares, when issued, shall be “restricted securities” under the federal securities Laws inasmuch as they are being acquired from Parent in a transaction not involving a public offering and that under such Laws such Parent Shares may be resold without registration under the Securities Act only in certain limited circumstances. Seller represents that it is familiar with Rule 144. Seller understands that such Parent Shares are being offered and issued to it in reliance on specific exemptions from the registration requirements of United States federal and state securities Laws and Buyer is relying in part upon the truth and accuracy of, and Seller’s compliance with, the representations, warranties, agreements, acknowledgements and understandings of Seller set forth in this Agreement in order to determine the availability of such exemptions and the eligibility of Seller to acquire such Parent Shares.
(h)Legends. Seller understands that any certificates or book entries representing the Registrable Shares shall, so long as required by this Section 13(h), bear the following legends:
(i) “THESE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933. THEY MAY NOT BE SOLD, OFFERED FOR SALE, PLEDGED OR HYPOTHECATED IN THE ABSENCE OF A REGISTRATION STATEMENT IN EFFECT WITH RESPECT TO THE SECURITIES UNDER THE SECURITIES ACT OR AN OPINION OF COUNSEL (WHICH COUNSEL SHALL BE REASONABLY SATISFACTORY TO BIOHAVEN LTD.) THAT SUCH REGISTRATION IS NOT REQUIRED OR UNLESS SOLD PURSUANT TO RULE 144 OF THE SECURITIES ACT.”
(ii) any legend required by applicable state securities Laws; and
(iii) “THE SECURITIES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO AND SHALL BE TRANSFERABLE ONLY UPON THE TERMS AND CONDITIONS OF A MEMBERSHIP INTEREST PURCHASE AGREEMENT DATED AS OF FEBRUARY 24, 2022 BY AND AMONG BIOHAVEN THERAPEUTICS LTD., KNOPP BIOSCIENCES LLC, BIOHAVEN PHARMACEUTICALS, INC. (AS SUCCESSOR TO CHANNEL BIOSCIENCES, LLC), AND, SOLELY FOR THE PURPOSES OF SECTION 9.14 THEREOF, BIOHAVEN LTD (AS SUCCESSOR TO BIOHAVEN PHARMACEUTICAL HOLDING COMPANY LTD.), AS AMENDED, A COPY OF WHICH IS ON FILE WITH THE SECRETARY OF BIOHAVEN LTD.”
Certificates or book-entry positions evidencing such shares shall not be required to contain such legends or any other legend (A) following any sale of such shares pursuant to an effective registration statement covering the resale of such shares, (B) following any sale of such shares pursuant to Rule 144, or (C) solely with respect to the legend described in Section 12(h)(i), if the holder of such shares is not (and for the preceding three months has not been) an affiliate of Parent, one (1) year following issuance (provided, however, that in the case of (A), (B) and (C), above, the holder provides Parent with customary legal representation letters reasonably acceptable to Parent). Whenever such restrictions shall cease and terminate as to any such shares, the holder of such shares shall be entitled to receive from Parent upon a written request in writing, without expense, new securities of like tenor not bearing the legends set forth herein. Parent shall cooperate with any holder of Registrable Securities being offered and, to the extent applicable, facilitate the timely preparation and delivery of certificates or book entries (not bearing any restrictive legend) representing the Registrable Securities to be offered pursuant to a registration statement and enable such certificates or book entries to be in such denominations or amounts, as the case may be, as the holder may reasonably request and registered in such names as such holder may request.
(i)Brokers and Finders. Except as set forth in Section 3.32 of the Company Disclosure Letter, neither Seller nor any of its Subsidiaries, or any of their respective directors or officers, as applicable, has employed any investment banker, broker or finder or incurred or will incur any liability for any brokerage payments, investment banking fees, commissions, finders’ fees or other similar payments in connection with the transactions contemplated by this Amendment. Seller and its Affiliates shall be solely responsible for the fees of any broker, finder, or agent engaged by Seller or any of its Affiliates in connection with the transactions contemplated by this Amendment or otherwise.
(j)No Other Representations or Warranties.
(i) Seller acknowledges and agrees that each Buyer Party has relied on the representations and warranties set forth in this Paragraph 12 in making the decision to enter into this Amendment. Except for the representations and warranties expressly set forth in this Paragraph 12 and in the Warrant, none of Seller, any of its Affiliates or any other Person makes (and Seller, on behalf of itself and its Affiliates, hereby disclaims) any other express or implied representation or warranty with respect to the transactions contemplated by this Amendment, Seller or any of its Affiliates or to any of their respective businesses, operations, assets, Liabilities, conditions (financial or otherwise) or prospects in connection with this Amendment or the transactions contemplated hereby (including any implied warranties that may otherwise be applicable because of the provisions of the Uniform Commercial Code or any other applicable Law, including the warranties of merchantability and fitness for a particular purpose).
(ii) Seller acknowledges and agrees that, except for the representations and warranties expressly set forth in Paragraph 13 of this Amendment and in the Warrant, none of the Buyer Parties or any other Person has made any express or implied representation or warranty with respect to the transactions contemplated by this Amendment, to the Buyer Parties or any of their respective Affiliates or to any of their respective businesses, operations, assets, Liabilities, conditions (financial or otherwise) or prospects in connection with this Amendment or the transactions contemplated hereby (including any implied warranties that may otherwise be applicable because of the provisions of the Uniform Commercial Code or any other applicable Law, including the warranties of merchantability and fitness for a particular purpose) and Seller has not relied on any representation or warranty other than those expressly set forth in Paragraph 13 of this Amendment and in the Warrant; provided, however, that notwithstanding anything to the contrary set forth in the foregoing provisions of this Paragraph 12(j)(ii), nothing in this Paragraph 12(j)(ii) shall limit Seller’s remedies with respect to claims of Fraud in connection with, arising out of or otherwise related to the express written representations and warranties made by Seller in this Amendment or the Warrant.
13.Buyer Parties Representations and Warranties. The Buyer Parties hereby jointly and severally represent to Seller as of the Amendment Effective Date as follows:
(a)Authority; Approval. The Buyer Parties each have all requisite corporate power and authority and have taken all corporate or similar action necessary in order to execute, deliver and perform its obligations under this Amendment. This Amendment has been duly executed and delivered by the Buyer Parties, and, when executed and delivered by the other Parties, will constitute a valid and binding agreement of the Buyer Parties enforceable against the Buyer Parties in accordance with its terms, subject to the Bankruptcy and Equity Exception.
(b)Valid Issuance of Parent Shares. When issued and delivered in accordance with the terms of the Agreement, the Additional Consideration Shares shall be duly authorized, validly issued, fully paid and nonassessable, free from any liens, encumbrances or restrictions on transfer, including preemptive rights, rights of first refusal or other similar rights, other than as arising pursuant to the Agreement, as a result of any action by Seller or under federal or state securities Laws.
(c)Governmental Filings; No Violations.Other than the Seller Approvals, no expirations of waiting periods under applicable Laws are required and notices, reports or other filings are required to be made by the Buyer Parties with, nor are any consents, registrations, approvals, permits or authorizations required to be obtained by the Buyer Parties from, any Governmental Entity in connection with the execution, delivery and performance of this Amendment by the Buyer Parties or the consummation of the transactions contemplated by this Amendment.
(ii) The execution, delivery and performance by the Buyer Parties of this Amendment does not, and the consummation of the Transactions will not, conflict with, or result in any breach or violation of, or default (with or without notice, lapse of time or both) under, or give rise to any right of termination, loss of rights, adverse modification of provisions, cancellation or acceleration of any obligations under, or result in the creation of a Lien on any of the assets of a Buyer Party under any provision of (i) its Organizational Documents, (ii) any Contract binding upon a Buyer Party or its Affiliates or (iii) assuming (solely with respect to the performance of
this Amendment and the consummation of the transactions contemplated hereby) receipt of the Seller Approvals, any Law to which a Buyer Party or its Affiliates is subject, except, in the case of clauses (ii) and (iii) above, for any such breach, violation, default, termination, loss, adverse modification, cancellation, acceleration or creation that would not, individually or in the aggregate, reasonably be expected to prevent, materially delay or materially impair the consummation of the transactions contemplated by this Amendment.
(d)Brokers and Finders. None of the Buyer Parties nor any of their Affiliates, nor any of their respective directors or employees (including any officers) has employed any broker, finder or investment bank or has incurred or will incur any obligation or liability for any brokerage fees, commissions or finders fees in connection with the transactions contemplated by this Amendment. The Buyer Parties shall be solely responsible for the fees of any broker, finder, or agent engaged by the Buyer Parties or any of their Affiliates in connection with the transactions contemplated by this Amendment or otherwise.
(e)No Other Representations or Warranties.
(i) Each Buyer Party acknowledges and agrees that Seller has relied on the representations and warranties set forth in this Paragraph 13 in making the decision to enter into this Amendment. Except for the representations and warranties expressly set forth in this Paragraph 13 and in the Warrant, none of the Buyer Parties, any of its Affiliates or any other Person makes (and each Buyer Party, on behalf of itself and its Affiliates, hereby disclaims) any other express or implied representation or warranty with respect to the transactions contemplated by this Amendment, any Buyer Party or any of their respective Affiliates or to any of their respective businesses, operations, assets, Liabilities, conditions (financial or otherwise) or prospects in connection with this Amendment or the transactions contemplated hereby (including any implied warranties that may otherwise be applicable because of the provisions of the Uniform Commercial Code or any other applicable Law, including the warranties of merchantability and fitness for a particular purpose).
(ii) Each Buyer Party acknowledges and agrees that, except for the representations and warranties expressly set forth in Paragraph 12 of this Amendment and in the Warrant, neither Seller nor or any other Person has made any express or implied representation or warranty with respect to the transactions contemplated by this Amendment, to Seller or any of its Affiliates or to any of their respective businesses, operations, assets, Liabilities, conditions (financial or otherwise) or prospects in connection with this Amendment or the transactions contemplated hereby (including any implied warranties that may otherwise be applicable because of the provisions of the Uniform Commercial Code or any other applicable Law, including the warranties of merchantability and fitness for a particular purpose) and the Buyer Parties have not relied on any representation or warranty other than those expressly set forth in Section 12 of this Amendment and in the Warrant; provided, however, that notwithstanding anything to the contrary set forth in the foregoing provisions of this Paragraph 13(e)(ii), nothing in this Paragraph 13(e)(ii) shall limit any Buyer Party’s remedies with respect to claims of Fraud in connection with, arising out of or otherwise related to the express written representations and warranties made by Seller in this Amendment or the Warrant.
14.Indemnification.
(a)Seller Indemnification. Seller shall indemnify, defend, hold harmless and reimburse Buyer Indemnified Parties for, from and against all Losses imposed on, incurred or suffered by or asserted against any Buyer Indemnified Party arising from any inaccuracy or breach of a representation in Paragraph 12 of this Amendment.
(b)Buyer Parties Indemnification. The Buyer Parties shall jointly and severally indemnify, defend, hold harmless and reimburse Seller Indemnified Parties for, from and against all Losses imposed on, incurred, suffered or asserted against any Seller Indemnified Party against any inaccuracy or breach of a representation in Paragraph 13 of this Amendment.
(c)Punitive Damages. No Party shall have any liability under this Paragraph 15 for any punitive damages, except to the extent actually awarded to a third party in a Third-Party Claim.
(d)Applicable Damages. Sections 8.4, 8.5, 8.8, 8.9 and 8.11 of the Agreement shall be applicable to claims for indemnification under this Paragraph 14. The rights of the Parties under this Paragraph 14 shall be in addition to, and not in lieu of, any rights under the Agreement.
(e)Exclusive Remedies and No Rights Against Nonparties.
(i) Following the Amendment Effective Date, no Party shall assert against any other Party any claim, cause of action, right or remedy, or any Action, relating to this Amendment, the Warrant, or the transactions contemplated hereby or thereby, other than claims pursuant to this Paragraph 14 or the right to seek specific performance pursuant to and subject to the terms of Paragraph 15(d). Following the Amendment Effective Date, the claims and remedies specified in the previous sentence shall constitute the Parties’ sole and exclusive rights and remedies available to the Indemnified Parties for any and all Losses or other claims relating to or arising out this Amendment, the Warrant, or the transactions contemplated hereby or thereby and shall supersede all other rights and remedies available at law or in equity (including any right of rescission). Accordingly, effective as of the Amendment Effective Date, each Party hereby irrevocably waives and discharges, and releases each other Party, to the fullest extent permitted under applicable Law, from, all other claims, causes of action and Actions relating thereto (but excluding, for the avoidance of doubt, any claims arising under the Agreement).
(ii) This Amendment may only be enforced against, and any Action, right or remedy that may be based upon, arise out of or relate to this Amendment, the Warrant or the transactions contemplated hereby or thereby, or the negotiation, execution or performance of this Amendment or the Warrant, may only be made against the Persons that are expressly identified as Parties in their capacities as parties to this Agreement, and no Party shall at any time assert against any Nonparty, any claim, cause of action, right or remedy, or any Action, relating to this Amendment, the Warrant, or the transactions contemplated hereby or thereby. Each Party hereby waives and discharges any such claim, cause of action, right, remedy and Action, and releases (and agrees to execute and deliver any instrument necessary to effectuate the release of) each Nonparty therefrom. The provisions of this Paragraph 14(e)(ii) are for the benefit of and shall be
enforceable by each Nonparty, which is an intended third-party beneficiary of this Paragraph 14(e)(ii).
15.Miscellaneous
(a)Except as expressly provided in this Amendment, the other terms and conditions of the Agreement are hereby ratified, affirmed and confirmed in all respects and shall remain in full force and effect.
(b)Sections 9.1, 9.2, 9.3, 9.4, 9.5, 9.6, 9.7, 9.9, 9.10, and 9.11 of the Agreement are incorporated herein mutatis mutandis.1
[Signature Page Follows]
IN WITNESS WHEREOF, the Parties have executed this Amendment as of the Amendment Effective Date.
| | | | | |
| BIOHAVEN THERAPEUTICS LTD. |
| |
| By: | /s/ Matt Buten |
| Name: | Matt Buten |
| Title: | Treasurer |
| | |
| BIOHAVEN LTD. |
| | |
| By: | /s/ Vlad Coric |
| Name: | Vlad Coric, M.D. |
| Title: | Chief Executive Officer |
| | |
| |
| BIOHAVEN PHARMACEUTICALS, INC. |
| |
| By: | /s/ Warren Volles |
| Name: | Warren Volles |
| Title: | Chief Legal Officer |
| | |
WITNESS to Signatures of Biohaven Ltd., Biohaven Therapeutics Ltd. and Biohaven Pharmaeuticals, Inc.: Location: Hamilton, Bermuda |
Date: April 30, 2024 By: /s/ Bruce Car Name: Bruce Car, Ph.D. Title: Chief Scientific Officer, Biohaven Ltd. |
| | | | | |
| KNOPP BIOSCIENCES LLC |
| |
| By: | /s/ Frank J. Lucchino Jr. |
| Name: | Frank J. Lucchino Jr. |
| Title: | President |
[Signature page to Amendment]
Exhibit A
Form of Warrant
WARRANT No. 1
THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW AND, IF THE COMPANY REQUESTS, AN OPINION SATISFACTORY TO THE COMPANY TO SUCH EFFECT HAS BEEN RENDERED BY COUNSEL.
Warrant Certificate No.: 1
Original Issue Date: May 1, 2024
FOR VALUE RECEIVED, BIOHAVEN LTD., a British Virgin Islands company (the “Company”), hereby certifies that KNOPP BIOSCIENCES LLC, a Delaware limited liability company, or its registered assigns (the “Holder”) is entitled to purchase from the Company 294,195 duly authorized, validly issued, fully paid and nonassessable Common Shares at a purchase price per share of sixty-seven and ninety-eight dollars and fifty-five cents ($67.98 USD) (subject to adjustment as provided herein, the “Exercise Price”), all subject to the terms, conditions and adjustments set forth below in this Warrant (including the Vesting Conditions set forth in Section 2 hereof). Certain capitalized terms used herein are defined in Section 1 hereof.
Company, Holder, Biohaven Therapeutics Ltd. (“BTL”) and Channel Biosciences, LLC are parties to that certain Membership Interest Purchase Agreement, dated February 24, 2022 (“Membership Interest Purchase Agreement”). This Warrant has been issued pursuant to the terms of the Amendment to the Membership Interest Purchase Agreement, dated May 1, 2024, among the Company, BTL and the Holder (the “Amendment”).
1.Definitions. As used in this Warrant, the following terms have the respective meanings set forth below:
“Aggregate Exercise Price” means an amount equal to the product of (a) the number of Warrant Shares in respect of which this Warrant is then being exercised pursuant to Section 3 hereof, multiplied by (b) the Exercise Price in effect as of the Exercise Date in accordance with the terms of this Warrant.
“Amendment” has the meaning set forth in the preamble.
“Board” means the board of directors of the Company.
“Business Day” means any day, except a Saturday, Sunday or legal holiday, on which banking institutions in New York City are authorized or obligated by law or executive order to close.
“Change of Control” means (a) a merger or consolidation of the Company with another Person other than (i) a merger in which the Company is the surviving Person and the holders of Common Shares immediately before the merger hold more than 50% of the Common Shares immediately after the merger or consolidation, or (ii) a merger solely for the purpose of changing the state of the Company’s incorporation, (b) a tender offer or similar transaction through which a Person (not including the Holder or a “group” within the meaning of Section 13(d)(3) under the Securities Exchange Act of 1934, as amended, of which the Holder is a member) acquires more than 50% of the outstanding Common Shares, or (c) a sale of all or substantially all of the assets of the Company.
“Common Shares” means the common shares, no par value, of the Company, and any capital stock into which such Common Shares shall have been converted, exchanged or reclassified following the date hereof.
“Company” has the meaning set forth in the preamble.
“Exercise Date” means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth in Section 3 shall have been satisfied at or prior to 5:00 p.m., New York time, on a Business Day, including, without limitation, the receipt by the Company of the Exercise Notice, the Warrant and the Aggregate Exercise Price.
“Exercise Notice” has the meaning set forth in Section 3(a)(i).
“Exercise Period” has the meaning set forth in Section 2.
“Exercise Price” has the meaning set forth in the preamble.
“Fair Market Value” means, as of any particular date: (a) the volume weighted average of the closing sales prices of the Common Shares for such day on all United States securities exchanges on which the Common Shares may at the time be listed; (b) if there have been no sales of the Common Shares on any such exchange on any such day, the average of the highest bid and lowest asked prices for the Common Shares on all such exchanges at the end of such day; (c) if on any such day the Common Shares are not listed on a United States securities exchange, the closing sales price of the Common Shares on the principal stock exchange on which the Common Shares may at the time be listed; (d) if on any such day the Common Shares are not listed on a United States securities exchange and there is no other principal stock exchange on which the Common Shares are listed, the closing sales price of the Common Shares as quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association for such day; or (d) if there have been no sales of the Common Shares on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association on such day, the average of the highest bid and lowest asked prices for the Common Shares quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association at the end of such day; in each case, averaged over twenty (20) consecutive Business Days ending on the Business Day immediately prior to the day as
of which “Fair Market Value” is being determined; provided, that if the Common Shares are listed on any securities exchange under clause (a), (b) or (c) above, the term “Business Day” as used in this sentence means Business Days on which such exchange is open for trading. If at any time the Common Shares are not listed on any United States securities exchange or any other principal stock exchange and are not quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association, the “Fair Market Value” of the Common Shares shall be the fair market value per share as determined jointly by the Board and the Holder; provided, that if the Board and the Holder are unable to agree on the fair market value per share of the Common Shares within a reasonable period of time (not to exceed ten (10) Business Days from the Company’s receipt of the Exercise Notice), such fair market value shall be determined by a nationally recognized investment banking, accounting or valuation firm jointly selected by the Board and the Holder. The determination of such firm shall be final and conclusive, and the fees and expenses of such valuation firm shall be borne equally by the Company and the Holder.
[***].
“Holder” has the meaning set forth in the preamble.
“Kv7 Compound” has the meaning set forth in the Membership Interest Purchase Agreement.
“Market Price” has the meaning set forth in the Membership Interest Purchase Agreement.
“Original Issue Date” has the meaning set forth on page 1 hereof.
“OTC Bulletin Board” means the Financial Industry Regulatory Authority OTC Bulletin Board electronic inter-dealer quotation system.
“Person” means any individual, sole proprietorship, partnership, limited liability company, corporation, joint venture, trust, incorporated organization or government or department or agency thereof.
[***].
“Pink OTC Markets” means the OTC Markets Group Inc. electronic inter-dealer quotation system, including OTCQX, OTCQB and OTC Pink.
[***].
[***].
[***].
“Vesting Conditions” has the meaning set forth in Section 2(b).
“Warrant” means this Warrant No. 1 and all warrants issued upon division or combination of, or in substitution for, this Warrant No. 1.
“Warrant Shares” means the Common Shares or other capital stock of the Company then purchasable upon exercise of this Warrant in accordance with the terms of this Warrant.
2.Term of Warrant.
(a) Subject to the terms and conditions hereof and the satisfaction of the Vesting Conditions described in clause (b) as to the applicable Warrant Shares, at any time or from time to time thereafter and prior to 5:00 p.m., New York time, on the tenth (10th) anniversary of the date hereof or, if such day is not a Business Day, on the closest preceding Business Day (the “Exercise Period”), the Holder of this Warrant may exercise this Warrant for all or any part of the vested Warrant Shares purchasable hereunder (subject to adjustment as provided herein).
(b) This Warrant shall be subject to achievement of both of the following vesting conditions (the “Vesting Conditions”):
(i) [***]; and
(ii) [***].
Notwithstanding the foregoing, this Warrant shall become fully vested and shall be automatically exercised pursuant to Section 3(b)(ii) immediately prior to the consummation of a Change of Control.
3.Exercise of Warrant.
(a) Exercise Procedure. This Warrant may be exercised from time to time on any Business Day during the Exercise Period, for all or any part of the unexercised Warrant Shares into which it is then exercisable, upon:
(i) surrender of this Warrant to the Company at its then principal executive offices (or an indemnification undertaking with respect to this Warrant in the case of its loss, theft or destruction), together with an Exercise Notice in the form attached hereto as Exhibit A (each, an “Exercise Notice”), duly completed (including specifying the number of Warrant Shares to be purchased) and executed; and
(ii) payment to the Company of the Aggregate Exercise Price in accordance with Section 3(b).
(b) Payment of the Aggregate Exercise Price. Payment of the Aggregate Exercise Price shall be made, at the option of the Holder as expressed in the Exercise Notice, by the following methods:
(i) by delivery to the Company of a certified or official bank check payable to the order of the Company or by wire transfer of immediately available funds to an account designated in writing by the Company, in the amount of such Aggregate Exercise Price; or
(ii) by instructing the Company to issue Warrant Shares then issuable upon all or any part of this Warrant on a net basis under clause (ii) such that, without payment of any cash consideration or other immediately available funds, the Holder shall surrender this
Warrant in exchange for the number of Warrant Shares as is computed using the following formula:
X = Y(A-B) ÷ A
where:
X = the number of Warrant Shares to be issued to the Holder;
Y = the total number of Warrant Shares for which the Holder has elected to exercise this Warrant pursuant to Section 3(a);
A = the Fair Market Value of one Warrant Share as of the applicable Exercise Date; and
B = the Exercise Price in effect under this Warrant as of the applicable Exercise Date.
In the event of any withholding of Warrant Shares to effect a net settlement pursuant to clause (ii) above where the number of shares issuable thereunder is not a whole number, the number of shares issued by the Company on a net basis shall be rounded down to the nearest whole share and the Company shall make a cash payment to the Holder (by delivery of a certified or official bank check or by wire transfer of immediately available funds) based on the incremental fraction of a share being so withheld by the Company in an amount equal to the product of (x) such incremental fraction of a share being so withheld multiplied by (y) the Fair Market Value per Warrant Share as of the Exercise Date.
(c) Fractional Shares. The Company shall not be required to issue a fractional Warrant Share upon exercise of any Warrant. As to any fraction of a Warrant Share that the Holder would otherwise be entitled to purchase upon such exercise, the Company shall pay to such Holder an amount in cash (by delivery of a certified or official bank check or by wire transfer of immediately available funds) equal to the product of (i) such fraction multiplied by (ii) the Fair Market Value of one Warrant Share on the Exercise Date.
(d) Delivery of New Warrant. Unless the purchase rights represented by this Warrant shall have expired or shall have been fully exercised, the Company shall, at the time of delivery of the certificate or certificates representing the Warrant Shares being issued in accordance with Section 3 hereof, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unexpired and unexercised Warrant Shares called for by this Warrant. Such new Warrant shall in all other respects be identical to this Warrant.
(e) Valid Issuance of Warrant and Warrant Shares; Payment of Taxes. With respect to the exercise of this warrant, the Company hereby represents, covenants and agrees:
(i) This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued.
(ii) All Warrant Shares issuable upon the exercise of this Warrant pursuant to the terms hereof shall be, upon issuance, and the Company shall take all such actions as may be necessary or appropriate in order that such Warrant Shares are, validly issued, fully paid
and non-assessable, issued without violation of any preemptive or similar rights of any shareholder of the Company and free and clear of all taxes, liens and charges.
(iii) The Company shall take all such actions as may be necessary to ensure that all such Warrant Shares are issued without violation by the Company of any applicable law or governmental regulation or any requirements of any primary securities exchange upon which Common Shares or other securities constituting Warrant Shares may be listed at the time of such exercise (except for official notice of issuance which shall be immediately delivered by the Company upon each such issuance).
(iv) The Company shall use reasonable commercial efforts to cause the Warrant Shares, immediately upon such exercise, to be listed on any primary securities exchange upon which Common Shares or other securities constituting Warrant Shares are listed at the time of such exercise.
(v) The Company shall pay all expenses in connection with, and all taxes and other governmental charges that may be imposed with respect to, the issuance or delivery of Warrant Shares upon exercise of this Warrant; provided, that the Company shall not be required to pay any tax or governmental charge that may be imposed with respect to any applicable withholding or the issuance or delivery of the Warrant Shares to any Person other than the Holder, and no such issuance or delivery shall be made unless and until the Person requesting such issuance has paid to the Company the amount of any such tax, or has established to the satisfaction of the Company that such tax has been paid.
(f) Conditional Exercise. Notwithstanding any other provision hereof, if an exercise of any portion of this Warrant is to be made in connection with a sale of the Company (pursuant to a merger, sale of stock, or otherwise), such exercise may at the election of the Holder be conditioned upon the consummation of such transaction, in which case such exercise shall not be deemed to be effective until immediately prior to the consummation of such transaction.
(g) Reservation of Shares. During the Exercise Period, the Company shall at all times reserve and keep available out of its authorized but unissued Common Shares or other securities constituting Warrant Shares, solely for the purpose of issuance upon the exercise of this Warrant, the maximum number of Warrant Shares issuable upon the exercise of this Warrant.
4.Adjustment to Exercise Price and Number of Warrant Shares. The Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant shall be subject to adjustment from time to time as provided in this Section 4 (in each case, after taking into consideration any prior adjustments pursuant to this Section 4).
(a) Adjustment to Exercise Price and Warrant Shares Upon Dividend, Subdivision or Combination of Common Shares. If the Company shall, at any time or from time to time after the Original Issue Date, (i) pay a dividend or make any other distribution upon the Common Shares or any other capital stock of the Company that is payable in Common Shares, or (ii) subdivide (by any stock split, recapitalization or otherwise) its outstanding Common Shares into a greater number of shares, the Exercise Price in effect
immediately prior to any such dividend, distribution or subdivision shall be proportionately reduced and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately increased. If the Company at any time combines (by combination, reverse stock split or otherwise) its outstanding Common Shares into a smaller number of shares, the Exercise Price in effect immediately prior to such combination shall be proportionately increased and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately decreased. Any adjustment under this Section 4(a) shall become effective at the close of business on the date the dividend, subdivision or combination becomes effective.
(b) Adjustment to Exercise Price and Warrant Shares Upon Reorganization, Reclassification, Consolidation or Merger. In the event of any (i) capital reorganization of the Company, (ii) reclassification of the stock of the Company (other than a change in par value or from par value to no par value or from no par value to par value or as a result of a stock dividend or subdivision, split-up or combination of shares), (iii) consolidation or merger of the Company with or into another Person, (iv) sale of all or substantially all of the Company’s assets to another Person or (v) other similar transaction (other than any such transaction covered by Section 4(a) or any Change of Control, which shall be governed by Section 2), in each case which entitles the holders of Common Shares to receive (either directly or upon subsequent liquidation) stock, securities or assets with respect to or in exchange for Common Shares, each Warrant shall, immediately after such reorganization, reclassification, consolidation, merger, sale or similar transaction, remain outstanding and shall thereafter, in lieu of or in addition to (as the case may be) the number of Warrant Shares then exercisable under this Warrant, be exercisable for the kind and number of shares of stock or other securities or assets of the Company or of the successor Person resulting from such transaction to which the Holder would have been entitled upon such reorganization, reclassification, consolidation, merger, sale or similar transaction if the Holder had exercised this Warrant in full immediately prior to the time of such reorganization, reclassification, consolidation, merger, sale or similar transaction and acquired the applicable number of Warrant Shares then issuable hereunder as a result of such exercise (without taking into account any limitations or restrictions on the exercisability of this Warrant); and, in such case, appropriate adjustment (in form and substance satisfactory to the Holder) shall be made with respect to the Holder’s rights under this Warrant to insure that the provisions of this Section 4 hereof shall thereafter be applicable, as nearly as possible, to this Warrant in relation to any shares of stock, securities or assets thereafter acquirable upon exercise of this Warrant (including, in the case of any consolidation, merger, sale or similar transaction in which the successor or purchasing Person is other than the Company, an immediate adjustment in the Exercise Price to the value per share for the Common Shares reflected by the terms of such consolidation, merger, sale or similar transaction, and a corresponding immediate adjustment to the number of Warrant Shares acquirable upon exercise of this Warrant without regard to any limitations or restrictions on exercise, if the value so reflected is less than the Exercise Price in effect immediately prior to such consolidation, merger, sale or similar transaction). The provisions of this Section 4(b) shall similarly apply to successive reorganizations, reclassifications, consolidations, mergers, sales or similar transactions. The Company shall not effect any such reorganization, reclassification, consolidation, merger, sale or similar transaction unless, prior to the consummation thereof, the successor Person (if other than the
Company) resulting from such reorganization, reclassification, consolidation, merger, sale or similar transaction, shall assume, by written instrument substantially similar in form and substance to this Warrant and satisfactory to the Holder, the obligation to deliver to the Holder such shares of stock, securities or assets which, in accordance with the foregoing provisions, such Holder shall be entitled to receive upon exercise of this Warrant. Notwithstanding anything to the contrary contained herein, with respect to any corporate event or other transaction contemplated by the provisions of this Section 4(b), the Holder shall have the right to elect prior to the consummation of such event or transaction, to give effect to the exercise rights contained in Section 2 instead of giving effect to the provisions contained in this Section 4(b) with respect to this Warrant.
(c) Certificate as to Adjustment.
(i) As promptly as reasonably practicable following any adjustment of the Exercise Price, but in any event not later than five (5) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer setting forth in reasonable detail such adjustment and the facts upon which it is based and certifying the calculation thereof. As promptly as reasonably practicable following the receipt by the Company of a written request by the Holder, but in any event not later than five (5) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer certifying the Exercise Price then in effect and the number of Warrant Shares or the amount, if any, of other shares of stock, securities or assets then issuable upon exercise of the Warrant.
6.Transfer of Warrant. Subject to the transfer conditions referred to in the legend endorsed hereon, this Warrant and all rights hereunder are transferable, in whole or in part, by the Holder without charge to the Holder, upon surrender of this Warrant to the Company at its then principal executive offices with a properly completed and duly executed Assignment in the form attached hereto as Exhibit B, together with funds sufficient to pay any transfer taxes described in Section 3(e)(v) in connection with the making of such transfer. Upon such compliance, surrender and delivery and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant, if any, not so assigned and this Warrant shall promptly be cancelled.
7.Holder Not Deemed a Shareholder; Limitations on Liability. Except as otherwise specifically provided herein and for common shares held directly by Holder not subject to this Warrant, prior to the issuance to the Holder of the Warrant Shares which the Holder is then entitled to receive upon the due exercise of this Warrant, the Holder shall not be entitled to vote or receive dividends or be deemed the holder of shares of capital stock of the Company for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, as such, any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of stock, consolidation, merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise. In addition, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder of
the Company, whether such liabilities are asserted by the Company or by creditors of the Company.
8.Replacement on Loss; Division and Combination.
(a) Replacement of Warrant on Loss. Upon receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and upon delivery of an indemnity reasonably satisfactory to it (it being understood that a written indemnification agreement or affidavit of loss of the Holder shall be a sufficient indemnity) and, in case of mutilation, upon surrender of such Warrant for cancellation to the Company, the Company at its own expense shall execute and deliver to the Holder, in lieu hereof, a new Warrant of like tenor and exercisable for an equivalent number of Warrant Shares as the Warrant so lost, stolen, mutilated or destroyed; provided, that, in the case of mutilation, no indemnity shall be required if this Warrant in identifiable form is surrendered to the Company for cancellation.
(b) Division and Combination of Warrant. Subject to compliance with the applicable provisions of this Warrant as to any transfer or other assignment which may be involved in such division or combination, this Warrant may be divided or, following any such division of this Warrant, subsequently combined with other Warrants, upon the surrender of this Warrant or Warrants to the Company at its then principal executive offices, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the respective Holders or their agents or attorneys. Subject to compliance with the applicable provisions of this Warrant as to any transfer or assignment which may be involved in such division or combination, the Company shall at its own expense execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants so surrendered in accordance with such notice. Such new Warrant or Warrants shall be of like tenor to the surrendered Warrant or Warrants and shall be exercisable in the aggregate for an equivalent number of Warrant Shares as the Warrant or Warrants so surrendered in accordance with such notice.
9.No Impairment. The Company shall not, by amendment of its Memorandum and Articles of Association, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities, or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed by it hereunder, but shall at all times in good faith assist in the carrying out of all the provisions of this Warrant and in the taking of all such action as may reasonably be requested by the Holder in order to protect the exercise rights of the Holder against dilution or other impairment, consistent with the tenor and purpose of this Warrant.
10.Compliance with the Securities Act.
(a) Agreement to Comply with the Securities Act; Legend. The Holder, by acceptance of this Warrant, agrees to comply in all respects with the provisions of this Section 10 and the restrictive legend requirements set forth on the face of this Warrant and further agrees that such Holder shall not offer, sell or otherwise dispose of this Warrant or any
Warrant Shares to be issued upon exercise hereof except under circumstances that will not result in a violation of the Securities Act of 1933, as amended (the “Securities Act”). This Warrant and all Warrant Shares issued upon exercise of this Warrant (unless registered under the Securities Act) shall be stamped or imprinted with a legend in substantially the following form:
“THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW AND, IF THE COMPANY REQUESTS, AN OPINION SATISFACTORY TO THE COMPANY TO SUCH EFFECT HAS BEEN RENDERED BY COUNSEL.”
Notwithstanding the forgoing, certificates evidencing the Warrant Shares shall not be required to contain such legend, (i) while a registration statement covering the resale of such Warrant Shares is effective under the Securities Act, (ii) following any sale of such Warrant Shares pursuant to Rule 144 (assuming cashless exercise of the Warrants), or (iii) if such Warrant Shares are eligible for sale under Rule 144 (assuming cashless exercise of the Warrants) (provided, however, that in the case of (i), (ii) and (iii), above, the holder provides the Company with customary legal representation letters reasonably acceptable to the Company). Whenever such restrictions shall cease and terminate as to any such Warrant Shares, the holder of such Warrant Shares shall be entitled to receive from the Company upon a written request in writing, without expense, new securities of like tenor not bearing the legend set forth herein. The Company shall cooperate with any holder of such Warrant Shares and, to the extent applicable, facilitate the timely preparation and delivery of certificates (not bearing any restrictive legend) representing the Warrant Shares and enable such certificates to be in such denominations or amounts, as the case may be, as the holder may reasonably request and registered in such names as such holder may request. Notwithstanding the forgoing, the Company’s obligations under this Section 10 with respect to Warrant Shares that may be sold under Rule 144 are contingent on the Company’s receipt from Holder of a customary Rule 144 representation letter in form and substance reasonably acceptable to the Company.
(b) Representations of the Holder. In connection with the issuance of this Warrant, the Holder specifically represents, as of the date hereof, to the Company by acceptance of this Warrant as follows:
(i) The Holder is an “accredited investor” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act. The Holder is acquiring this Warrant and the Warrant Shares to be issued upon exercise hereof for investment for its own account and not with a view towards, or for resale in connection with, the public sale or distribution of this
Warrant or the Warrant Shares, except pursuant to sales registered or exempted under the Securities Act.
(ii) The Holder understands and acknowledges that this Warrant and the Warrant Shares to be issued upon exercise hereof are “restricted securities” under the federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that, under such laws and applicable regulations, such securities may be resold without registration under the Securities Act only in certain limited circumstances. In addition, the Holder represents that it is familiar with Rule 144 under the Securities Act, as presently in effect, and understands the resale limitations imposed thereby and by the Securities Act.
(iii) The Holder acknowledges that it can bear the economic and financial risk of its investment for an indefinite period, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Warrant and the Warrant Shares. The Holder has had an opportunity to ask questions and receive answers from the Company regarding the terms and conditions of the offering of the Warrant and the business, properties, prospects and financial condition of the Company.
11.Warrant Register. The Company shall keep and properly maintain at its principal executive offices books for the registration of the Warrant and any transfers thereof. The Company may deem and treat the Person in whose name the Warrant is registered on such register as the Holder thereof for all purposes, and the Company shall not be affected by any notice to the contrary, except any assignment, division, combination or other transfer of the Warrant effected in accordance with the provisions of this Warrant.
12.Notices. All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given:
(a) when delivered by hand (with written confirmation of receipt); (b) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); (c) on the date sent by facsimile or e-mail of a PDF document (with confirmation of transmission) if sent during normal business hours of the recipient, and on the next Business Day if sent after normal business hours of the recipient; or (d) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the addresses indicated below (or at such other address for a party as shall be specified in a notice given in accordance with this Section 12).
| | | | | |
If to the Company: | Biohaven Ltd. c/o Biohaven Pharmaceuticals, Inc. 215 Church Street New Haven, CT 06510 E-mail: matt.buten@biohavenpharma.com Attention: CFO |
If to the Holder: | Knopp Biosciences LLC 2403 Sidney St. Suite 206 Pittsburgh, PA 15203 Email: FJLucchino@knoppbio.com Attention: President |
13.Cumulative Remedies. Except to the extent expressly provided in Section 7 to the contrary, the rights and remedies provided in this Warrant are cumulative and are not exclusive of, and are in addition to and not in substitution for, any other rights or remedies available at law, in equity or otherwise.
14.Equitable Relief. Each of the Company and the Holder acknowledges that a breach or threatened breach by such party of any of its obligations under this Warrant would give rise to irreparable harm to the other party hereto for which monetary damages would not be an adequate remedy and hereby agrees that in the event of a breach or a threatened breach by such party of any such obligations, the other party hereto shall, in addition to any and all other rights and remedies that may be available to it in respect of such breach, be entitled to equitable relief, including a restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction.
15.Entire Agreement. This Warrant, together with the Amendment, constitutes the sole and entire agreement of the parties to this Warrant with respect to the subject matter contained herein, and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter. In the event of any inconsistency between the statements in the body of this Warrant and the Amendment, the statements in the body of this Warrant shall control.
16.Successor and Assigns. This Warrant and the rights evidenced hereby shall be binding upon and shall inure to the benefit of the parties hereto and the successors of the Company and the successors and permitted assigns of the Holder. Such successors and/or permitted assigns of the Holder shall be deemed to be a Holder for all purposes hereunder.
17.No Third-Party Beneficiaries. This Warrant is for the sole benefit of the Company and the Holder and their respective successors and, in the case of the Holder, permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Warrant.
18.Headings. The headings in this Warrant are for reference only and shall not affect the interpretation of this Warrant.
19.Amendment and Modification; Waiver. Except as otherwise provided herein, this Warrant may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by the Company or the Holder of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any rights, remedy, power or privilege arising from this Warrant shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
20.Severability. If any term or provision of this Warrant is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Warrant or invalidate or render unenforceable such term or provision in any other jurisdiction.
21.Governing Law. This Warrant shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the domestic substantive laws of any other jurisdiction.
22.Submission to Jurisdiction. Any legal suit, action or proceeding arising out of or based upon this Warrant or the transactions contemplated hereby may be instituted in the courts located in New Castle County, Delaware, as well as to the jurisdiction of all courts to which an appeal may be taken from such courts, and each party irrevocably submits to the exclusive jurisdiction of such courts in any such suit, action or proceeding. Service of process, summons, notice or other document by certified or registered mail to such party’s address set forth herein shall be effective service of process for any suit, action or other proceeding brought in any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or any proceeding in such courts and irrevocably waive and agree not to plead or claim in any such court that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
23.Waiver of Jury Trial. Each party acknowledges and agrees that any controversy which may arise under this Warrant is likely to involve complicated and difficult issues and, therefore, each such party irrevocably and unconditionally waives any right it may have to a trial by jury in respect of any legal action arising out of or relating to this Warrant or the transactions contemplated hereby.
24.Counterparts. This Warrant may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Warrant delivered by facsimile, e-mail or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Warrant.
25.No Strict Construction. This Warrant shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted.
[signature page follows]
IN WITNESS WHEREOF, the Company has duly executed this Warrant on the Original Issue Date.
| | |
BIOHAVEN LTD. |
By: _______________________ Name: Matt Buten Title: Chief Financial Officer |
| | |
WITNESS to Signature of Biohaven Ltd.: Location:_______________________ |
Date:_______________________ By:_______________________ Name: Warren Volles Title: Chief Legal Officer Biohaven Ltd. |
Accepted and agreed,
| | | | | |
| KNOPP BIOSCIENCES LLC |
| |
| By: | ___________________ |
| |
| Name: | Frank J. Lucchino Jr. |
| Title: | President |
[Signature Page to Warrant]
EXHIBIT A
Form of Exercise Notice
(To be executed by the Holder to purchase Common Shares under the foregoing Warrant)
Ladies and Gentlemen:
(1)The undersigned is the Holder of Warrant No. 1 (the “Warrant”) issued by Biohaven Ltd., a British Virgin Islands Company (the “Company”). Capitalized terms used herein and not otherwise defined herein have the respective meanings set forth in the Warrant.
(2)The undersigned hereby exercises its right to purchase Warrant Shares pursuant to the Warrant.
(3)The Holder intends that payment of the Exercise Price shall be made as (check one):
[ ] Cash Exercise
[ ] “Cashless Exercise” under Section 3(b)(ii)
(4)If the Holder has elected a Cash Exercise, the Holder shall pay the sum of $ in immediately available funds to the Company in accordance with the terms of the Warrant.
(5)Pursuant to this Exercise Notice, the Company shall deliver to the Holder Warrant Shares determined in accordance with the terms of the Warrant.
(6)DWAC Account Number (if applicable):
| | |
____________________________ |
|
____________________________ |
|
____________________________ |
|
| | | | | |
| Dated: | ______________________, 20____ |
| Name of Holder: | ____________________________ |
| By: | ____________________________ |
| Name | ____________________________ |
| Title | ____________________________ |
| Address | ____________________________ |
| ____________________________ |
(Signature must be made by an authorized representative of the Holder.)
Exhibit B
Biohaven Ltd.
FORM OF ASSIGNMENT
[To be completed and signed only upon transfer of Warrant]
FOR VALUE RECEIVED, the undersigned hereby sells, assigns and transfers unto
(the “Transferee”) the right represented by the within Warrant to purchase up to ____________ shares of Common Stock of Biohaven Ltd. (the “Company”) to which the within Warrant relates and appoints _______________attorney to transfer said right on the books of the Company with full power of substitution in the premises.
| | | | | | | | |
| Dated: | ______________________, 20____ | |
| | |
| ___________________________________ |
| (Signature must be made by an authorized representative of the Holder.) |
| | |
| ___________________________________ |
| Address of Transferee |
| In the presence of: | |
___________________________________ | |
Exhibit 4.1
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
WARRANT No. 1
THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW AND, IF THE COMPANY REQUESTS, AN OPINION SATISFACTORY TO THE COMPANY TO SUCH EFFECT HAS BEEN RENDERED BY COUNSEL.
Warrant Certificate No.: 1
Original Issue Date: May 1, 2024
FOR VALUE RECEIVED, BIOHAVEN LTD., a British Virgin Islands company (the “Company”), hereby certifies that KNOPP BIOSCIENCES LLC, a Delaware limited liability company, or its registered assigns (the “Holder”) is entitled to purchase from the Company 294,195 duly authorized, validly issued, fully paid and nonassessable Common Shares at a purchase price per share of sixty-seven dollars and ninety-eight cents ($67.98 USD) (subject to adjustment as provided herein, the “Exercise Price”), all subject to the terms, conditions and adjustments set forth below in this Warrant (including the Vesting Conditions set forth in Section 2 hereof). Certain capitalized terms used herein are defined in Section 1 hereof.
Company, Holder, Biohaven Therapeutics Ltd. (“BTL”) and Channel Biosciences, LLC are parties to that certain Membership Interest Purchase Agreement, dated February 24, 2022 (“Membership Interest Purchase Agreement”). This Warrant has been issued pursuant to the terms of the Amendment to the Membership Interest Purchase Agreement, dated May 1, 2024, among the Company, BTL and the Holder (the “Amendment”).
1.Definitions. As used in this Warrant, the following terms have the respective meanings set forth below:
“Aggregate Exercise Price” means an amount equal to the product of (a) the number of Warrant Shares in respect of which this Warrant is then being exercised pursuant to Section 3 hereof, multiplied by (b) the Exercise Price in effect as of the Exercise Date in accordance with the terms of this Warrant.
“Amendment” has the meaning set forth in the preamble.
“Board” means the board of directors of the Company.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
“Business Day” means any day, except a Saturday, Sunday or legal holiday, on which banking institutions in New York City are authorized or obligated by law or executive order to close.
“Change of Control” means (a) a merger or consolidation of the Company with another Person other than (i) a merger in which the Company is the surviving Person and the holders of Common Shares immediately before the merger hold more than 50% of the Common Shares immediately after the merger or consolidation, or (ii) a merger solely for the purpose of changing the state of the Company’s incorporation, (b) a tender offer or similar transaction through which a Person (not including the Holder or a “group” within the meaning of Section 13(d)(3) under the Securities Exchange Act of 1934, as amended, of which the Holder is a member) acquires more than 50% of the outstanding Common Shares, or (c) a sale of all or substantially all of the assets of the Company.
“Common Shares” means the common shares, no par value, of the Company, and any capital stock into which such Common Shares shall have been converted, exchanged or reclassified following the date hereof.
“Company” has the meaning set forth in the preamble.
“Exercise Date” means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth in Section 3 shall have been satisfied at or prior to 5:00 p.m., New York time, on a Business Day, including, without limitation, the receipt by the Company of the Exercise Notice, the Warrant and the Aggregate Exercise Price.
“Exercise Notice” has the meaning set forth in Section 3(a)(i).
“Exercise Period” has the meaning set forth in Section 2.
“Exercise Price” has the meaning set forth in the preamble.
“Fair Market Value” means, as of any particular date: (a) the volume weighted average of the closing sales prices of the Common Shares for such day on all United States securities exchanges on which the Common Shares may at the time be listed; (b) if there have been no sales of the Common Shares on any such exchange on any such day, the average of the highest bid and lowest asked prices for the Common Shares on all such exchanges at the end of such day; (c) if on any such day the Common Shares are not listed on a United States securities exchange, the closing sales price of the Common Shares on the principal stock exchange on which the Common Shares may at the time be listed; (d) if on any such day the Common Shares are not listed on a United States securities exchange and there is no other principal stock exchange on which the Common Shares are listed, the closing sales price of the Common Shares as quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association for such day; or (d) if there have been no sales of the Common Shares on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association on such day, the average of the highest bid and lowest asked prices for the Common Shares quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association at the end of such day; in each case, averaged over twenty (20) consecutive Business Days ending on the Business Day immediately prior to the day as
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
of which “Fair Market Value” is being determined; provided, that if the Common Shares are listed on any securities exchange under clause (a), (b) or (c) above, the term “Business Day” as used in this sentence means Business Days on which such exchange is open for trading. If at any time the Common Shares are not listed on any United States securities exchange or any other principal stock exchange and are not quoted on the OTC Bulletin Board, the Pink OTC Markets or similar quotation system or association, the “Fair Market Value” of the Common Shares shall be the fair market value per share as determined jointly by the Board and the Holder; provided, that if the Board and the Holder are unable to agree on the fair market value per share of the Common Shares within a reasonable period of time (not to exceed ten (10) Business Days from the Company’s receipt of the Exercise Notice), such fair market value shall be determined by a nationally recognized investment banking, accounting or valuation firm jointly selected by the Board and the Holder. The determination of such firm shall be final and conclusive, and the fees and expenses of such valuation firm shall be borne equally by the Company and the Holder.
[***].
“Holder” has the meaning set forth in the preamble.
“Kv7 Compound” has the meaning set forth in the Membership Interest Purchase Agreement.
“Market Price” has the meaning set forth in the Membership Interest Purchase Agreement.
“Original Issue Date” has the meaning set forth on page 1 hereof.
“OTC Bulletin Board” means the Financial Industry Regulatory Authority OTC Bulletin Board electronic inter-dealer quotation system.
“Person” means any individual, sole proprietorship, partnership, limited liability company, corporation, joint venture, trust, incorporated organization or government or department or agency thereof.
[***].
“Pink OTC Markets” means the OTC Markets Group Inc. electronic inter-dealer quotation system, including OTCQX, OTCQB and OTC Pink.
[***].
[***].
[***].
“Vesting Conditions” has the meaning set forth in Section 2(b).
“Warrant” means this Warrant No. 1 and all warrants issued upon division or combination of, or in substitution for, this Warrant No. 1.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
“Warrant Shares” means the Common Shares or other capital stock of the Company then purchasable upon exercise of this Warrant in accordance with the terms of this Warrant.
2.Term of Warrant.
(a) Subject to the terms and conditions hereof and the satisfaction of the Vesting Conditions described in clause (b) as to the applicable Warrant Shares, at any time or from time to time thereafter and prior to 5:00 p.m., New York time, on the tenth (10th) anniversary of the date hereof or, if such day is not a Business Day, on the closest preceding Business Day (the “Exercise Period”), the Holder of this Warrant may exercise this Warrant for all or any part of the vested Warrant Shares purchasable hereunder (subject to adjustment as provided herein).
(b) This Warrant shall be subject to achievement of both of the following vesting conditions (the “Vesting Conditions”):
(i) [***]; and
(ii) [***].
Notwithstanding the foregoing, this Warrant shall become fully vested and shall be automatically exercised pursuant to Section 3(b)(ii) immediately prior to the consummation of a Change of Control.
3.Exercise of Warrant.
(a) Exercise Procedure. This Warrant may be exercised from time to time on any Business Day during the Exercise Period, for all or any part of the unexercised Warrant Shares into which it is then exercisable, upon:
(i) surrender of this Warrant to the Company at its then principal executive offices (or an indemnification undertaking with respect to this Warrant in the case of its loss, theft or destruction), together with an Exercise Notice in the form attached hereto as Exhibit A (each, an “Exercise Notice”), duly completed (including specifying the number of Warrant Shares to be purchased) and executed; and
(ii) payment to the Company of the Aggregate Exercise Price in accordance with Section 3(b).
(b) Payment of the Aggregate Exercise Price. Payment of the Aggregate Exercise Price shall be made, at the option of the Holder as expressed in the Exercise Notice, by the following methods:
(i) by delivery to the Company of a certified or official bank check payable to the order of the Company or by wire transfer of immediately available funds to an account designated in writing by the Company, in the amount of such Aggregate Exercise Price; or
(ii) by instructing the Company to issue Warrant Shares then issuable upon all or any part of this Warrant on a net basis under clause (ii) such that, without payment of any cash consideration or other immediately available funds, the Holder shall surrender this
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
Warrant in exchange for the number of Warrant Shares as is computed using the following formula:
X = Y(A-B) ÷ A
where:
X = the number of Warrant Shares to be issued to the Holder;
Y = the total number of Warrant Shares for which the Holder has elected to exercise this Warrant pursuant to Section 3(a);
A = the Fair Market Value of one Warrant Share as of the applicable Exercise Date; and
B = the Exercise Price in effect under this Warrant as of the applicable Exercise Date.
In the event of any withholding of Warrant Shares to effect a net settlement pursuant to clause (ii) above where the number of shares issuable thereunder is not a whole number, the number of shares issued by the Company on a net basis shall be rounded down to the nearest whole share and the Company shall make a cash payment to the Holder (by delivery of a certified or official bank check or by wire transfer of immediately available funds) based on the incremental fraction of a share being so withheld by the Company in an amount equal to the product of (x) such incremental fraction of a share being so withheld multiplied by (y) the Fair Market Value per Warrant Share as of the Exercise Date.
(c) Fractional Shares. The Company shall not be required to issue a fractional Warrant Share upon exercise of any Warrant. As to any fraction of a Warrant Share that the Holder would otherwise be entitled to purchase upon such exercise, the Company shall pay to such Holder an amount in cash (by delivery of a certified or official bank check or by wire transfer of immediately available funds) equal to the product of (i) such fraction multiplied by (ii) the Fair Market Value of one Warrant Share on the Exercise Date.
(d) Delivery of New Warrant. Unless the purchase rights represented by this Warrant shall have expired or shall have been fully exercised, the Company shall, at the time of delivery of the certificate or certificates representing the Warrant Shares being issued in accordance with Section 3 hereof, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unexpired and unexercised Warrant Shares called for by this Warrant. Such new Warrant shall in all other respects be identical to this Warrant.
(e) Valid Issuance of Warrant and Warrant Shares; Payment of Taxes. With respect to the exercise of this warrant, the Company hereby represents, covenants and agrees:
(i) This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued.
(ii) All Warrant Shares issuable upon the exercise of this Warrant pursuant to the terms hereof shall be, upon issuance, and the Company shall take all such actions as may be necessary or appropriate in order that such Warrant Shares are, validly issued, fully paid
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
and non-assessable, issued without violation of any preemptive or similar rights of any shareholder of the Company and free and clear of all taxes, liens and charges.
(iii) The Company shall take all such actions as may be necessary to ensure that all such Warrant Shares are issued without violation by the Company of any applicable law or governmental regulation or any requirements of any primary securities exchange upon which Common Shares or other securities constituting Warrant Shares may be listed at the time of such exercise (except for official notice of issuance which shall be immediately delivered by the Company upon each such issuance).
(iv) The Company shall use reasonable commercial efforts to cause the Warrant Shares, immediately upon such exercise, to be listed on any primary securities exchange upon which Common Shares or other securities constituting Warrant Shares are listed at the time of such exercise.
(v) The Company shall pay all expenses in connection with, and all taxes and other governmental charges that may be imposed with respect to, the issuance or delivery of Warrant Shares upon exercise of this Warrant; provided, that the Company shall not be required to pay any tax or governmental charge that may be imposed with respect to any applicable withholding or the issuance or delivery of the Warrant Shares to any Person other than the Holder, and no such issuance or delivery shall be made unless and until the Person requesting such issuance has paid to the Company the amount of any such tax, or has established to the satisfaction of the Company that such tax has been paid.
(f) Conditional Exercise. Notwithstanding any other provision hereof, if an exercise of any portion of this Warrant is to be made in connection with a sale of the Company (pursuant to a merger, sale of stock, or otherwise), such exercise may at the election of the Holder be conditioned upon the consummation of such transaction, in which case such exercise shall not be deemed to be effective until immediately prior to the consummation of such transaction.
(g) Reservation of Shares. During the Exercise Period, the Company shall at all times reserve and keep available out of its authorized but unissued Common Shares or other securities constituting Warrant Shares, solely for the purpose of issuance upon the exercise of this Warrant, the maximum number of Warrant Shares issuable upon the exercise of this Warrant.
4.Adjustment to Exercise Price and Number of Warrant Shares. The Exercise Price and the number of Warrant Shares issuable upon exercise of this Warrant shall be subject to adjustment from time to time as provided in this Section 4 (in each case, after taking into consideration any prior adjustments pursuant to this Section 4).
(a) Adjustment to Exercise Price and Warrant Shares Upon Dividend, Subdivision or Combination of Common Shares. If the Company shall, at any time or from time to time after the Original Issue Date, (i) pay a dividend or make any other distribution upon the Common Shares or any other capital stock of the Company that is payable in Common Shares, or (ii) subdivide (by any stock split, recapitalization or otherwise) its outstanding Common Shares into a greater number of shares, the Exercise Price in effect
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
immediately prior to any such dividend, distribution or subdivision shall be proportionately reduced and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately increased. If the Company at any time combines (by combination, reverse stock split or otherwise) its outstanding Common Shares into a smaller number of shares, the Exercise Price in effect immediately prior to such combination shall be proportionately increased and the number of Warrant Shares issuable upon exercise of this Warrant shall be proportionately decreased. Any adjustment under this Section 4(a) shall become effective at the close of business on the date the dividend, subdivision or combination becomes effective.
(b) Adjustment to Exercise Price and Warrant Shares Upon Reorganization, Reclassification, Consolidation or Merger. In the event of any (i) capital reorganization of the Company, (ii) reclassification of the stock of the Company (other than a change in par value or from par value to no par value or from no par value to par value or as a result of a stock dividend or subdivision, split-up or combination of shares), (iii) consolidation or merger of the Company with or into another Person, (iv) sale of all or substantially all of the Company’s assets to another Person or (v) other similar transaction (other than any such transaction covered by Section 4(a) or any Change of Control, which shall be governed by Section 2), in each case which entitles the holders of Common Shares to receive (either directly or upon subsequent liquidation) stock, securities or assets with respect to or in exchange for Common Shares, each Warrant shall, immediately after such reorganization, reclassification, consolidation, merger, sale or similar transaction, remain outstanding and shall thereafter, in lieu of or in addition to (as the case may be) the number of Warrant Shares then exercisable under this Warrant, be exercisable for the kind and number of shares of stock or other securities or assets of the Company or of the successor Person resulting from such transaction to which the Holder would have been entitled upon such reorganization, reclassification, consolidation, merger, sale or similar transaction if the Holder had exercised this Warrant in full immediately prior to the time of such reorganization, reclassification, consolidation, merger, sale or similar transaction and acquired the applicable number of Warrant Shares then issuable hereunder as a result of such exercise (without taking into account any limitations or restrictions on the exercisability of this Warrant); and, in such case, appropriate adjustment (in form and substance satisfactory to the Holder) shall be made with respect to the Holder’s rights under this Warrant to insure that the provisions of this Section 4 hereof shall thereafter be applicable, as nearly as possible, to this Warrant in relation to any shares of stock, securities or assets thereafter acquirable upon exercise of this Warrant (including, in the case of any consolidation, merger, sale or similar transaction in which the successor or purchasing Person is other than the Company, an immediate adjustment in the Exercise Price to the value per share for the Common Shares reflected by the terms of such consolidation, merger, sale or similar transaction, and a corresponding immediate adjustment to the number of Warrant Shares acquirable upon exercise of this Warrant without regard to any limitations or restrictions on exercise, if the value so reflected is less than the Exercise Price in effect immediately prior to such consolidation, merger, sale or similar transaction). The provisions of this Section 4(b) shall similarly apply to successive reorganizations, reclassifications, consolidations, mergers, sales or similar transactions. The Company shall not effect any such reorganization, reclassification, consolidation, merger, sale or similar transaction unless, prior to the consummation thereof, the successor Person (if other than the
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
Company) resulting from such reorganization, reclassification, consolidation, merger, sale or similar transaction, shall assume, by written instrument substantially similar in form and substance to this Warrant and satisfactory to the Holder, the obligation to deliver to the Holder such shares of stock, securities or assets which, in accordance with the foregoing provisions, such Holder shall be entitled to receive upon exercise of this Warrant. Notwithstanding anything to the contrary contained herein, with respect to any corporate event or other transaction contemplated by the provisions of this Section 4(b), the Holder shall have the right to elect prior to the consummation of such event or transaction, to give effect to the exercise rights contained in Section 2 instead of giving effect to the provisions contained in this Section 4(b) with respect to this Warrant.
(c) Certificate as to Adjustment.
(i) As promptly as reasonably practicable following any adjustment of the Exercise Price, but in any event not later than five (5) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer setting forth in reasonable detail such adjustment and the facts upon which it is based and certifying the calculation thereof. As promptly as reasonably practicable following the receipt by the Company of a written request by the Holder, but in any event not later than five (5) Business Days thereafter, the Company shall furnish to the Holder a certificate of an executive officer certifying the Exercise Price then in effect and the number of Warrant Shares or the amount, if any, of other shares of stock, securities or assets then issuable upon exercise of the Warrant.
6.Transfer of Warrant. Subject to the transfer conditions referred to in the legend endorsed hereon, this Warrant and all rights hereunder are transferable, in whole or in part, by the Holder without charge to the Holder, upon surrender of this Warrant to the Company at its then principal executive offices with a properly completed and duly executed Assignment in the form attached hereto as Exhibit B, together with funds sufficient to pay any transfer taxes described in Section 3(e)(v) in connection with the making of such transfer. Upon such compliance, surrender and delivery and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant, if any, not so assigned and this Warrant shall promptly be cancelled.
7.Holder Not Deemed a Shareholder; Limitations on Liability. Except as otherwise specifically provided herein and for common shares held directly by Holder not subject to this Warrant, prior to the issuance to the Holder of the Warrant Shares which the Holder is then entitled to receive upon the due exercise of this Warrant, the Holder shall not be entitled to vote or receive dividends or be deemed the holder of shares of capital stock of the Company for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, as such, any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of stock, consolidation, merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise. In addition, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a stockholder of
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
the Company, whether such liabilities are asserted by the Company or by creditors of the Company.
8.Replacement on Loss; Division and Combination.
(a) Replacement of Warrant on Loss. Upon receipt of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant and upon delivery of an indemnity reasonably satisfactory to it (it being understood that a written indemnification agreement or affidavit of loss of the Holder shall be a sufficient indemnity) and, in case of mutilation, upon surrender of such Warrant for cancellation to the Company, the Company at its own expense shall execute and deliver to the Holder, in lieu hereof, a new Warrant of like tenor and exercisable for an equivalent number of Warrant Shares as the Warrant so lost, stolen, mutilated or destroyed; provided, that, in the case of mutilation, no indemnity shall be required if this Warrant in identifiable form is surrendered to the Company for cancellation.
(b) Division and Combination of Warrant. Subject to compliance with the applicable provisions of this Warrant as to any transfer or other assignment which may be involved in such division or combination, this Warrant may be divided or, following any such division of this Warrant, subsequently combined with other Warrants, upon the surrender of this Warrant or Warrants to the Company at its then principal executive offices, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the respective Holders or their agents or attorneys. Subject to compliance with the applicable provisions of this Warrant as to any transfer or assignment which may be involved in such division or combination, the Company shall at its own expense execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants so surrendered in accordance with such notice. Such new Warrant or Warrants shall be of like tenor to the surrendered Warrant or Warrants and shall be exercisable in the aggregate for an equivalent number of Warrant Shares as the Warrant or Warrants so surrendered in accordance with such notice.
9.No Impairment. The Company shall not, by amendment of its Memorandum and Articles of Association, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities, or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed by it hereunder, but shall at all times in good faith assist in the carrying out of all the provisions of this Warrant and in the taking of all such action as may reasonably be requested by the Holder in order to protect the exercise rights of the Holder against dilution or other impairment, consistent with the tenor and purpose of this Warrant.
10.Compliance with the Securities Act.
(a) Agreement to Comply with the Securities Act; Legend. The Holder, by acceptance of this Warrant, agrees to comply in all respects with the provisions of this Section 10 and the restrictive legend requirements set forth on the face of this Warrant and further agrees that such Holder shall not offer, sell or otherwise dispose of this Warrant or any
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
Warrant Shares to be issued upon exercise hereof except under circumstances that will not result in a violation of the Securities Act of 1933, as amended (the “Securities Act”). This Warrant and all Warrant Shares issued upon exercise of this Warrant (unless registered under the Securities Act) shall be stamped or imprinted with a legend in substantially the following form:
“THIS WARRANT AND THE SECURITIES ISSUABLE UPON EXERCISE OF THIS WARRANT HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR QUALIFIED UNDER ANY STATE OR FOREIGN SECURITIES LAWS AND MAY NOT BE OFFERED FOR SALE, SOLD, PLEDGED, HYPOTHECATED OR OTHERWISE TRANSFERRED OR ASSIGNED UNLESS (I) A REGISTRATION STATEMENT COVERING SUCH SHARES IS EFFECTIVE UNDER THE ACT AND IS QUALIFIED UNDER APPLICABLE STATE AND FOREIGN LAW OR (II) THE TRANSACTION IS EXEMPT FROM THE REGISTRATION AND PROSPECTUS DELIVERY REQUIREMENTS UNDER THE ACT AND THE QUALIFICATION REQUIREMENTS UNDER APPLICABLE STATE AND FOREIGN LAW AND, IF THE COMPANY REQUESTS, AN OPINION SATISFACTORY TO THE COMPANY TO SUCH EFFECT HAS BEEN RENDERED BY COUNSEL.”
Notwithstanding the forgoing, certificates evidencing the Warrant Shares shall not be required to contain such legend, (i) while a registration statement covering the resale of such Warrant Shares is effective under the Securities Act, (ii) following any sale of such Warrant Shares pursuant to Rule 144 (assuming cashless exercise of the Warrants), or (iii) if such Warrant Shares are eligible for sale under Rule 144 (assuming cashless exercise of the Warrants) (provided, however, that in the case of (i), (ii) and (iii), above, the holder provides the Company with customary legal representation letters reasonably acceptable to the Company). Whenever such restrictions shall cease and terminate as to any such Warrant Shares, the holder of such Warrant Shares shall be entitled to receive from the Company upon a written request in writing, without expense, new securities of like tenor not bearing the legend set forth herein. The Company shall cooperate with any holder of such Warrant Shares and, to the extent applicable, facilitate the timely preparation and delivery of certificates (not bearing any restrictive legend) representing the Warrant Shares and enable such certificates to be in such denominations or amounts, as the case may be, as the holder may reasonably request and registered in such names as such holder may request. Notwithstanding the forgoing, the Company’s obligations under this Section 10 with respect to Warrant Shares that may be sold under Rule 144 are contingent on the Company’s receipt from Holder of a customary Rule 144 representation letter in form and substance reasonably acceptable to the Company.
(b) Representations of the Holder. In connection with the issuance of this Warrant, the Holder specifically represents, as of the date hereof, to the Company by acceptance of this Warrant as follows:
(i) The Holder is an “accredited investor” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act. The Holder is acquiring this Warrant and the Warrant Shares to be issued upon exercise hereof for investment for its own account and not with a view towards, or for resale in connection with, the public sale or distribution of this
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
Warrant or the Warrant Shares, except pursuant to sales registered or exempted under the Securities Act.
(ii) The Holder understands and acknowledges that this Warrant and the Warrant Shares to be issued upon exercise hereof are “restricted securities” under the federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that, under such laws and applicable regulations, such securities may be resold without registration under the Securities Act only in certain limited circumstances. In addition, the Holder represents that it is familiar with Rule 144 under the Securities Act, as presently in effect, and understands the resale limitations imposed thereby and by the Securities Act.
(iii) The Holder acknowledges that it can bear the economic and financial risk of its investment for an indefinite period, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment in the Warrant and the Warrant Shares. The Holder has had an opportunity to ask questions and receive answers from the Company regarding the terms and conditions of the offering of the Warrant and the business, properties, prospects and financial condition of the Company.
11.Warrant Register. The Company shall keep and properly maintain at its principal executive offices books for the registration of the Warrant and any transfers thereof. The Company may deem and treat the Person in whose name the Warrant is registered on such register as the Holder thereof for all purposes, and the Company shall not be affected by any notice to the contrary, except any assignment, division, combination or other transfer of the Warrant effected in accordance with the provisions of this Warrant.
12.Notices. All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given:
(a) when delivered by hand (with written confirmation of receipt); (b) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); (c) on the date sent by facsimile or e-mail of a PDF document (with confirmation of transmission) if sent during normal business hours of the recipient, and on the next Business Day if sent after normal business hours of the recipient; or (d) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the addresses indicated below (or at such other address for a party as shall be specified in a notice given in accordance with this Section 12).
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
| | | | | |
If to the Company: | Biohaven Ltd. c/o Biohaven Pharmaceuticals, Inc. 215 Church Street New Haven, CT 06510 E-mail: matt.buten@biohavenpharma.com Attention: CFO |
If to the Holder: | Knopp Biosciences LLC 2403 Sidney St. Suite 206 Pittsburgh, PA 15203 Email: FJLucchino@knoppbio.com Attention: President |
13.Cumulative Remedies. Except to the extent expressly provided in Section 7 to the contrary, the rights and remedies provided in this Warrant are cumulative and are not exclusive of, and are in addition to and not in substitution for, any other rights or remedies available at law, in equity or otherwise.
14.Equitable Relief. Each of the Company and the Holder acknowledges that a breach or threatened breach by such party of any of its obligations under this Warrant would give rise to irreparable harm to the other party hereto for which monetary damages would not be an adequate remedy and hereby agrees that in the event of a breach or a threatened breach by such party of any such obligations, the other party hereto shall, in addition to any and all other rights and remedies that may be available to it in respect of such breach, be entitled to equitable relief, including a restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction.
15.Entire Agreement. This Warrant, together with the Amendment, constitutes the sole and entire agreement of the parties to this Warrant with respect to the subject matter contained herein, and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter. In the event of any inconsistency between the statements in the body of this Warrant and the Amendment, the statements in the body of this Warrant shall control.
16.Successor and Assigns. This Warrant and the rights evidenced hereby shall be binding upon and shall inure to the benefit of the parties hereto and the successors of the Company and the successors and permitted assigns of the Holder. Such successors and/or permitted assigns of the Holder shall be deemed to be a Holder for all purposes hereunder.
17.No Third-Party Beneficiaries. This Warrant is for the sole benefit of the Company and the Holder and their respective successors and, in the case of the Holder, permitted assigns and nothing herein, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Warrant.
18.Headings. The headings in this Warrant are for reference only and shall not affect the interpretation of this Warrant.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
19.Amendment and Modification; Waiver. Except as otherwise provided herein, this Warrant may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by the Company or the Holder of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any rights, remedy, power or privilege arising from this Warrant shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
20.Severability. If any term or provision of this Warrant is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Warrant or invalidate or render unenforceable such term or provision in any other jurisdiction.
21.Governing Law. This Warrant shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the domestic substantive laws of any other jurisdiction.
22.Submission to Jurisdiction. Any legal suit, action or proceeding arising out of or based upon this Warrant or the transactions contemplated hereby may be instituted in the courts located in New Castle County, Delaware, as well as to the jurisdiction of all courts to which an appeal may be taken from such courts, and each party irrevocably submits to the exclusive jurisdiction of such courts in any such suit, action or proceeding. Service of process, summons, notice or other document by certified or registered mail to such party’s address set forth herein shall be effective service of process for any suit, action or other proceeding brought in any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or any proceeding in such courts and irrevocably waive and agree not to plead or claim in any such court that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
23.Waiver of Jury Trial. Each party acknowledges and agrees that any controversy which may arise under this Warrant is likely to involve complicated and difficult issues and, therefore, each such party irrevocably and unconditionally waives any right it may have to a trial by jury in respect of any legal action arising out of or relating to this Warrant or the transactions contemplated hereby.
24.Counterparts. This Warrant may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Warrant delivered by facsimile, e-mail or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Warrant.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
25.No Strict Construction. This Warrant shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted.
[signature page follows]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
***Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(B)(4) and 240.24b-2
IN WITNESS WHEREOF, the Company has duly executed this Warrant on the Original Issue Date.
| | |
BIOHAVEN LTD. |
By: /s/ Matt Buten Name: Matt Buten Title: Chief Financial Officer |
| | |
WITNESS to Signature of Biohaven Ltd.: Location: Hamilton, Bermuda |
Date: April 30, 2024
By: /s/ Warren Volles Name: Warren Volles Title: Chief Legal Officer Biohaven Ltd. |
Accepted and agreed,
| | | | | |
| KNOPP BIOSCIENCES LLC |
| |
| By: | /s/ Frank J. Lucchino Jr. |
| |
| Name: | Frank J. Lucchino Jr. |
| Title: | President |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
EXHIBIT A
Form of Exercise Notice
(To be executed by the Holder to purchase Common Shares under the foregoing Warrant)
Ladies and Gentlemen:
(1)The undersigned is the Holder of Warrant No. 1 (the “Warrant”) issued by Biohaven Ltd., a British Virgin Islands Company (the “Company”). Capitalized terms used herein and not otherwise defined herein have the respective meanings set forth in the Warrant.
(2)The undersigned hereby exercises its right to purchase Warrant Shares pursuant to the Warrant.
(3)The Holder intends that payment of the Exercise Price shall be made as (check one):
[ ] Cash Exercise
[ ] “Cashless Exercise” under Section 3(b)(ii)
(4)If the Holder has elected a Cash Exercise, the Holder shall pay the sum of $ in immediately available funds to the Company in accordance with the terms of the Warrant.
(5)Pursuant to this Exercise Notice, the Company shall deliver to the Holder Warrant Shares determined in accordance with the terms of the Warrant.
(6)DWAC Account Number (if applicable):
| | |
____________________________ |
|
____________________________ |
|
____________________________ |
|
| | | | | |
| Dated: | ______________________, 20____ |
| Name of Holder: | ____________________________ |
| By: | ____________________________ |
| Name | ____________________________ |
| Title | ____________________________ |
| Address | ____________________________ |
| ____________________________ |
(Signature must be made by an authorized representative of the Holder.)
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
Exhibit B
Biohaven Ltd.
FORM OF ASSIGNMENT
[To be completed and signed only upon transfer of Warrant]
FOR VALUE RECEIVED, the undersigned hereby sells, assigns and transfers unto
(the “Transferee”) the right represented by the within Warrant to purchase up to ____________ shares of Common Stock of Biohaven Ltd. (the “Company”) to which the within Warrant relates and appoints _______________attorney to transfer said right on the books of the Company with full power of substitution in the premises.
| | | | | | | | |
| Dated: | ______________________, 20____ | |
| | |
| ___________________________________ |
| (Signature must be made by an authorized representative of the Holder.) |
| | |
| ___________________________________ |
| Address of Transferee |
| In the presence of: | |
___________________________________ | |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
Exhibit 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Vlad Coric, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q for the period ended June 30, 2024 of Biohaven Ltd. (the "registrant");
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5. The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: August 9, 2024 | | | | | | | | |
| | /s/ VLAD CORIC, M.D. |
| | | Vlad Coric, M.D. |
| | President and Chief Executive Officer |
| | (principal executive officer) |
Exhibit 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Matthew Buten, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q for the period ended June 30, 2024 of Biohaven Ltd. (the "registrant");
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5. The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: August 9, 2024 | | | | | | | | |
| | /s/ MATTHEW BUTEN |
| | | Matthew Buten |
| | Chief Financial Officer |
| | (principal financial officer) |
Exhibit 32.1
CERTIFICATIONS OF
PRINCIPAL EXECUTIVE OFFICER AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, (the "Exchange Act") and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Vlad Coric, M.D., President and Chief Executive Officer of Biohaven Ltd. (the "Company"), and Matthew Buten, Chief Financial Officer of the Company, each hereby certifies that, to the best of his knowledge:
1. The Company's Quarterly Report on Form 10-Q for the period ended June 30, 2024, to which this Certification is attached as Exhibit 32.1 (the "Periodic Report"), fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and
2. The information contained in the Periodic Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
IN WITNESS WHEREOF, the undersigned have set their hands hereto as of the 9 day of August 2024.
| | | | | | | | |
| /s/ VLAD CORIC, M.D. | | /s/ MATTHEW BUTEN |
| Vlad Coric, M.D. | | Matthew Buten |
| President and Chief Executive Officer | | Chief Financial Officer |
| (principal executive officer) | | (principal financial officer) |
* This certification accompanies the Form 10-Q to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-Q), irrespective of any general incorporation language contained in such filing.
v3.24.2.u1
COVER - shares
|
6 Months Ended |
|
Jun. 30, 2024 |
Aug. 06, 2024 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Document Quarterly Report |
true
|
|
| Document Period End Date |
Jun. 30, 2024
|
|
| Document Transition Report |
false
|
|
| Entity File Number |
001-41477
|
|
| Entity Registrant Name |
Biohaven Ltd.
|
|
| Entity Incorporation, State or Country Code |
D8
|
|
| Entity Address, Address Line One |
c/o Biohaven Pharmaceuticals, Inc.
|
|
| Entity Address, Address Line Two |
215 Church Street
|
|
| Entity Address, City or Town |
New Haven
|
|
| Entity Address, State or Province |
CT
|
|
| Entity Address, Postal Zip Code |
06510
|
|
| City Area Code |
203
|
|
| Local Phone Number |
404-0410
|
|
| Title of 12(b) Security |
Common Shares, no par value
|
|
| Trading Symbol |
BHVN
|
|
| Security Exchange Name |
NYSE
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Large Accelerated Filer
|
|
| Entity Small Business |
false
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
94,542,253
|
| Entity Central Index Key |
0001935979
|
|
| Document Fiscal Year Focus |
2024
|
|
| Document Fiscal Period Focus |
Q2
|
|
| Amendment Flag |
false
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
CONDENSED CONSOLIDATED BALANCE SHEETS - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 239,147
|
$ 248,402
|
| Marketable securities |
197,801
|
133,417
|
| Prepaid expenses |
59,532
|
35,242
|
| Income tax receivable |
7,522
|
13,252
|
| Other current assets |
7,266
|
12,133
|
| Total current assets |
511,268
|
442,446
|
| Property and equipment, net |
18,665
|
17,191
|
| Intangible assets |
18,400
|
18,400
|
| Goodwill |
1,390
|
1,390
|
| Other non-current assets |
32,918
|
33,785
|
| Total assets |
582,641
|
513,212
|
| Current liabilities: |
|
|
| Accounts payable |
17,259
|
15,577
|
| Accrued expenses and other current liabilities |
57,728
|
39,846
|
| Forward contract and derivative liabilities |
81,220
|
0
|
| Total current liabilities |
156,207
|
55,423
|
| Non-current operating lease liabilities |
26,193
|
27,569
|
| Derivative liability, non-current |
12,180
|
0
|
| Other non-current liabilities |
4,321
|
2,245
|
| Total liabilities |
198,901
|
85,237
|
| Commitments and contingencies (Note 11) |
|
|
| Shareholders' Equity: |
|
|
| Preferred shares, no par value; 10,000,000 shares authorized, no shares issued and outstanding as of June 30, 2024 and December 31, 2023 |
0
|
0
|
| Common shares, no par value; 200,000,000 shares authorized as of June 30, 2024 and December 31, 2023; 92,346,332 and 81,115,723 shares issued and outstanding as of June 30, 2024 and December 31, 2023, respectively |
1,298,553
|
887,528
|
| Additional paid-in capital |
83,832
|
39,804
|
| Accumulated deficit |
(998,567)
|
(499,292)
|
| Accumulated other comprehensive loss |
(78)
|
(65)
|
| Total shareholders' equity |
383,740
|
427,975
|
| Total liabilities and shareholders' equity |
$ 582,641
|
$ 513,212
|
| X |
- DefinitionForward Contract and Derivative Liability, Current
| Name: |
bhvn_ForwardContractAndDerivativeLiabilityCurrent |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset, expected to be settled after one year or the normal operating cycle, if longer. Includes assets not subject to a master netting arrangement and not elected to be offset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483466/210-20-50-3
| Name: |
us-gaap_DerivativeLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated impairment loss, of asset representing future economic benefit arising from other asset acquired in business combination or from joint venture formation or both, that is not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482598/350-20-45-1
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482573/350-20-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in marketable security, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_MarketableSecuritiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due after one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
CONDENSED CONSOLIDATED BALANCE SHEETS (Parenthetical) - $ / shares
|
Jun. 30, 2024 |
Dec. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, par value (in dollars per share) |
$ 0
|
$ 0
|
| Preferred stock, authorized (in shares) |
10,000,000
|
10,000,000
|
| Preferred stock issued (share) |
0
|
0
|
| Preferred stock outstanding (share) |
0
|
0
|
| Common shares, par value (in dollars per share) |
$ 0
|
$ 0
|
| Common shares, authorized (shares) |
200,000,000
|
200,000,000
|
| Common shares, issued (shares) |
92,346,332
|
81,115,723
|
| Common shares, outstanding (shares) |
92,346,332
|
81,115,723
|
| X |
- DefinitionFace amount per share of no-par value common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockNoParValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount per share of no-par value preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockNoParValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Operating expenses: |
|
|
|
|
| Research and development |
$ 314,819
|
$ 79,490
|
$ 470,791
|
$ 142,951
|
| General and administrative |
18,953
|
14,521
|
46,221
|
28,842
|
| Total operating expenses |
333,772
|
94,011
|
517,012
|
171,793
|
| Loss from operations |
(333,772)
|
(94,011)
|
(517,012)
|
(171,793)
|
| Other income, net |
14,178
|
5,842
|
18,483
|
14,071
|
| Loss before provision for income taxes |
(319,594)
|
(88,169)
|
(498,529)
|
(157,722)
|
| Provision for income taxes |
177
|
2,177
|
746
|
3,116
|
| Net loss |
$ (319,771)
|
$ (90,346)
|
$ (499,275)
|
$ (160,838)
|
| Net loss per share attributable to common shareholders of Biohaven Ltd. — basic (in dollars per share) |
$ (3.64)
|
$ (1.32)
|
$ (5.93)
|
$ (2.36)
|
| Net loss per share attributable to common shareholders of Biohaven Ltd. — diluted (in dollars per share) |
$ (3.64)
|
$ (1.32)
|
$ (5.93)
|
$ (2.36)
|
| Weighted average common shares outstanding—basic (in shares) |
87,766,069
|
68,248,023
|
84,174,099
|
68,227,564
|
| Weighted average common shares outstanding—diluted (in shares) |
87,766,069
|
68,248,023
|
84,174,099
|
68,227,564
|
| Net loss |
$ (319,771)
|
$ (90,346)
|
$ (499,275)
|
$ (160,838)
|
| Other comprehensive income (loss), net of tax |
28
|
(146)
|
(13)
|
(264)
|
| Comprehensive loss |
$ (319,743)
|
$ (90,492)
|
$ (499,288)
|
$ (161,102)
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionTotal costs of sales and operating expenses for the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of other comprehensive income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-4
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482739/220-10-55-15
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS - USD ($)
$ in Thousands |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Cash flows from operating activities: |
|
|
| Net loss |
$ (499,275)
|
$ (160,838)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation and amortization |
3,400
|
3,480
|
| Non-cash share-based compensation |
47,109
|
8,460
|
| Issuance of common shares as payment for acquisition of IPR&D asset |
10,793
|
0
|
| Issuance of common shares as payment under license and other agreements |
71,618
|
0
|
| Fair value of forward contract and derivative liabilities under license agreement |
102,529
|
0
|
| Change in fair value of forward contract and derivative liabilities |
(9,129)
|
0
|
| Issuance of warrant under license agreement |
3,340
|
0
|
| Other non-cash items, net |
(3,129)
|
(3,682)
|
| Changes in operating assets and liabilities, net of effects of acquisition: |
|
|
| Prepaid expenses and other current and non-current assets |
(13,491)
|
39,283
|
| Accounts payable |
(60)
|
1,336
|
| Accrued expenses and other current and non-current liabilities |
15,860
|
(10,070)
|
| Net cash used in operating activities |
(270,435)
|
(122,031)
|
| Cash flows from investing activities: |
|
|
| Proceeds from maturities of marketable securities |
182,364
|
124,977
|
| Proceeds from sales of marketable securities |
0
|
4,920
|
| Purchases of marketable securities |
(243,266)
|
(53,372)
|
| Purchases of property and equipment |
(3,389)
|
(1,330)
|
| Cash acquired from acquisition of IPR&D asset |
391
|
0
|
| Net cash (used in) provided by investing activities |
(63,900)
|
75,195
|
| Cash flows from financing activities: |
|
|
| Proceeds from issuance of common shares |
318,520
|
0
|
| Payment of issuance costs |
(800)
|
0
|
| Change in restricted cash due to Former Parent |
0
|
5,203
|
| Proceeds from equity incentive plan and employee share purchase plan |
4,568
|
1,126
|
| Other financing activities, net |
2,258
|
0
|
| Net cash provided by financing activities |
324,546
|
6,329
|
| Effects of exchange rates on cash, cash equivalents, and restricted cash |
(13)
|
(147)
|
| Net decrease in cash, cash equivalents, and restricted cash |
(9,802)
|
(40,654)
|
| Cash, cash equivalents, and restricted cash at beginning of period |
252,120
|
242,604
|
| Cash, cash equivalents, and restricted cash at end of period |
$ 242,318
|
$ 201,950
|
| X |
- DefinitionFair Value Adjustment of Forward Contract and Derivative Liabilities
| Name: |
bhvn_FairValueAdjustmentOfForwardContractAndDerivativeLiabilities |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease (Decrease) in Fair Value of Derivative Liabilities
| Name: |
bhvn_IncreaseDecreaseInFairValueOfDerivativeLiabilities |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIssuance of Common Shares for Acquisition of Research and Development Asset
| Name: |
bhvn_IssuanceOfCommonSharesForAcquisitionOfResearchAndDevelopmentAsset |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from Issuance of Common Stock for License Agreements
| Name: |
bhvn_ProceedsFromIssuanceOfCommonStockForLicenseAgreements |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the acquisition of business during the period (for example, cash that was held by the acquired business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_CashAcquiredFromAcquisition |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in accrued expenses, and obligations classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilitiesAndOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based compensation granted to nonemployees as payment for services rendered or acknowledged claims. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IssuanceOfStockAndWarrantsForServicesOrClaims |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) included in net income that results in no cash inflow (outflow), classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OtherNoncashIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481830/320-10-45-11
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-11
| Name: |
us-gaap_PaymentsToAcquireAvailableForSaleSecuritiesDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from maturity, prepayment and call of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481830/320-10-45-11
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromMaturitiesPrepaymentsAndCallsOfAvailableForSaleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 15
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_ProceedsFromPaymentsForOtherFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481830/320-10-45-11
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-9
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-11
| Name: |
us-gaap_ProceedsFromSaleOfAvailableForSaleSecuritiesDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.2.u1
Nature of the Business and Basis of Presentation
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Nature of the Business and Basis of Presentation |
Nature of the Business and Basis of Presentation Biohaven Ltd. (“we,” “us," "our," "Biohaven" or the “Company”) was incorporated in Tortola, British Virgin Islands in May 2022. Biohaven is a biopharmaceutical company focused on the discovery, development and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. The Company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple, proprietary drug development platforms. Biohaven's extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; Transient Receptor Potential Melastatin 3 ("TRPM3") antagonism for migraine and neuropathic pain; Tyrosine Kinase 2/Janus Kinase 1 ("TYK2/JAK1") inhibition for neuroinflammatory disorders; glutamate modulation for obsessive compulsive disorder ("OCD") and spinocerebellar ataxia ("SCA"); myostatin inhibition for neuromuscular and metabolic diseases, including spinal muscular atrophy ("SMA") and obesity; and antibody recruiting bispecific molecules ("ARMs") and antibody drug conjugates ("ADCs") for cancer. The Company is subject to risks and uncertainties common to companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations and the ability to secure additional capital to fund operations. Product candidates currently under development will require significant additional research and development efforts, including preclinical and clinical testing and regulatory approval, prior to commercialization. These efforts may require additional capital, additional personnel and infrastructure, and further regulatory and other capabilities. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales. Separation from Biohaven Pharmaceutical Holding Company Ltd. On October 3, 2022, Biohaven Pharmaceutical Holding Company Ltd. (the “Former Parent”) completed the distribution (the “Distribution”) to holders of its common shares of all of the outstanding common shares of Biohaven Ltd. and the spin-off of Biohaven Ltd. from the Former Parent (the “Spin-Off”) described in Biohaven’s Information Statement attached as Exhibit 99.1 to Biohaven’s Registration Statement on Form 10, as amended (Reg. No. 001-41477). Collectively, we refer to the Distribution and Spin-Off throughout this Quarterly Report on Form 10-Q as the "Separation." As a result of the Separation, Biohaven Ltd. became an independent, publicly traded company as of October 3, 2022, and commenced regular way trading under the symbol “BHVN”’ on the New York Stock Exchange (the "NYSE") on October 4, 2022. Where we describe historical business activities in this report, we do so as if the Former Parent’s activities related to such assets and liabilities had been performed by the Company. Basis of Presentation The accompanying condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and pursuant to the rules and regulations of the SEC. The accompanying condensed consolidated financial statements include the accounts of Biohaven Ltd. and our wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation. Going Concern In accordance with Accounting Standards Codification (“ASC”) 205-40, Going Concern, the Company has evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the condensed consolidated financial statements are issued. Through August 9, 2024, the Company has funded its operations primarily with funding from the Former Parent, proceeds from the sale of its common shares, and the cash contribution received from the Former Parent at the Separation. The Company has incurred recurring losses since its inception and expects to continue to generate operating losses for the foreseeable future. As of the date of issuance of these condensed consolidated financial statements, the Company expects its existing cash, cash equivalents, and marketable securities will be sufficient to fund operating and financial commitments, and other cash requirements for at least one year after the issuance date of these financial statements. To execute its business plans, the Company will require funding to support its continuing operations and pursue its growth strategy. Until such time as the Company can generate significant revenue from product sales or royalties, if ever, it expects to finance its operations through the sale of public or private equity, debt financings or other capital sources, including collaborations with other companies or other strategic transactions. The Company may not be able to obtain financing on acceptable terms, or at all. The terms of any financing may adversely affect the holdings or the rights of the Company’s shareholders. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate some or all of its research and development programs, product portfolio expansion or commercialization efforts, which could adversely affect its business prospects, or the Company may be unable to continue operations.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Summary of Significant Accounting Policies
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
Summary of Significant Accounting Policies Our significant accounting policies are described in Note 2, "Summary of Significant Accounting Policies" to the consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023 (the "2023 Form 10-K"). Updates to our accounting policies are discussed below in this Note 2. Unaudited Interim Condensed Consolidated Financial Information The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with GAAP for interim financial information. The accompanying unaudited condensed consolidated financial statements do not include all of the information and footnotes required by GAAP for complete consolidated financial statements. The accompanying year-end condensed consolidated balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America. The unaudited interim condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of June 30, 2024, the results of its operations for the three and six months ended June 30, 2024 and 2023, and its cash flows for the six months ended June 30, 2024 and 2023. The results for the three and six months ended June 30, 2024 are not necessarily indicative of results to be expected for the year ending December 31, 2024, any other interim periods or any future year or period. The financial information included herein should be read in conjunction with the financial statements and notes in the Company's Annual Report on Form 10-K for the year ended December 31, 2023. Reclassifications Certain items in the prior period’s condensed consolidated financial statements have been reclassified to conform to the current year presentation. Use of Estimates The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of expenses during the reporting periods. Significant estimates and assumptions reflected in these condensed consolidated financial statements include, but are not limited to, the accrual for research and development expenses and valuation of forward contract and derivative liabilities. In addition, management’s assessment of the Company’s ability to continue as a going concern involves the estimation of the amount and timing of future cash inflows and outflows. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates. Restricted Cash Restricted cash included in other current assets in the condensed consolidated balance sheets consists primarily of employee contributions to the Company's employee share purchase plan held for future purchases of the Company's outstanding shares. Restricted cash included in other non-current assets in the condensed consolidated balance sheets represents collateral held by banks for a letter of credit ("LOC") issued in connection with the leased office space in Yardley, Pennsylvania and LOCs issued in connection with the leased office and lab spaces in Cambridge, Massachusetts and Pittsburgh, Pennsylvania. See Note 11, ‘‘Commitments and Contingencies’’ for additional information on the real estate leases. The following represents a reconciliation of cash and cash equivalents in the condensed consolidated balance sheets to total cash, cash equivalents and restricted cash as of June 30, 2024 and June 30, 2023, respectively, in the condensed consolidated statements of cash flows: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of June 30, 2023 | | Cash and cash equivalents | | $ | 239,147 | | | $ | 147,612 | | | Restricted cash held on behalf of Former Parent | | — | | | 40,415 | | | Restricted cash (included in other current assets) | | 156 | | | 11,574 | | | Restricted cash (included in other non-current assets) | | 3,015 | | | 2,349 | | | Total cash, cash equivalents and restricted cash at the end of the period in the condensed consolidated statement of cash flows | | $ | 242,318 | | | $ | 201,950 | |
Forward Contracts and Derivative Liabilities The Company evaluates certain of its financial and business development transactions to determine the accounting classification of equity linked instruments issued in those transactions. The Company first assesses whether a freestanding equity linked instrument meets liability classification in accordance with ASC 480-10 Distinguishing Liabilities from Equity and ASC 815-40 Derivatives and Hedging - Contracts in Entity's Own Equity. Under ASC 480-10 an instrument is considered liability classified if the instrument is mandatorily redeemable, obligate the issuer to settle an instrument or the underlying shares by paying cash or other assets, or must or may require settlement by issuing variable number of shares. If an instrument does not meet liability classification under ASC 480-10, the Company assesses the requirements under ASC 815-40, which states that contracts that require or may require net cash settlement are liabilities recorded at fair value. If the instrument does not require liability classification under ASC 815-40, in order to conclude equity classification, the Company assesses whether the instrument is indexed to its common stock and whether the instrument is classified as equity under ASC 815-40 or other applicable GAAP. Equity linked instruments that are accounted for in accordance with ASC 815-40 are reported as liabilities on the condensed consolidated balance sheets at fair value. Any change in fair value, as determined at each measurement period, is recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. Changes in fair value are reported on the condensed consolidated statements of cash flows as change in fair value of forward contract and derivative liabilities. The Company accounted for certain consideration agreed to in connection with the Amendment, dated as of May 1, 2024 (the "Knopp Amendment"), to the Membership Interest Purchase Agreement, dated as of February 24, 2022 (the "Purchase Agreement") entered into with Knopp Biosciences, LLC ("Knopp") as derivative liabilities under ASC 815 (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements"). Warrants The Company evaluates warrants issued as either equity instruments, liabilities or derivative liabilities in accordance with ASC Topic 480, Distinguishing Liabilities from Equity ("ASC 480") and/or ASC Topic 815, Derivatives and Hedging ("ASC 815"), depending on the specific terms of the warrant agreement. The Company assessed the warrants issued to Knopp in May 2024 (the "Knopp Warrants") and concluded that they met the criteria for equity classification under ASC 480 and ASC 815. Accordingly, the Knopp Warrants were recorded within additional paid-in capital on the condensed consolidated balance sheet at the time of issuance and are not subject to remeasurement. See Note 6, Shareholders' Equity for further detail on the Knopp Warrants. Fair Value Measurements Certain assets and liabilities of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable: •Level 1—Quoted prices in active markets for identical assets or liabilities. •Level 2—Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. •Level 3—Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. The carrying values of other current assets, accounts payable, and accrued expenses approximate their fair values due to the short-term nature of these assets and liabilities. The Company has accounted for certain consideration agreed to in connection with the Knopp Amendment as forward contract liabilities, which are recorded on the condensed consolidated balance sheets at fair value. Any changes in fair value, as determined each measurement period, are recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. The fair value of the forward contract liabilities is determined based on significant inputs not observable in the market, and therefore represents a Level 3 measurement within the fair value hierarchy. The valuations are based on a Monte Carlo simulation of the Company's share price, which requires judgement and assumption on the volatility of Biohaven's share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk since payable in a variable number of shares. (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements"). Recently Issued Accounting Pronouncements In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting—Improvements to Reportable Segment Disclosures, which improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses. The amendments in ASU No. 2023-07 apply to public entities, including those with a single reportable segment, and are effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact ASU No. 2023-07 will have on its consolidated financial statements In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, to improve the transparency of income tax disclosures by requiring consistent categories and greater disaggregation of information in the rate reconciliation and income taxes paid disaggregated by jurisdiction. The ASU also includes certain other amendments to improve the effectiveness of income tax disclosures. The amendments in ASU 2023-09 are effective for fiscal years beginning after December 15, 2024, with early adoption permitted for annual financial statements that have not yet been issued or made available for issuance. The Company is currently evaluating the impact ASU No. 2023-09 will have on its consolidated financial statements. |
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Marketable Securities
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Investments, Debt and Equity Securities [Abstract] |
|
| Marketable Securities |
Marketable Securities The amortized cost, gross unrealized holding gains, gross unrealized holding losses and fair value of debt securities available-for-sale by type of security at June 30, 2024 and December 31, 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Amortized Cost | | Allowance for Credit Losses | | Net Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value | | June 30, 2024 | | | | | | | | | | | | | Debt securities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | U.S. treasury bills | | $ | 234,748 | | | $ | — | | | $ | 234,748 | | | $ | 2 | | | $ | (5) | | | $ | 234,745 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | | | | | | Debt securities | | | | | | | | | | | | | U.S. corporate bonds | | $ | 46,228 | | | $ | — | | | $ | 46,228 | | | $ | 7 | | | $ | (24) | | | $ | 46,211 | | Foreign corporate bonds | | 7,180 | | | — | | | 7,180 | | | — | | | (7) | | | 7,173 | | | | | | | | | | | | | | | U.S. treasury bills | | 113,908 | | | — | | | 113,908 | | | 27 | | | — | | | 113,935 | | | | | | | | | | | | | | | | Total | | $ | 167,316 | | | $ | — | | | $ | 167,316 | | | $ | 34 | | | $ | (31) | | | $ | 167,319 | |
The fair value of debt securities available-for-sale by classification in the condensed consolidated balance sheets was as follows: | | | | | | | | | | | | | | | | | | | June 30, 2024 | | December 31, 2023 | | | | Cash and cash equivalents | | $ | 36,944 | | | $ | 33,902 | | | | | Marketable securities | | 197,801 | | | 133,417 | | | | | Total | | $ | 234,745 | | | $ | 167,319 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The net amortized cost and fair value of debt securities available-for-sale at June 30, 2024 and December 31, 2023 are shown below by contractual maturity. Actual maturities may differ from contractual maturities because securities may be restructured, called or prepaid, or the Company intends to sell a security prior to maturity. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | June 30, 2024 | | December 31, 2023 | | | Net Amortized Cost | | Fair Value | | Net Amortized Cost | | Fair Value | | Due to mature: | | | | | | | | | | Less than one year | | $ | 234,748 | | | $ | 234,745 | | | $ | 167,316 | | | $ | 167,319 | | | | | | | | | | | | | | | | | | | |
Summarized below are the debt securities available-for-sale the Company held at June 30, 2024 and December 31, 2023 that were in an unrealized loss position, aggregated by the length of time the investments have been in that position: | | | | | | | | | | | | | | | | | | | | | | | Less than 12 months | | | Number of Securities | | Fair Value | | Unrealized Losses | | June 30, 2024 | | | | | | | | Debt securities | | | | | | | | | | | | | | | | | | | | | | U.S. treasury bills | | 11 | | | $ | 133,504 | | | $ | (5) | | | | | | | | | | | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | Debt securities | | | | | | | | U.S. corporate bonds | | 6 | | | $ | 29,537 | | | $ | (24) | | | Foreign corporate bonds | | 1 | | | 7,173 | | | (7) | | | | | | | | | | | | | | | | | | | | | | | | Total | | 7 | | | $ | 36,710 | | | $ | (31) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The Company did not have any investments in a continuous unrealized loss position for more than twelve months as of June 30, 2024 or December 31, 2023. The Company reviewed the securities in the table above and concluded that they are performing assets, considering factors such as the credit quality of the investment security based on research performed by external rating agencies and the prospects of realizing the carrying value of the security based on the investment’s current prospects for recovery. As of June 30, 2024, the Company did not intend to sell these securities and did not believe it was more likely than not that it would be required to sell these securities prior to the anticipated recovery of their amortized cost basis. Net Investment Income Gross investment income includes income from debt securities available-for-sale, money-market funds, cash and restricted cash. Net investment income included in other income, net in the condensed consolidated statements of operations and comprehensive loss for the three and six months ended June 30, 2024 and 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | Debt securities (including realized losses) | | $ | 3,000 | | | $ | 2,225 | | | $ | 5,069 | | | $ | 5,634 | | Other investments | | 2,140 | | | 1,941 | | | 4,402 | | | 2,727 | | Gross investment income (including realized losses) | | 5,140 | | | 4,166 | | | 9,471 | | | 8,361 | | Investment expenses | | (40) | | | (68) | | | (70) | | | (138) | | | Net investment income | | $ | 5,100 | | | $ | 4,098 | | | $ | 9,401 | | | $ | 8,223 | |
We utilize the specific identification method in computing realized gains and losses. The proceeds from the sale of available-for-sale debt securities and the related gross realized capital losses for the three and six months ended June 30, 2024 and 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | | Proceeds from sales | | $ | — | | | $ | 2,464 | | | $ | — | | | $ | 4,920 | | | | | | | | | | | | Gross realized capital losses | | $ | — | | | $ | 17 | | | $ | — | | | $ | 39 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for investments in certain debt and equity securities. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 940
-SubTopic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/940-320/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1403
-Paragraph b
-Publisher SEC
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/320/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-10
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6B
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/942-320/tableOfContent
| Name: |
us-gaap_InvestmentsInDebtAndMarketableEquitySecuritiesAndCertainTradingAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Fair Value of Financial Assets and Liabilities
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value of Financial Assets and Liabilities |
Fair Value of Financial Assets and Liabilities The preparation of the Company’s condensed consolidated financial statements in accordance with GAAP requires certain assets and liabilities to be reflected at their fair value and others to be reflected on another basis, such as an adjusted historical cost basis. In this note, the Company provides details on the fair value of financial assets and liabilities and how it determines those fair values. Financial Instruments Measured at Fair Value on the Condensed Consolidated Balance Sheets Certain assets of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable: •Level 1 — Quoted prices in active markets for identical assets or liabilities. •Level 2 — Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. •Level 3 — Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. Financial assets measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 and December 31, 2023 and financial liabilities measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 were as follows : | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement Using: | | Balance Sheet Classification | | Type of Instrument | | Level 1 | | Level 2 | | Level 3 | | Total | | June 30, 2024 | | | | | | | | | | | | Assets: | | | | | | | | | | | | Cash and cash equivalents | | Money market funds | | $ | 113,379 | | | $ | — | | | $ | — | | | $ | 113,379 | | Cash and cash equivalents | | U.S. treasury bills | | — | | | 36,944 | | | — | | | 36,944 | | | | | | | | | | | | | | Marketable securities | | U.S. treasury bills | | 29,701 | | | 168,100 | | | — | | | 197,801 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Other non-current assets | | Money market funds | | 2,515 | | | — | | | — | | | 2,515 | | | Total assets | | | | $ | 145,595 | | | $ | 205,044 | | | $ | — | | | $ | 350,639 | | | | | | | | | | | | | | Liabilities: | | | | | | | | | | | Forward contract and derivative liabilities | | Forward contract, current and written put option, current | | $ | — | | | $ | — | | | $ | 81,220 | | | $ | 81,220 | | | | | | | | | | | | | Derivative liability, non-current | | Written put option, non-current | | — | | | — | | | 12,180 | | | 12,180 | | | | | | | | | | | | | | Total liabilities | | | | $ | — | | | $ | — | | | $ | 93,400 | | | $ | 93,400 | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | | | | | Assets: | | | | | | | | | | | | Cash and cash equivalents | | Money market funds | | $ | 59,199 | | | $ | — | | | $ | — | | | $ | 59,199 | | | Cash and cash equivalents | | U.S. treasury bills | | — | | | 27,901 | | | — | | | 27,901 | | | Cash and cash equivalents | | U.S. corporate bonds | | — | | | 6,001 | | | — | | | 6,001 | | | Marketable securities | | U.S. treasury bills | | 9,874 | | | 76,160 | | | — | | | 86,034 | | | Marketable securities | | U.S. corporate bonds | | — | | | 40,210 | | | — | | | 40,210 | | | Marketable securities | | Foreign corporate bonds | | — | | | 7,173 | | | — | | | 7,173 | | | Other non-current assets | | Money market funds | | 1,900 | | | — | | | — | | | 1,900 | | | Total assets | | | | $ | 70,973 | | | $ | 157,445 | | | $ | — | | | $ | 228,418 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The Company had no financial liabilities measured at fair value on a recurring basis on the condensed consolidated balance sheets at December 31, 2023. There were no securities transferred into or out of Level 3 during the three and six months ended June 30, 2024 or 2023. The following is a description, including valuation methodology, of the financial assets measured at fair value on a recurring basis: Cash Equivalents Cash equivalents consisted of cash invested in short-term money market funds and debt securities with an original maturity of 90 days or less at the date of purchase. The carrying value of cash equivalents approximates fair value as maturities are less than three months. When quoted prices are available in an active market, cash equivalents are classified in Level 1 of the fair value hierarchy. Fair values of cash equivalent instruments that do not trade on a regular basis in active markets are classified as Level 2. Marketable Securities and Other Non-Current Assets Quoted prices for identical assets in active markets are considered Level 1 and consist of on-the-run U.S. Treasuries and money market funds. The fair values of the Company’s Level 2 debt securities are obtained from quoted market prices of debt securities with similar characteristics, quoted prices from identical assets in inactive markets, or discounted cash flows to estimate fair value. Forward Contract and Derivative Liabilities In connection with the Knopp Amendment, Knopp has the option to request a one-time cash true-up payment from the Company in December 2024, as defined in the agreement (the "2024 Additional Consideration True-up"). The Company also agreed to issue to Knopp additional common shares of the Company with an approximate value of $75,000 within 60 days of the first anniversary of execution of the Knopp Amendment (the “2025 Additional Consideration”). In addition, Knopp has the option to request a one-time cash true-up payment from the Company in December 2025, as defined in the agreement (the "2025 Additional Consideration True-up"). See Note 10, "License, Acquisitions and Other Agreements," for further details. The following table provides a roll forward of the fair value of the Company's forward contract and derivative liabilities related to the Knopp Amendment for which fair value is determined by Level 3 inputs for the three months ended June 30, 2024: | | | | | | | | | | | | | Carrying Value | | | Fair value at May 1, 2024 | | $ | 93,290 | | | | Change in fair value of derivative in other income (expense), net | | 110 | | | | Fair value at June 30, 2024 | | $ | 93,400 | | | |
The fair value of the forward contract and derivative liabilities recognized in connection with the Knopp Amendment were determined based on significant inputs not observable in the market, and therefore represents a Level 3 measurement within the fair value hierarchy. The valuation is based on a Monte Carlo simulation of Biohaven's share price, which requires judgement and assumption on the volatility of Biohaven's share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk since payable in a variable number of shares for the 2025 Additional Consideration or potentially cash for the 2024 Additional Consideration True-up and 2025 Additional Consideration True-up. A summary of the unobservable inputs (Level 3 inputs) used in measuring the Company’s forward contract and derivative liabilities related to the Knopp Amendment as of June 30, 2024 and May 1, 2024 are as follows, presented on a weighted-average basis: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of May 1, 2024 | | | | | | Time to payment and potential payment (years) | | 0.91 | | 1.08 | Volatility (annual) | | 70.0 | % | | 80.0 | % | Discount rate | | 15.6 | % | | 14.8 | % |
Our expectations of the volatility of Biohaven's share price at the reporting date could be materially different than our actual future volatility, and if so, would mean the estimated fair value could be significantly higher or lower than the fair value determined. The Company expects the fair value of the forward contract to be approximately $75,000 on the first anniversary of the Knopp Amendment. An increase in the derivative liabilities related to the 2024 Additional Consideration True-up and 2025 Additional Consideration True-up between the reporting date and settlement date of the derivatives would have a material adverse effect on the Company's financial performance.
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Balance Sheet Components
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Balance Sheet Components |
Balance Sheet Components Property and Equipment, Net Property and equipment, net consisted of the following: | | | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | Building and land | | $ | 14,078 | | | $ | 11,728 | | | | Leasehold improvements | | 824 | | | 802 | | | | | Computer hardware and software | | 875 | | | 875 | | | | | Office and lab equipment | | 11,027 | | | 9,961 | | | | | Furniture and fixtures | | 1,793 | | | 1,550 | | | | | | $ | 28,597 | | | $ | 24,916 | | | | | Accumulated depreciation | | (10,201) | | | (8,283) | | | | | | 18,396 | | | 16,633 | | | | | Equipment not yet in service | | 269 | | | 558 | | | | | Property and equipment, net | | $ | 18,665 | | | $ | 17,191 | | | |
Depreciation expense was $977 and $1,918 for the three and six months ended June 30, 2024, respectively, and $800 and $1,564 for the three and six months ended June 30, 2023, respectively. Equipment not yet in service primarily consisted of lab equipment that had not been placed into service as of June 30, 2024 and December 31, 2023. Other Non-current Assets Other non-current assets consisted of the following: | | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | | | | | | | | | | | | | | | | | | Operating lease right-of-use assets | | $ | 29,903 | | | $ | 31,385 | | | | Other | | 3,015 | | | 2,400 | | | | Other non-current assets | | $ | 32,918 | | | $ | 33,785 | | |
Accrued Expenses and Other Current Liabilities Accrued expenses and other current liabilities consisted of the following: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | | | | Accrued employee compensation and benefits | | $ | 10,845 | | | $ | 837 | | | Accrued clinical trial costs | | 36,446 | | | 29,501 | | | | | | | | | | | | | | | | | | | | | | | | | | | Operating lease liabilities - current portion | | 3,550 | | | 3,308 | | | Other accrued expenses and other current liabilities | | 6,887 | | | 6,200 | | | Accrued expenses and other current liabilities | | $ | 57,728 | | | $ | 39,846 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for supplemental balance sheet disclosures, including descriptions and amounts for assets, liabilities, and equity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/210/tableOfContent
| Name: |
us-gaap_SupplementalBalanceSheetDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Shareholders' Equity
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Stockholders' Equity Note [Abstract] |
|
| Shareholders' Equity |
Shareholders' Equity Changes in shareholders’ equity for the three and six months ended June 30, 2024 and June 30, 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Common Shares | | | | | | | | | | | | | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Loss | | Total Shareholders' Equity | | Balances as of December 31, 2023 | | 81,115,723 | | | $ | 887,528 | | | | | $ | 39,804 | | | $ | (499,292) | | | $ | (65) | | | $ | 427,975 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Net loss | | — | | | — | | | | | — | | | (179,504) | | | — | | | (179,504) | | Issuance of common shares as payment for acquisition of IPR&D asset | | 242,958 | | | 10,347 | | | | | — | | | — | | | — | | | 10,347 | | Issuance of common shares as payment under license and other agreements | | 97,233 | | | 5,637 | | | | | — | | | — | | | — | | | 5,637 | | | Issuance of common shares under 2022 Equity Incentive Plan | | 351,307 | | | 7,452 | | | | | (5,296) | | | — | | | — | | | 2,156 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 34,877 | | | — | | | — | | | 34,877 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (41) | | | (41) | | | Balances as of March 31, 2024 | | 81,807,221 | | | 910,964 | | | | | 69,385 | | | (678,796) | | | (106) | | | 301,447 | | | Net loss | | — | | | — | | | | | — | | | (319,771) | | | — | | | (319,771) | | | Issuance of common shares, net of offering costs | | 8,544,951 | | | 317,720 | | | | | — | | | — | | | — | | | 317,720 | | | Issuance of common shares as payment for acquisition of IPR&D asset | | 10,452 | | | 446 | | | | | — | | | — | | | — | | | 446 | | | Issuance of common shares as payment under license and other agreements | | 1,872,874 | | | 65,981 | | | | | — | | | — | | | — | | | 65,981 | | | Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 110,834 | | | 3,442 | | | | | (1,125) | | | — | | | — | | | 2,317 | | | Issuance of warrant as payment under license agreement | | — | | | — | | | | | 3,340 | | | — | | | — | | | 3,340 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 12,232 | | | — | | | — | | | 12,232 | | | Other comprehensive income | | — | | | — | | | | | — | | | — | | | 28 | | | 28 | | | Balances as of June 30, 2024 | | 92,346,332 | | | $ | 1,298,553 | | | | | $ | 83,832 | | | $ | (998,567) | | | $ | (78) | | | $ | 383,740 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Common Shares | | | | | | | | | | | | | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income | | Total Shareholders' Equity | Balances as of December 31, 2022 | | 68,190,479 | | | $ | 615,742 | | | | | $ | 13,869 | | | $ | (91,124) | | | $ | 284 | | | $ | 538,771 | | | Net loss | | — | | | — | | | | | — | | | (70,492) | | | — | | | (70,492) | | | Issuance of common shares under 2022 Equity Incentive Plan | | 22,000 | | | 504 | | | | | (172) | | | — | | | — | | | 332 | | Non-cash share-based compensation expense | | — | | | — | | | | | 3,765 | | | — | | | — | | | 3,765 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (118) | | | (118) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Balances as of March 31, 2023 | | 68,212,479 | | | 616,246 | | | | | 17,462 | | | (161,616) | | | 166 | | | 472,258 | | | Net loss | | — | | | — | | | | | — | | | (90,346) | | | — | | | (90,346) | | | Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 104,474 | | | 1,264 | | | | | (470) | | | — | | | — | | | 794 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 4,695 | | | — | | | — | | | 4,695 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (146) | | | (146) | | Balance as of June 30, 2023 | | 68,316,953 | | | $ | 617,510 | | | | | $ | 21,687 | | | $ | (251,962) | | | $ | 20 | | | $ | 387,255 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Knopp Amendment In May 2024, the Company entered into the Knopp Amendment under which the parties thereto agreed to revise the success-based payment and royalty payment obligations under the Membership Purchase Agreement. As consideration, the Company issued 1,872,874 Biohaven Shares to Knopp, valued at approximately $65,981 in May 2024. As further consideration for the revisions to the success-based payment and royalty payment obligations in the Knopp Amendment, the Company issued to Knopp a warrant to purchase 294,195 of the Company's common shares with a purchase price of $67.98, subject to certain specified development milestones and the Company achieving a specified market capitalization. The warrant was recorded at its initial fair value of $3,340 within additional paid-in capital on the condensed consolidated balance sheet as of June 30,2024 and is not subject to remeasurement. See Note 10 for further detail on the Knopp Amendment. April 2024 Public Offering On April 22, 2024, the Company closed an underwritten public offering of 6,451,220 of its common shares, which included the exercise in full of the underwriters' option to purchase additional shares, at a price of $41.00 per share. The net proceeds raised in the offering, after deducting underwriting discounts and expenses of the offering payable by Biohaven, were approximately $247,830. The Company intends to use the net proceeds received from the offering for general corporate purposes. Pyramid Acquisition In January 2024, the Company acquired Pyramid pursuant to the Pyramid Agreement. In consideration for the Pyramid acquisition, Biohaven made an upfront payment of 255,794 Company common shares, valued at approximately $10,894. As of June 30, 2024, 253,410 of these common shares have been issued by the Company. During the first quarter of 2024, the Company recorded $5,689 of R&D expense in the condensed consolidated statement of operations and comprehensive loss for a developmental milestone which became due under the Pyramid Agreement, to be paid in 98,129 Company common shares. As of June 30, 2024, 97,233 of these common shares have been issued by the Company. Refer to Note 10, "License, Acquisitions and Other Agreements" for further discussion of the Pyramid acquisition. Equity Distribution Agreement In October 2023, the Company entered into an equity distribution agreement pursuant to which the Company may offer and sell common shares having an aggregate offering price of up to $150,000 from time to time through or to the sales agent, acting as its agent or principal (the "Equity Distribution Agreement"). Sales of the Company's common shares, if any, will be made in sales deemed to be “at-the-market offerings”. The sales agent is not required to sell any specific amount of securities but will act as the Company's sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between the sales agent and the Company. The Company currently plans to use the net proceeds from any at-the-market offerings of its common shares for general corporate purposes. As of June 30, 2024, the Company sold and issued 2,093,731 common shares under the Equity Distribution Agreement for net proceeds of approximately $69,890. Subsequent to June 30, 2024, the Company issued and sold an additional 2,154,857 common shares for net proceeds of $76,360. As of August 9, 2024, the date of this report, the Company issued and sold a total of 4,248,588 common shares under the Equity Distribution Agreement for total net proceeds of $146,250, and no shares remain available to be issued.
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Accumulated Other Comprehensive (Loss) Income
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Accumulated Other Comprehensive (Loss) Income |
Accumulated Other Comprehensive (Loss) Income Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2024: | | | | | | | | | | | | | | | | | Three Months Ended June 30, 2024 | | Six Months Ended June 30, 2024 | | | | | | | Net unrealized investment gains (losses): | | | | | | Beginning of period balance | | $ | (32) | | | $ | 3 | | | | | | | | | | | | Other comprehensive income (loss)(1)(2) | | 29 | | | (6) | | | End of period balance | | (3) | | | (3) | | | | | | | | Foreign currency translation adjustments: | | | | | | Beginning of period balance | | (74) | | | (68) | | Other comprehensive loss(1) | | (1) | | | (7) | | | | | | | | End of period balance | | (75) | | | (75) | | | | | | | Total beginning of period accumulated other comprehensive loss | | (106) | | | (65) | | Total other comprehensive income (loss) | | 28 | | | (13) | | Total end of period accumulated other comprehensive loss | | $ | (78) | | | $ | (78) | | | | | | |
(1) There was no tax on other comprehensive loss and no amounts reclassified from accumulated other comprehensive (loss) income during the period. Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2023: | | | | | | | | | | | | | | | | | Three Months Ended June 30, 2023 | | Six Months Ended June 30, 2023 | | | | | | | Net unrealized investment gains (losses): | | | | | | Beginning of period balance | | $ | (278) | | | $ | (145) | | Other comprehensive loss before reclassifications(1) | | (1) | | | (156) | | Amounts reclassified from accumulated other comprehensive loss(1)(2) | | 17 | | | 39 | | Other comprehensive income (loss)(1) | | 16 | | | (117) | | | End of period balance | | (262) | | | (262) | | | | | | | | Foreign currency translation adjustments: | | | | | | Beginning of period balance | | 444 | | | 429 | | Other comprehensive loss(1) | | (162) | | | (147) | | | | | | | | End of period balance | | 282 | | | 282 | | | | | | | Total beginning of period accumulated other comprehensive income | | 166 | | | 284 | | Total other comprehensive loss | | (146) | | | (264) | | Total end of period accumulated other comprehensive income | | $ | 20 | | | $ | 20 | | | | | | |
(1) There was no tax on other comprehensive income (loss) and immaterial tax on amounts reclassified from accumulated other comprehensive income (loss) during the period. (2) Amounts reclassified from accumulated other comprehensive income (loss) for specifically identified debt securities are included in other income (expense), net on the condensed consolidated statement of operations and comprehensive loss.
|
| X |
- DefinitionThe entire disclosure for comprehensive income, which includes, but is not limited to, 1) the amount of income tax expense or benefit allocated to each component of other comprehensive income, including reclassification adjustments, 2) the reclassification adjustments for each classification of other comprehensive income and 3) the ending accumulated balances for each component of comprehensive income. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/220/tableOfContent
| Name: |
us-gaap_ComprehensiveIncomeNoteTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Non-Cash Share-Based Compensation
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Non-Cash Share-Based Compensation |
Non-Cash Share-Based Compensation Non-Cash Share-based Compensation Expense The Company measures non-cash share-based compensation at the grant date based on the fair value of the award and recognizes non-cash shared-based compensation as expense over the requisite service period of the award (generally three years) using the straight-line method. Non-cash share-based compensation expense, consisting of expense for share options, restricted share units ("RSUs"), performance share options, and the Employee Share Purchase Plan ("ESPP"), was classified in the condensed consolidated statements of operations and comprehensive loss as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | Research and development expenses | | $ | 7,071 | | | $ | 2,457 | | | $ | 28,362 | | | $ | 4,698 | | General and administrative expenses | | 5,161 | | | 2,238 | | | 18,747 | | | 3,762 | | | Total non-cash share-based compensation expense | | $ | 12,232 | | | $ | 4,695 | | | $ | 47,109 | | | $ | 8,460 | |
Share Options All share option grants are awarded at fair value on the date of grant. The fair value of share options is estimated using the Black-Scholes option pricing model. Stock options generally expire 10 years after the grant date. The aggregate intrinsic value of share options is calculated as the difference between the exercise price of the share options and the fair value of the Company's common shares for those share options that had exercise prices lower than the fair value of the Company's common shares at June 30, 2024. As of June 30, 2024, total unrecognized compensation cost related to the unvested share options was $90,206, which is expected to be recognized over a weighted average period of 2.33 years, which does not consider the impact of a change in control. The weighted average grant date fair value per share of share options granted under the Company's share option plan during the six months ended June 30, 2024 and 2023 was $30.76 and $10.16, respectively. The Company expects approximately 7,978,678 of the unvested stock options to vest over the requisite service period. The following table is a summary of the Company's share option activity for the six months ended June 30, 2024: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Number of Shares | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term | | Aggregate Intrinsic Value | | | | | | | | (in years) | | | Outstanding as of December 31, 2023 | | 11,379,429 | | $ | 11.48 | | | | | | | Granted | | 2,500,161 | | $ | 42.05 | | | | | | | Exercised | | (295,890) | | $ | 9.00 | | | | | | | Forfeited | | (38,684) | | $ | 20.38 | | | | | | Outstanding as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | | Options exercisable as of June 30, 2024 | | 5,566,338 | | $ | 13.44 | | | 8.54 | | $ | 122,803 | | Vested and expected to vest as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | |
Restricted Share Units The Company’s RSUs are considered nonvested share awards and require no payment from the employee. For each RSU, employees receive one common share at the end of the vesting period. The employee can elect to receive the one common share net of taxes or pay for taxes separately and receive the entire share. Compensation cost is recorded based on the market price of the Company’s common shares on the grant date and is recognized on a straight-line basis over the requisite service period. As of June 30, 2024, there was $9,405 of total unrecognized compensation cost related to Company RSUs that are expected to vest. These costs are expected to be recognized over a weighted-average period of 2.53 years, which does not consider the impact of a change in control. The total fair value of RSUs vested during the three and six months ended June 30, 2024 was $3,770. The following table is a summary of the RSU activity for the six months ended June 30, 2024:
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Net Loss Per Share
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Net Loss Per Share |
Net Loss Per Share Basic and diluted net loss per share attributable to common shareholders of Biohaven was calculated as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | | Numerator: | | | | | | | | | | | | | | | | | | | Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (499,275) | | | $ | (160,838) | | | Denominator: | | | | | | | | | Weighted average common shares outstanding—basic and diluted | | 87,766,069 | | | 68,248,023 | | | 84,174,099 | | | 68,227,564 | | | Net loss per share — basic and diluted | | $ | (3.64) | | | $ | (1.32) | | | $ | (5.93) | | | $ | (2.36) | |
The Company's potential dilutive securities include share options which have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted average number of common shares outstanding used to calculate both basic and diluted net loss per share attributable to common shareholders of the Company is the same. The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect: | | | | | | | | | | | | | | | | | | As of June 30, | | | | 2024 | | 2023 | | Options to purchase common shares | | 13,545,016 | | | 9,639,557 | | | Warrants to purchase common shares | | 294,195 | | | — | | Restricted share units | | 263,672 | | | — | | | Total | | 14,102,883 | | | 9,639,557 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
License, Acquisitions and Other Agreements
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| License, Acquisitions and Other Agreements |
License, Acquisitions and Other Agreements The Company has entered into various licensing, developmental and acquisition agreements which provide the Company with rights to certain know-how, technology and patent rights. The agreements generally include upfront fees, milestone payments upon achievement of certain developmental, regulatory and commercial and sales milestones, as well as sales- based royalties, with percentages that vary by agreement. License and Other Agreements As of June 30, 2024, the Company has potential future developmental, regulatory and commercial milestone payments under its license and other agreements of up to approximately $140,650, $641,975, and $2,150,450, respectively. See below for a detailed discussion of these agreements. The Company has not recorded these potential contingent consideration payments as liabilities in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024. Yale Agreements In September 2013, the Company entered into an exclusive license agreement (the "Yale Agreement") with Yale University to obtain a license to certain patent rights for the commercial development, manufacture, distribution, use and sale of products and processes resulting from the development of those patent rights, related to the use of riluzole in treating various neurological conditions, such as general anxiety disorder, post-traumatic stress disorder and depression. The Yale Agreement was amended and restated in May 2019. As of June 30, 2024, under the amended Yale Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $2,000 and annual royalty payments of a low single-digit percentage based on net sales of riluzole-based products from the licensed patents or from products based on troriluzole. Under the amended and restated agreement, the royalty rates are reduced as compared to the original agreement. In addition, under the amended and restated agreement, the Company may develop products based on riluzole or troriluzole. The amended and restated agreement retains a minimum annual royalty of up to $1,000 per year, beginning after the first sale of product under the agreement. If the Company grants any sublicense rights under the Yale Agreement, it must pay Yale University a low single-digit percentage of sublicense income that it receives. For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the Yale Agreement. In January 2021, the Company entered into a worldwide, exclusive license agreement with Yale University for the development and commercialization of a novel Molecular Degrader of Extracellular Protein ("MoDE") platform (the "Yale MoDE Agreement"). The platform pertains to the clearance of disease-causing protein and other biomolecules by targeting them for lysosomal degradation using multi-functional molecules. The Yale MoDE Agreement includes an obligation to pay a minimum annual royalty of up to $1,000 per year, and low single digit royalties on the net sales of licensed products. If the Company grants any sublicense rights under the Yale MoDE Agreement, it must pay Yale University a low single-digit percentage of sublicense income that it receives. As of June 30, 2024, under the Yale MoDE Agreement, the Company has remaining contingent development and commercial milestone payments of up to $650 and $2,950, respectively. The Yale MoDE Agreement terminates on the later of twenty years from the effective date, twenty years from the filing date of the first investigational new drug application for a licensed product or the last to expire of a licensed patent. The Company did not record any material milestone or royalty payments under the Yale MoDE Agreement for the three months ended June 30, 2024. For the six months ended June 30, 2024, the Company recorded research and development expense of $150 related to the achievement of a developmental milestone under the Yale MoDE Agreement. For the three and six months ended June 30, 2023, the Company did not record any material milestone or royalty payments under the Yale MoDE Agreement. ALS Biopharma Agreement In August 2015, the Company entered into an agreement (the "ALS Biopharma Agreement") with ALS Biopharma and Fox Chase Chemical Diversity Center Inc. ("FCCDC"), pursuant to which ALS Biopharma and FCCDC assigned the Company their worldwide patent rights to a family of over 300 prodrugs of glutamate modulating agents, including troriluzole, as well as other innovative technologies. Under the ALS Biopharma Agreement, the Company is obligated to use commercially reasonable efforts to commercialize and develop markets for the patent products. As of June 30, 2024, under the ALS Biopharma Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $4,000, as well as royalty payments of a low single-digit percentage based on net sales of products licensed under the ALS Biopharma Agreement, payable on a quarterly basis. The ALS Biopharma Agreement terminates on a country-by-country basis as the last patent rights expire in each such country. If the Company abandons its development, research, licensing or sale of all products covered by one or more claims of any patent or patent application assigned under the ALS Biopharma Agreement, or if the Company ceases operations, it has agreed to reassign the applicable patent rights back to ALS Biopharma. For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the ALS Biopharma Agreement. Taldefgrobep Alfa License Agreement In February 2022, following the transfer of intellectual property, the Company announced that it entered into a worldwide license agreement with BMS for the development and commercialization rights to taldefgrobep alfa (also known as BMS-986089), a novel, Phase 3-ready anti-myostatin adnectin (the "Taldefgrobep Alfa License Agreement"). As of June 30, 2024, under the Taldefgrobep Alfa License Agreement, the Company has remaining contingent regulatory approval milestone payments of up to $200,000, as well as tiered, sales-based royalty percentages from the high teens to the low twenties. There were no upfront or contingent payments to BMS related to the Taldefgrobep Alfa License Agreement. For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments under the Taldefgrobep Alfa License Agreement. Agreement with Hangzhou Highlightll Pharmaceutical Co. Ltd. In March 2023, the Company and Hangzhou Highlightll Pharmaceutical Co. Ltd. ("Highlightll") entered into an exclusive, worldwide (excluding People’s Republic of China and its territories and possessions) license agreement (the "Highlightll Agreement") pursuant to which Biohaven obtained the right to research, develop, manufacture and commercialize Highlightll’s brain penetrant dual TYK2/JAK1 inhibitor program. In connection with the Highlightll Agreement, the Company was obligated to pay Highlightll a cash payment of $10,000 and 721,136 common shares (collectively, "the Highlightll Upfront Payments"), upon the completion of certain post-closing activities. In December 2023, the Company entered into a second amendment to the Highlightll Agreement, which granted the Company an exclusive option and right of first refusal to any Selective TYK2 Inhibitor being developed by or on behalf of Highlightll or its affiliates and provided for the payment of the Highlightll Upfront Payments. As a result, the Company made a $10,000 cash payment and issued 721,136 shares, valued at $21,814 to Highlightll during the fourth quarter of 2023, which was recorded as R&D expense during the fourth quarter of 2023. As of June 30, 2024, under the Highlightll Agreement, the Company has remaining contingent development, regulatory approval, and commercial milestone payments of up to $75,000, $37,500, and $837,500, respectively. Additionally, the Company has agreed to make tiered royalty payments as a percentage of net sales starting at mid single digits and peaking at low teens digits. During the royalty term, if the Company offers to include China clinical sites in its Phase 3 study sufficient for submission to Chinese National Medical Products Administration and Highlightll, at its sole discretion, agrees, then Highlightll will pay royalties in the low tens digits to the Company on China sales upon approval. The Highlightll Agreement terminates on a country-by-country basis upon expiration of the royalty term and can also be terminated if certain events occur, e.g., material breach or insolvency. For the three and six months ended June 30, 2024 and 2023, the Company did not record any material milestone or royalty payments related to the Highlightll Agreement. Other Agreements In addition to the agreements detailed above, the Company has entered into various other license agreements and development programs. The Company records milestones and other payments, including funding for research arrangements, which become due under these agreements to research and development expense in the condensed consolidated statements of operations and comprehensive loss. Amounts recorded for the period were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | Six Months Ended June 30, | | | | 2024 | | 2023 | 2024 | | 2023 | Milestone payments | | $ | 375 | | | $ | — | | $ | 1,875 | | | $ | — | | | | | | | | | |
For the three and six months ended June 30, 2024 and 2023, the Company did not make any upfront payments under these agreements. Acquisitions Kv7 Platform Acquisition In April 2022, the Company closed the acquisition from Knopp of Channel Biosciences, LLC (“Channel”), a wholly owned subsidiary of Knopp owning the assets of Knopp’s Kv7 channel targeting platform (the “Kv7 Platform Acquisition”), pursuant to the Purchase Agreement, dated February 24, 2022. Under the Purchase Agreement, the Company agreed to make success-based payments based on developmental and regulatory milestones through approvals in the United States, Europe, the Middle East and Asia ("EMEA") and Japan for the lead asset, BHV-7000 (formerly known as KB-3061), developmental and regulatory milestones for the Kv7 pipeline development in other indications and additional country approvals, and commercial sales-based milestones of BHV-7000. Additionally, the Company agreed to make scaled royalty payments in cash for BHV-7000 and the pipeline programs, with percentages starting at high single digits and peaking at low teens for BHV-7000 and starting at mid-single digits and peaking at low tens digits for the pipeline programs. In May 2024, the Company entered into the Knopp Amendment under which the parties thereto agreed to replace the scaled high single digit to low teens royalty payment obligations with a flat royalty payment in the mid-single digits for BHV-7000 and the pipeline programs. The parties also agreed to reduce the success-based payments payable under the Purchase Agreement. The Company retains the ability to pay these contingent milestone payments in cash or in the Company's common shares at Biohaven's election, subject to the same increases if the Company elects to pay in the Company's common shares. As of June 30, 2024, under the Purchase Agreement, as amended, the Company had remaining success-based payments comprised of (i) to up to $185,000 based on regulatory approvals in the United States and EMEA for BHV-7000 and (ii) up to an additional $60,000 based on regulatory approval in the United States for the other Kv7 pipeline programs. In consideration of the revisions to the success-based payment and royalty payment obligations, the Company agreed to issue to Knopp 1,872,874 Company common shares, valued at approximately $75,000, through a private placement within 60 days of the date of execution of the Knopp Amendment (the “2024 Additional Consideration”) and additional Company common shares with an approximate value of $75,000 within 60 days of the first anniversary of execution of the Knopp Amendment (the “2025 Additional Consideration”). The Company has also given Knopp the option to request a one-time cash true-up payment from the Company in December 2024 in the event that Knopp continues to hold the Company's common shares representing the 2024 Additional Consideration and the value of such shares has declined (the "2024 Knopp True-Up"), and a one-time cash true-up payment from the Company in December 2025 in the event that Knopp continues to hold the Company's common shares representing the 2025 Additional Consideration and the value of such shares has declined (the "2025 Knopp True-Up"), in each case, subject to certain conditions. The Company concluded that the agreement to issue the 2024 Additional Consideration at a future date represented a fixed forward contract under ASC 815 and classified the commitment as a forward contract liability on its condensed consolidated balance sheet on the execution date of the Knopp Amendment. The Company initially measured the forward contract associated with the 2024 Additional Consideration at a fair value of $75,220, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. In May 2024, the Company issued the 2024 Additional Consideration at an approximate value of $65,981 and recognized a $9,239 gain upon settlement of the forward contract in other income (expense), net in its condensed consolidated statement of operations and comprehensive loss during the three months ended June 30, 2024. The gain on settlement of the 2024 Additional Consideration is due to the decline in fair value of the 2024 Additional Consideration from the execution date to the issuance date due to a decline in Biohaven's share price. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" and Note 6, "Shareholders' Equity" for further discussion. The 2024 Additional Consideration True-up represents a net cash settled written put option measured at fair value on a recurring basis. The Company has concluded that the 2024 Additional Consideration True-up represents a net cash settled written put option on the Company’s shares and is a freestanding derivative liability under ASC 815. Accordingly, the Company classified the 2024 Additional Consideration True-up as a current derivative liability on its condensed consolidated balance sheet. The Company initially recorded the 2024 Additional Consideration True-up at a fair value of $15,540, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. The Company subsequently remeasures the fair value of the derivative liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized expense of $590 for the three months ended June 30, 2024, related to the 2024 Additional Consideration True-up. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion. The Company has concluded that the agreement to issue the 2025 Additional Consideration at a future date represents a forward contract settleable in a variable number of shares under ASC 480, and classified the commitment as a current forward contract liability on its condensed consolidated balance sheet. The Company initially measured the 2025 Additional Consideration at a fair value of $63,940, which was recorded as R&D expense during the three months ended June 30, 2024 in its condensed consolidated statements of operations and comprehensive loss. The Company subsequently remeasures the fair value of the forward contract liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized expense of $1,150 for the three months ended June 30, 2024, related to the 2025 Additional Consideration. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion. The Company has concluded that the 2025 Additional Consideration True-up represents a net cash settled written put option on the Company’s shares and is a freestanding derivative liability under ASC 815. Accordingly, the Company classified the 2025 Additional Consideration True-up as a non-current derivative liability on its condensed consolidated balance sheet. The Company initially recorded the 2025 Additional Consideration True-up at a fair value of $13,810, which was recorded as R&D expense during the three months ended June 30, 2024. The Company subsequently remeasures the fair value of the derivative liability and recognizes any gains or losses through other income (expense), net in its condensed consolidated statement of operations and comprehensive loss. The Company recognized a gain of $1,630 for the three months ended June 30, 2024, related to the 2025 Additional Consideration True-up. Refer to Note 4, "Fair Value of Financial Assets and Liabilities" for further discussion. As further consideration for the revisions to the success-based payment and royalty payment obligations in the Knopp Amendment, the Company issued to Knopp a warrant (the “Warrant”) to purchase 294,195 Company common shares at a purchase price per share of $67.98, subject to certain specified development milestones and the Company achieving a specified market capitalization. Refer to Note 6, "Shareholders' Equity" for further discussion. The Company has not recorded any of the remaining contingent consideration payments to Knopp as a liability in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024. Pyramid Acquisition In January 2024, the Company acquired Pyramid Biosciences, Inc. (“Pyramid”), pursuant to an Agreement and Plan of Merger, dated January 7, 2024 ("the Pyramid Agreement"). In consideration for the Pyramid acquisition, Biohaven made an upfront payment of 255,794 Company common shares, valued at approximately $10,894. The Company accounted for this purchase as an asset acquisition as substantially all of the fair value of the gross assets acquired was concentrated in a single identifiable asset, IPR&D. The IPR&D asset has no alternative future use and relates primarily to BHV-1510. There was no material value assigned to any other assets or liabilities acquired in the acquisition. As such, the upfront payment discussed above was recorded as a charge to R&D expense in the accompanying condensed consolidated statements of operations and comprehensive loss during the three months ended March 31, 2024. As of June 30, 2024, under the Pyramid Agreement, the Company has remaining success-based payments comprised of (i) up to $5,000 based on developmental and regulatory milestones for the lead asset, BHV-1510 (formerly known as PBI-410), (ii) up to an additional $30,000 based on developmental and regulatory milestones for a second asset (formerly known as PBI-200) and (iii) up to $40,000 for commercial sales-based milestones of BHV-1510. Contingent developmental and regulatory milestone payments may be paid in cash or Biohaven common shares at the election of Biohaven and commercial sales-based milestones are to be made in cash. The Company has not recorded any of the remaining contingent consideration payments as a liability in the accompanying condensed consolidated balance sheet as none of the future events which would trigger a milestone payment were considered probable of occurring at June 30, 2024. During the first quarter of 2024, the Company recorded $5,689 of R&D expense in the condensed consolidated statement of operations and comprehensive loss for a developmental milestone which became due under the Pyramid Agreement, to be paid in 98,129 common shares of the Company. See Note 6, "Shareholders' Equity" for discussion of common shares issued to as part of the Pyramid Agreement.
|
| X |
- DefinitionLicense, Acquisitions and Other Agreements
| Name: |
bhvn_LicenseAcquisitionsAndOtherAgreementsTextBlock |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Commitments and Contingencies
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Commitments and Contingencies Lease Agreements The Company leases certain office and laboratory space. Other than the Pittsburgh Centre Avenue Lease described below, there have been no material changes to the lease obligations from those disclosed in Note 11, "Commitments and Contingencies" to the consolidated financial statements included in the 2023 Form 10-K. Pittsburgh Centre Avenue Lease Agreement In March 2024, the Company entered into a lease agreement in Pittsburgh, Pennsylvania for lab space (the "Pittsburgh Centre Avenue Lease"), which will be used to support the research and development of the Company's ion channel platform and replace the Company's current operating lease in Pittsburgh. The lease is expected to commence in mid 2025 after substantial completion of building improvements, and has a term of 122 months, with an option to extend for one additional period of 60 months. The Company expects to record the Pittsburgh Centre Avenue Lease as an operating lease. The Company has annual commitments relating to the Pittsburgh Centre Avenue Lease ranging from $1,859 to $2,373, excluding any additional tenant improvement allowance that would increase the base rent. Research Commitments The Company has agreements with several contract manufacturing organizations ("CMOs") and contract research organizations ("CROs") to provide products and services in connection with the Company’s preclinical studies and clinical trials. As of June 30, 2024, the Company had no remaining maximum research commitments in excess of one year. Indemnification Agreements In the ordinary course of business, the Company may provide indemnification of varying scope and terms to vendors, lessors, business partners and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. The Company’s amended and restated memorandum and articles of association also provide for indemnification of directors and officers in specific circumstances. To date, the Company has not incurred any material costs as a result of such indemnification provisions. The Company does not believe that the outcome of any claims under indemnification arrangements will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its condensed consolidated financial statements as of June 30, 2024 or December 31, 2023. License, Acquisition and Other Agreements The Company has entered into licensing, developmental and acquisition agreements with various parties under which it is obligated to make contingent and non-contingent payments (see Note 10, "License, Acquisitions and Other Agreements"). Other Agreements Moda Agreement On January 1, 2021, the Company entered into a consulting services agreement (the "Moda Agreement") with Moda Pharmaceuticals LLC ("Moda") to further the scientific advancement of technology, drug discovery platforms (including the technology licensed under the Yale MoDE Agreement), product candidates and related intellectual property owned or controlled by the Company. Under the Moda Agreement, the Company paid Moda an upfront cash payment of $2,700 and 37,836 shares of the Former Parent valued at approximately $3,243. In addition, Moda will be eligible to receive additional development and regulatory milestone payments of up to $81,612 and commercial milestone payments of up to $30,171. The Moda Agreement has a term of four years and may be terminated earlier by the Company or Moda under certain circumstances including, for example, the Company's discontinuation of research on the MoDE platform or default. In August 2023, the Company entered into an amendment to the Moda Agreement with Moda. Under the amendment, Moda will be eligible to receive development and regulatory milestone payments up to $25,200 and commercial milestone payments up to $23,000, in addition to the milestones noted above. The Company did not record any material milestone payments related to the Moda Agreement for the three months ended June 30, 2024. For the six months ended June 30, 2024, the Company recorded research and development expense of $850 related to developmental milestones under the Moda Agreement. For the three and six months ended June 30, 2023, the Company did not record any material milestone payments related to the Moda Agreement. Legal Proceedings From time to time, in the ordinary course of business, the Company is subject to litigation and regulatory examinations as well as information gathering requests, inquiries and investigations. As of June 30, 2024, there were no matters which would have a material impact on the Company’s financial
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Income Taxes
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Income Taxes The following table provides a comparative summary of the Company's income tax provision and effective income tax rate for the three and six months ended June 30, 2024 and 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | Six Months Ended June 30, | | | 2024 | | 2023 | 2024 | | 2023 | Income tax provision | | $ | 177 | | | $ | 2,177 | | $ | 746 | | | $ | 3,116 | | | Effective income tax rate | | 0.1 | % | | 2.5 | % | 0.1 | % | | 2.0 | % |
The decrease in income tax provisions for the three and six months ended June 30, 2024 as compared to the same periods in 2023 was primarily attributable to the Company adopting the guidance contained in a Notice of Proposed Rule Making issued during the third quarter of 2023 by the United States Internal Revenue Service ("the Notice"). The Notice allows the Company to immediately deduct certain R&D expenditures which were incurred in the US and reimbursed by the Company’s foreign parent. Previously, these expenditures were required to be capitalized under the Tax Cuts and Jobs Act, which was effective for tax years beginning on or after January 1, 2022.
|
| X |
- DefinitionThe entire disclosure for income tax. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 231
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482663/740-10-55-231
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477891/740-270-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/740/tableOfContent
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-14
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-21
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Related Party Transactions
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Related Party Transactions [Abstract] |
|
| Related Party Transactions |
Related Party Transactions Relationship with the Former Parent Upon the effectiveness of the Separation on October 3, 2022, the Former Parent ceased to be a related party of the Company. On October 3, 2022, the Company entered into agreements with the Former Parent in connection with the Separation, including a Transition Services Agreement. For a full discussion of agreements entered into with the Former Parent, refer to Note 14, "Related Party Transactions" to the consolidated financial statements included in the 2023 Form 10-K. The Company did not record any material income for transition services provided to the Former Parent during the three and six months ended June 30, 2024. For the three and six months ended June 30, 2023, the Company recorded $1,674 and $5,559, respectively, in other income reflecting transition services provided to the Former Parent. Related Party Agreements License Agreement with Yale University On September 30, 2013, the Company entered into the Yale Agreement with Yale University (see Note 10). The Company’s Chief Executive Officer is one of the inventors of the patents that the Company has licensed from Yale University and, as such, is entitled to a specified share of the glutamate product-related royalty revenues that may be received by Yale University under the Yale Agreement. In January 2021, the Company entered into the Yale MoDE Agreement with Yale University (see Note 10 for details). Under the license agreement, the Company acquired exclusive, worldwide rights to Yale University's intellectual property directed to its MoDE platform. Under the Yale MoDE Agreement, the Company entered into the Yale MoDE SRA (see Note 10 for details), which included funding of up to $4,000 over the life of the agreement. In May 2023, the Company entered into an additional sponsored research agreement with Yale University (the "2023 Yale SRA"), which includes funding of up to $612 over the life of the agreement. For the three and six months ended June 30, 2024, the Company recorded $582 and $1,027, respectively, in R&D expense, including certain administrative expenses, related to the Yale MoDE Agreement, the Yale Agreement, and the 2023 Yale SRA (collectively, the "Yale Agreements"). For the three and six months ended June 30, 2023, the Company recorded $947 and $1,699, respectively, in research and development expense, including certain administrative expenses, related to the Yale Agreements. As of June 30, 2024, the Company did not owe any amounts to Yale University. |
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Pay vs Performance Disclosure - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Pay vs Performance Disclosure |
|
|
|
|
| Net loss |
$ (319,771)
|
$ (90,346)
|
$ (499,275)
|
$ (160,838)
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.2.u1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Summary of Significant Accounting Policies (Policies)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Unaudited Interim Condensed Consolidated Financial Information |
Unaudited Interim Condensed Consolidated Financial Information The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with GAAP for interim financial information. The accompanying unaudited condensed consolidated financial statements do not include all of the information and footnotes required by GAAP for complete consolidated financial statements. The accompanying year-end condensed consolidated balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America. The unaudited interim condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of June 30, 2024, the results of its operations for the three and six months ended June 30, 2024 and 2023, and its cash flows for the six months ended June 30, 2024 and 2023. The results for the three and six months ended June 30, 2024 are not necessarily indicative of results to be expected for the year ending December 31, 2024, any other interim periods or any future year or period. The financial information included herein should be read in conjunction with the financial statements and notes in the Company's Annual Report on Form 10-K for the year ended December 31, 2023
|
| Reclassifications |
Reclassifications Certain items in the prior period’s condensed consolidated financial statements have been reclassified to conform to the current year presentation.
|
| Use of Estimates |
Use of Estimates The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of expenses during the reporting periods. Significant estimates and assumptions reflected in these condensed consolidated financial statements include, but are not limited to, the accrual for research and development expenses and valuation of forward contract and derivative liabilities. In addition, management’s assessment of the Company’s ability to continue as a going concern involves the estimation of the amount and timing of future cash inflows and outflows. Estimates are periodically reviewed in light of changes in circumstances, facts and experience. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates.
|
| Restricted Cash |
Restricted Cash Restricted cash included in other current assets in the condensed consolidated balance sheets consists primarily of employee contributions to the Company's employee share purchase plan held for future purchases of the Company's outstanding shares. Restricted cash included in other non-current assets in the condensed consolidated balance sheets represents collateral held by banks for a letter of credit ("LOC") issued in connection with the leased office space in Yardley, Pennsylvania and LOCs issued in connection with the leased office and lab spaces in Cambridge, Massachusetts and Pittsburgh, Pennsylvania. See Note 11, ‘‘Commitments and Contingencies’’ for additional information on the real estate leases.
|
| Forward Contracts and Derivative Liabilities |
Forward Contracts and Derivative Liabilities The Company evaluates certain of its financial and business development transactions to determine the accounting classification of equity linked instruments issued in those transactions. The Company first assesses whether a freestanding equity linked instrument meets liability classification in accordance with ASC 480-10 Distinguishing Liabilities from Equity and ASC 815-40 Derivatives and Hedging - Contracts in Entity's Own Equity. Under ASC 480-10 an instrument is considered liability classified if the instrument is mandatorily redeemable, obligate the issuer to settle an instrument or the underlying shares by paying cash or other assets, or must or may require settlement by issuing variable number of shares. If an instrument does not meet liability classification under ASC 480-10, the Company assesses the requirements under ASC 815-40, which states that contracts that require or may require net cash settlement are liabilities recorded at fair value. If the instrument does not require liability classification under ASC 815-40, in order to conclude equity classification, the Company assesses whether the instrument is indexed to its common stock and whether the instrument is classified as equity under ASC 815-40 or other applicable GAAP. Equity linked instruments that are accounted for in accordance with ASC 815-40 are reported as liabilities on the condensed consolidated balance sheets at fair value. Any change in fair value, as determined at each measurement period, is recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. Changes in fair value are reported on the condensed consolidated statements of cash flows as change in fair value of forward contract and derivative liabilities. The Company accounted for certain consideration agreed to in connection with the Amendment, dated as of May 1, 2024 (the "Knopp Amendment"), to the Membership Interest Purchase Agreement, dated as of February 24, 2022 (the "Purchase Agreement") entered into with Knopp Biosciences, LLC ("Knopp") as derivative liabilities under ASC 815 (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements")
|
| Warrants |
Warrants The Company evaluates warrants issued as either equity instruments, liabilities or derivative liabilities in accordance with ASC Topic 480, Distinguishing Liabilities from Equity ("ASC 480") and/or ASC Topic 815, Derivatives and Hedging ("ASC 815"), depending on the specific terms of the warrant agreement. The Company assessed the warrants issued to Knopp in May 2024 (the "Knopp Warrants") and concluded that they met the criteria for equity classification under ASC 480 and ASC 815. Accordingly, the Knopp Warrants were recorded within additional paid-in capital on the condensed consolidated balance sheet at the time of issuance and are not subject to remeasurement. See Note 6, Shareholders' Equity for further detail on the Knopp Warrants.
|
| Fair Value Measurements |
Fair Value Measurements Certain assets and liabilities of the Company are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. Financial assets and liabilities carried at fair value are to be classified and disclosed in one of the following three levels of the fair value hierarchy, of which the first two are considered observable and the last is considered unobservable: •Level 1—Quoted prices in active markets for identical assets or liabilities. •Level 2—Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. •Level 3—Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. The carrying values of other current assets, accounts payable, and accrued expenses approximate their fair values due to the short-term nature of these assets and liabilities. The Company has accounted for certain consideration agreed to in connection with the Knopp Amendment as forward contract liabilities, which are recorded on the condensed consolidated balance sheets at fair value. Any changes in fair value, as determined each measurement period, are recorded as a component of other income (expense), net on the condensed consolidated statements of operations and comprehensive loss. The fair value of the forward contract liabilities is determined based on significant inputs not observable in the market, and therefore represents a Level 3 measurement within the fair value hierarchy. The valuations are based on a Monte Carlo simulation of the Company's share price, which requires judgement and assumption on the volatility of Biohaven's share price, discounted to present value using a risk-free rate plus Biohaven specific credit risk since payable in a variable number of shares. (see Notes 4, "Fair Value of Financial Assets and Liabilities" and 10, "License, Acquisitions and Other Agreements").
|
| Recently Issued Accounting Pronouncements |
Recently Issued Accounting Pronouncements In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting—Improvements to Reportable Segment Disclosures, which improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses. The amendments in ASU No. 2023-07 apply to public entities, including those with a single reportable segment, and are effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact ASU No. 2023-07 will have on its consolidated financial statements In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, to improve the transparency of income tax disclosures by requiring consistent categories and greater disaggregation of information in the rate reconciliation and income taxes paid disaggregated by jurisdiction. The ASU also includes certain other amendments to improve the effectiveness of income tax disclosures. The amendments in ASU 2023-09 are effective for fiscal years beginning after December 15, 2024, with early adoption permitted for annual financial statements that have not yet been issued or made available for issuance. The Company is currently evaluating the impact ASU No. 2023-09 will have on its consolidated financial statements.
|
| X |
- DefinitionForward Contract and Derivative Liability, Policy
| Name: |
bhvn_ForwardContractAndDerivativeLiabilityPolicyPolicyTextBlock |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
bhvn_WarrantsPolicyPolicyTextBlock |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEntity's cash and cash equivalents accounting policy with respect to restricted balances. Restrictions may include legally restricted deposits held as compensating balances against short-term borrowing arrangements, contracts entered into with others, or company statements of intention with regard to particular deposits; however, time deposits and short-term certificates of deposit are not generally included in legally restricted deposits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsRestrictedCashAndCashEquivalentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for reclassification affecting comparability of financial statement. Excludes amendment to accounting standards, other change in accounting principle, and correction of error. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 205
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483504/205-10-50-1
| Name: |
us-gaap_PriorPeriodReclassificationAdjustmentDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Summary of Significant Accounting Policies (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Schedule of reconciliation of total cash, cash equivalents and restricted cash |
The following represents a reconciliation of cash and cash equivalents in the condensed consolidated balance sheets to total cash, cash equivalents and restricted cash as of June 30, 2024 and June 30, 2023, respectively, in the condensed consolidated statements of cash flows: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of June 30, 2023 | | Cash and cash equivalents | | $ | 239,147 | | | $ | 147,612 | | | Restricted cash held on behalf of Former Parent | | — | | | 40,415 | | | Restricted cash (included in other current assets) | | 156 | | | 11,574 | | | Restricted cash (included in other non-current assets) | | 3,015 | | | 2,349 | | | Total cash, cash equivalents and restricted cash at the end of the period in the condensed consolidated statement of cash flows | | $ | 242,318 | | | $ | 201,950 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of cash and cash equivalents.
| Name: |
us-gaap_ScheduleOfCashAndCashEquivalentsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Marketable Securities (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Investments, Debt and Equity Securities [Abstract] |
|
| Schedule of reconciliation of available-for-sale debt securities from amortized cost to fair value |
The amortized cost, gross unrealized holding gains, gross unrealized holding losses and fair value of debt securities available-for-sale by type of security at June 30, 2024 and December 31, 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Amortized Cost | | Allowance for Credit Losses | | Net Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value | | June 30, 2024 | | | | | | | | | | | | | Debt securities | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | U.S. treasury bills | | $ | 234,748 | | | $ | — | | | $ | 234,748 | | | $ | 2 | | | $ | (5) | | | $ | 234,745 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | | | | | | Debt securities | | | | | | | | | | | | | U.S. corporate bonds | | $ | 46,228 | | | $ | — | | | $ | 46,228 | | | $ | 7 | | | $ | (24) | | | $ | 46,211 | | Foreign corporate bonds | | 7,180 | | | — | | | 7,180 | | | — | | | (7) | | | 7,173 | | | | | | | | | | | | | | | U.S. treasury bills | | 113,908 | | | — | | | 113,908 | | | 27 | | | — | | | 113,935 | | | | | | | | | | | | | | | | Total | | $ | 167,316 | | | $ | — | | | $ | 167,316 | | | $ | 34 | | | $ | (31) | | | $ | 167,319 | |
The fair value of debt securities available-for-sale by classification in the condensed consolidated balance sheets was as follows: | | | | | | | | | | | | | | | | | | | June 30, 2024 | | December 31, 2023 | | | | Cash and cash equivalents | | $ | 36,944 | | | $ | 33,902 | | | | | Marketable securities | | 197,801 | | | 133,417 | | | | | Total | | $ | 234,745 | | | $ | 167,319 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Schedule of available-for-sale debt securities by contractual maturity |
The net amortized cost and fair value of debt securities available-for-sale at June 30, 2024 and December 31, 2023 are shown below by contractual maturity. Actual maturities may differ from contractual maturities because securities may be restructured, called or prepaid, or the Company intends to sell a security prior to maturity. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | June 30, 2024 | | December 31, 2023 | | | Net Amortized Cost | | Fair Value | | Net Amortized Cost | | Fair Value | | Due to mature: | | | | | | | | | | Less than one year | | $ | 234,748 | | | $ | 234,745 | | | $ | 167,316 | | | $ | 167,319 | | | | | | | | | | | | | | | | | | | |
|
| Summary of debt securities available-for-sale in an unrealized loss position, aggregated by the length of time |
Summarized below are the debt securities available-for-sale the Company held at June 30, 2024 and December 31, 2023 that were in an unrealized loss position, aggregated by the length of time the investments have been in that position: | | | | | | | | | | | | | | | | | | | | | | | Less than 12 months | | | Number of Securities | | Fair Value | | Unrealized Losses | | June 30, 2024 | | | | | | | | Debt securities | | | | | | | | | | | | | | | | | | | | | | U.S. treasury bills | | 11 | | | $ | 133,504 | | | $ | (5) | | | | | | | | | | | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | Debt securities | | | | | | | | U.S. corporate bonds | | 6 | | | $ | 29,537 | | | $ | (24) | | | Foreign corporate bonds | | 1 | | | 7,173 | | | (7) | | | | | | | | | | | | | | | | | | | | | | | | Total | | 7 | | | $ | 36,710 | | | $ | (31) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Schedule of net investment income |
Net investment income included in other income, net in the condensed consolidated statements of operations and comprehensive loss for the three and six months ended June 30, 2024 and 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | Debt securities (including realized losses) | | $ | 3,000 | | | $ | 2,225 | | | $ | 5,069 | | | $ | 5,634 | | Other investments | | 2,140 | | | 1,941 | | | 4,402 | | | 2,727 | | Gross investment income (including realized losses) | | 5,140 | | | 4,166 | | | 9,471 | | | 8,361 | | Investment expenses | | (40) | | | (68) | | | (70) | | | (138) | | | Net investment income | | $ | 5,100 | | | $ | 4,098 | | | $ | 9,401 | | | $ | 8,223 | |
|
| Schedule of proceeds from sale of available-for-sale debt securities and related gross realized capital gains and losses |
The proceeds from the sale of available-for-sale debt securities and the related gross realized capital losses for the three and six months ended June 30, 2024 and 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | | Proceeds from sales | | $ | — | | | $ | 2,464 | | | $ | — | | | $ | 4,920 | | | | | | | | | | | | Gross realized capital losses | | $ | — | | | $ | 17 | | | $ | — | | | $ | 39 | |
|
| X |
- DefinitionTabular disclosure of fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of investment income, including, but not limited to, interest and dividend income and amortization of discount (premium) derived from debt and equity securities. Excludes realized and unrealized gain (loss) on investments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_InvestmentIncomeTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of maturities of an entity's investments as well as any other information pertinent to the investments.
| Name: |
us-gaap_InvestmentsClassifiedByContractualMaturityDateTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation of available-for-sale securities from cost basis to fair value.
| Name: |
us-gaap_ScheduleOfAvailableForSaleSecuritiesReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the proceeds from sales of available-for-sale securities and the gross realized gains and gross realized losses that have been included in earnings as a result of those sales. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-9
| Name: |
us-gaap_ScheduleOfRealizedGainLossTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Fair Value of Financial Assets and Liabilities (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of financial assets and liabilities measured at fair value on a recurring basis |
Financial assets measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 and December 31, 2023 and financial liabilities measured at fair value on a recurring basis on the condensed consolidated balance sheets at June 30, 2024 were as follows : | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement Using: | | Balance Sheet Classification | | Type of Instrument | | Level 1 | | Level 2 | | Level 3 | | Total | | June 30, 2024 | | | | | | | | | | | | Assets: | | | | | | | | | | | | Cash and cash equivalents | | Money market funds | | $ | 113,379 | | | $ | — | | | $ | — | | | $ | 113,379 | | Cash and cash equivalents | | U.S. treasury bills | | — | | | 36,944 | | | — | | | 36,944 | | | | | | | | | | | | | | Marketable securities | | U.S. treasury bills | | 29,701 | | | 168,100 | | | — | | | 197,801 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Other non-current assets | | Money market funds | | 2,515 | | | — | | | — | | | 2,515 | | | Total assets | | | | $ | 145,595 | | | $ | 205,044 | | | $ | — | | | $ | 350,639 | | | | | | | | | | | | | | Liabilities: | | | | | | | | | | | Forward contract and derivative liabilities | | Forward contract, current and written put option, current | | $ | — | | | $ | — | | | $ | 81,220 | | | $ | 81,220 | | | | | | | | | | | | | Derivative liability, non-current | | Written put option, non-current | | — | | | — | | | 12,180 | | | 12,180 | | | | | | | | | | | | | | Total liabilities | | | | $ | — | | | $ | — | | | $ | 93,400 | | | $ | 93,400 | | | | | | | | | | | | | | December 31, 2023 | | | | | | | | | | | | Assets: | | | | | | | | | | | | Cash and cash equivalents | | Money market funds | | $ | 59,199 | | | $ | — | | | $ | — | | | $ | 59,199 | | | Cash and cash equivalents | | U.S. treasury bills | | — | | | 27,901 | | | — | | | 27,901 | | | Cash and cash equivalents | | U.S. corporate bonds | | — | | | 6,001 | | | — | | | 6,001 | | | Marketable securities | | U.S. treasury bills | | 9,874 | | | 76,160 | | | — | | | 86,034 | | | Marketable securities | | U.S. corporate bonds | | — | | | 40,210 | | | — | | | 40,210 | | | Marketable securities | | Foreign corporate bonds | | — | | | 7,173 | | | — | | | 7,173 | | | Other non-current assets | | Money market funds | | 1,900 | | | — | | | — | | | 1,900 | | | Total assets | | | | $ | 70,973 | | | $ | 157,445 | | | $ | — | | | $ | 228,418 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| Schedule of aggregate fair value of liability determined by Level 3 inputs |
The following table provides a roll forward of the fair value of the Company's forward contract and derivative liabilities related to the Knopp Amendment for which fair value is determined by Level 3 inputs for the three months ended June 30, 2024: | | | | | | | | | | | | | Carrying Value | | | Fair value at May 1, 2024 | | $ | 93,290 | | | | Change in fair value of derivative in other income (expense), net | | 110 | | | | Fair value at June 30, 2024 | | $ | 93,400 | | | |
|
| Schedule of fair value measurement inputs and valuation techniques |
A summary of the unobservable inputs (Level 3 inputs) used in measuring the Company’s forward contract and derivative liabilities related to the Knopp Amendment as of June 30, 2024 and May 1, 2024 are as follows, presented on a weighted-average basis: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of May 1, 2024 | | | | | | Time to payment and potential payment (years) | | 0.91 | | 1.08 | Volatility (annual) | | 70.0 | % | | 80.0 | % | Discount rate | | 15.6 | % | | 14.8 | % |
|
| X |
- DefinitionTabular disclosure of financial instrument measured at fair value on recurring or nonrecurring basis. Includes, but is not limited to, instrument classified in shareholders' equity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of input and valuation technique used to measure fair value and change in valuation approach and technique for each separate class of asset and liability measured on recurring and nonrecurring basis. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 103
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-103
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the fair value measurement of liabilities using significant unobservable inputs (Level 3), a reconciliation of the beginning and ending balances, separately presenting changes attributable to the following: (1) total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings (or changes in net assets), and gains or losses recognized in other comprehensive income (loss) and a description of where those gains or losses included in earnings (or changes in net assets) are reported in the statement of income (or activities); (2) purchases, sales, issues, and settlements (each type disclosed separately); and (3) transfers in and transfers out of Level 3 (for example, transfers due to changes in the observability of significant inputs) by class of liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisUnobservableInputReconciliationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Balance Sheet Components (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Schedule of property and equipment, net |
Property and equipment, net consisted of the following: | | | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | Building and land | | $ | 14,078 | | | $ | 11,728 | | | | Leasehold improvements | | 824 | | | 802 | | | | | Computer hardware and software | | 875 | | | 875 | | | | | Office and lab equipment | | 11,027 | | | 9,961 | | | | | Furniture and fixtures | | 1,793 | | | 1,550 | | | | | | $ | 28,597 | | | $ | 24,916 | | | | | Accumulated depreciation | | (10,201) | | | (8,283) | | | | | | 18,396 | | | 16,633 | | | | | Equipment not yet in service | | 269 | | | 558 | | | | | Property and equipment, net | | $ | 18,665 | | | $ | 17,191 | | | |
|
| Schedule of other noncurrent assets |
Other non-current assets consisted of the following: | | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | | | | | | | | | | | | | | | | | | Operating lease right-of-use assets | | $ | 29,903 | | | $ | 31,385 | | | | Other | | 3,015 | | | 2,400 | | | | Other non-current assets | | $ | 32,918 | | | $ | 33,785 | | |
|
| Schedule of accrued expenses and other current liabilities |
Accrued expenses and other current liabilities consisted of the following: | | | | | | | | | | | | | | | | | As of June 30, 2024 | | As of December 31, 2023 | | | | | | | Accrued employee compensation and benefits | | $ | 10,845 | | | $ | 837 | | | Accrued clinical trial costs | | 36,446 | | | 29,501 | | | | | | | | | | | | | | | | | | | | | | | | | | | Operating lease liabilities - current portion | | 3,550 | | | 3,308 | | | Other accrued expenses and other current liabilities | | 6,887 | | | 6,200 | | | Accrued expenses and other current liabilities | | $ | 57,728 | | | $ | 39,846 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of noncurrent assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfOtherAssetsNoncurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Shareholders' Equity (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Stockholders' Equity Note [Abstract] |
|
| Schedule of changes in shareholders' equity (deficit) |
Changes in shareholders’ equity for the three and six months ended June 30, 2024 and June 30, 2023 were as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Common Shares | | | | | | | | | | | | | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Loss | | Total Shareholders' Equity | | Balances as of December 31, 2023 | | 81,115,723 | | | $ | 887,528 | | | | | $ | 39,804 | | | $ | (499,292) | | | $ | (65) | | | $ | 427,975 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Net loss | | — | | | — | | | | | — | | | (179,504) | | | — | | | (179,504) | | Issuance of common shares as payment for acquisition of IPR&D asset | | 242,958 | | | 10,347 | | | | | — | | | — | | | — | | | 10,347 | | Issuance of common shares as payment under license and other agreements | | 97,233 | | | 5,637 | | | | | — | | | — | | | — | | | 5,637 | | | Issuance of common shares under 2022 Equity Incentive Plan | | 351,307 | | | 7,452 | | | | | (5,296) | | | — | | | — | | | 2,156 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 34,877 | | | — | | | — | | | 34,877 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (41) | | | (41) | | | Balances as of March 31, 2024 | | 81,807,221 | | | 910,964 | | | | | 69,385 | | | (678,796) | | | (106) | | | 301,447 | | | Net loss | | — | | | — | | | | | — | | | (319,771) | | | — | | | (319,771) | | | Issuance of common shares, net of offering costs | | 8,544,951 | | | 317,720 | | | | | — | | | — | | | — | | | 317,720 | | | Issuance of common shares as payment for acquisition of IPR&D asset | | 10,452 | | | 446 | | | | | — | | | — | | | — | | | 446 | | | Issuance of common shares as payment under license and other agreements | | 1,872,874 | | | 65,981 | | | | | — | | | — | | | — | | | 65,981 | | | Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 110,834 | | | 3,442 | | | | | (1,125) | | | — | | | — | | | 2,317 | | | Issuance of warrant as payment under license agreement | | — | | | — | | | | | 3,340 | | | — | | | — | | | 3,340 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 12,232 | | | — | | | — | | | 12,232 | | | Other comprehensive income | | — | | | — | | | | | — | | | — | | | 28 | | | 28 | | | Balances as of June 30, 2024 | | 92,346,332 | | | $ | 1,298,553 | | | | | $ | 83,832 | | | $ | (998,567) | | | $ | (78) | | | $ | 383,740 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Common Shares | | | | | | | | | | | | | Shares | | Amount | | | | Additional Paid-in Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income | | Total Shareholders' Equity | Balances as of December 31, 2022 | | 68,190,479 | | | $ | 615,742 | | | | | $ | 13,869 | | | $ | (91,124) | | | $ | 284 | | | $ | 538,771 | | | Net loss | | — | | | — | | | | | — | | | (70,492) | | | — | | | (70,492) | | | Issuance of common shares under 2022 Equity Incentive Plan | | 22,000 | | | 504 | | | | | (172) | | | — | | | — | | | 332 | | Non-cash share-based compensation expense | | — | | | — | | | | | 3,765 | | | — | | | — | | | 3,765 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (118) | | | (118) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Balances as of March 31, 2023 | | 68,212,479 | | | 616,246 | | | | | 17,462 | | | (161,616) | | | 166 | | | 472,258 | | | Net loss | | — | | | — | | | | | — | | | (90,346) | | | — | | | (90,346) | | | Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan | | 104,474 | | | 1,264 | | | | | (470) | | | — | | | — | | | 794 | | | Non-cash share-based compensation expense | | — | | | — | | | | | 4,695 | | | — | | | — | | | 4,695 | | | Other comprehensive loss | | — | | | — | | | | | — | | | — | | | (146) | | | (146) | | Balance as of June 30, 2023 | | 68,316,953 | | | $ | 617,510 | | | | | $ | 21,687 | | | $ | (251,962) | | | $ | 20 | | | $ | 387,255 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| X |
- DefinitionTabular disclosure of changes in the separate accounts comprising stockholders' equity (in addition to retained earnings) and of the changes in the number of shares of equity securities during at least the most recent annual fiscal period and any subsequent interim period presented is required to make the financial statements sufficiently informative if both financial position and results of operations are presented. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
| Name: |
us-gaap_ScheduleOfStockholdersEquityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Accumulated Other Comprehensive (Loss) Income (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Schedule of activity in accumulated other comprehensive income (loss) |
Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2024: | | | | | | | | | | | | | | | | | Three Months Ended June 30, 2024 | | Six Months Ended June 30, 2024 | | | | | | | Net unrealized investment gains (losses): | | | | | | Beginning of period balance | | $ | (32) | | | $ | 3 | | | | | | | | | | | | Other comprehensive income (loss)(1)(2) | | 29 | | | (6) | | | End of period balance | | (3) | | | (3) | | | | | | | | Foreign currency translation adjustments: | | | | | | Beginning of period balance | | (74) | | | (68) | | Other comprehensive loss(1) | | (1) | | | (7) | | | | | | | | End of period balance | | (75) | | | (75) | | | | | | | Total beginning of period accumulated other comprehensive loss | | (106) | | | (65) | | Total other comprehensive income (loss) | | 28 | | | (13) | | Total end of period accumulated other comprehensive loss | | $ | (78) | | | $ | (78) | | | | | | |
(1) There was no tax on other comprehensive loss and no amounts reclassified from accumulated other comprehensive (loss) income during the period. Shareholders’ equity included the following activity in accumulated other comprehensive (loss) income for the three and six months ended June 30, 2023: | | | | | | | | | | | | | | | | | Three Months Ended June 30, 2023 | | Six Months Ended June 30, 2023 | | | | | | | Net unrealized investment gains (losses): | | | | | | Beginning of period balance | | $ | (278) | | | $ | (145) | | Other comprehensive loss before reclassifications(1) | | (1) | | | (156) | | Amounts reclassified from accumulated other comprehensive loss(1)(2) | | 17 | | | 39 | | Other comprehensive income (loss)(1) | | 16 | | | (117) | | | End of period balance | | (262) | | | (262) | | | | | | | | Foreign currency translation adjustments: | | | | | | Beginning of period balance | | 444 | | | 429 | | Other comprehensive loss(1) | | (162) | | | (147) | | | | | | | | End of period balance | | 282 | | | 282 | | | | | | | Total beginning of period accumulated other comprehensive income | | 166 | | | 284 | | Total other comprehensive loss | | (146) | | | (264) | | Total end of period accumulated other comprehensive income | | $ | 20 | | | $ | 20 | | | | | | |
(1) There was no tax on other comprehensive income (loss) and immaterial tax on amounts reclassified from accumulated other comprehensive income (loss) during the period. (2) Amounts reclassified from accumulated other comprehensive income (loss) for specifically identified debt securities are included in other income (expense), net on the condensed consolidated statement of operations and comprehensive loss.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accumulated other comprehensive income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
| Name: |
us-gaap_ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Non-Cash Share-Based Compensation (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Schedule of non-cash share-based compensation expense |
Non-cash share-based compensation expense, consisting of expense for share options, restricted share units ("RSUs"), performance share options, and the Employee Share Purchase Plan ("ESPP"), was classified in the condensed consolidated statements of operations and comprehensive loss as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | Research and development expenses | | $ | 7,071 | | | $ | 2,457 | | | $ | 28,362 | | | $ | 4,698 | | General and administrative expenses | | 5,161 | | | 2,238 | | | 18,747 | | | 3,762 | | | Total non-cash share-based compensation expense | | $ | 12,232 | | | $ | 4,695 | | | $ | 47,109 | | | $ | 8,460 | |
|
| Schedule of stock option activity |
The following table is a summary of the Company's share option activity for the six months ended June 30, 2024: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Number of Shares | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term | | Aggregate Intrinsic Value | | | | | | | | (in years) | | | Outstanding as of December 31, 2023 | | 11,379,429 | | $ | 11.48 | | | | | | | Granted | | 2,500,161 | | $ | 42.05 | | | | | | | Exercised | | (295,890) | | $ | 9.00 | | | | | | | Forfeited | | (38,684) | | $ | 20.38 | | | | | | Outstanding as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | | Options exercisable as of June 30, 2024 | | 5,566,338 | | $ | 13.44 | | | 8.54 | | $ | 122,803 | | Vested and expected to vest as of June 30, 2024 | | 13,545,016 | | $ | 17.15 | | | 8.68 | | $ | 256,022 | |
|
| Schedule of RSU activity |
The following table is a summary of the RSU activity for the six months ended June 30, 2024: | | | | | | | | | | | | | | | | | Number of shares | | Weighted Average Grant Date Fair Value | Unvested as of December 31, 2023 | | — | | | $ | — | | Granted | | 356,011 | | | $ | 42.34 | | Forfeited | | (3,280) | | | $ | 41.93 | | Vested | | (89,059) | | | $ | 42.34 | | Unvested as of June 30, 2024 | | 263,672 | | | $ | 42.34 | |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the number and weighted-average grant date fair value for restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock units that were granted, vested, or forfeited during the year. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Net Loss Per Share (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Schedule of basic and diluted net loss per share |
Basic and diluted net loss per share attributable to common shareholders of Biohaven was calculated as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | | Six Months Ended June 30, | | | 2024 | | 2023 | | 2024 | | 2023 | | Numerator: | | | | | | | | | | | | | | | | | | | Net loss | | $ | (319,771) | | | $ | (90,346) | | | $ | (499,275) | | | $ | (160,838) | | | Denominator: | | | | | | | | | Weighted average common shares outstanding—basic and diluted | | 87,766,069 | | | 68,248,023 | | | 84,174,099 | | | 68,227,564 | | | Net loss per share — basic and diluted | | $ | (3.64) | | | $ | (1.32) | | | $ | (5.93) | | | $ | (2.36) | |
|
| Schedule of potentially anti-dilutive securities excluded from calculation of diluted net loss per share |
The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect: | | | | | | | | | | | | | | | | | | As of June 30, | | | | 2024 | | 2023 | | Options to purchase common shares | | 13,545,016 | | | 9,639,557 | | | Warrants to purchase common shares | | 294,195 | | | — | | Restricted share units | | 263,672 | | | — | | | Total | | 14,102,883 | | | 9,639,557 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
| X |
- DefinitionSchedule of Milestone Payments
| Name: |
bhvn_ScheduleOfMilestonePaymentsTableTextBlock |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Income Taxes (Tables)
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of income tax provision and effective tax rate |
The following table provides a comparative summary of the Company's income tax provision and effective income tax rate for the three and six months ended June 30, 2024 and 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months Ended June 30, | Six Months Ended June 30, | | | 2024 | | 2023 | 2024 | | 2023 | Income tax provision | | $ | 177 | | | $ | 2,177 | | $ | 746 | | | $ | 3,116 | | | Effective income tax rate | | 0.1 | % | | 2.5 | % | 0.1 | % | | 2.0 | % |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Summary of Significant Accounting Policies - Cash, Cash Equivalents and Restricted Cash (Details) - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
Jun. 30, 2023 |
Dec. 31, 2022 |
| Accounting Policies [Abstract] |
|
|
|
|
| Cash and cash equivalents |
$ 239,147
|
$ 248,402
|
$ 147,612
|
|
| Restricted cash held on behalf of Former Parent |
0
|
|
40,415
|
|
| Restricted cash (included in other current assets) |
156
|
|
11,574
|
|
| Restricted cash (included in other non-current assets) |
3,015
|
|
2,349
|
|
| Total cash, cash equivalents and restricted cash at the end of the period in the condensed consolidated statement of cash flows |
$ 242,318
|
$ 252,120
|
$ 201,950
|
$ 242,604
|
| X |
- DefinitionRestricted Cash Included in Other Current Assets
| Name: |
bhvn_RestrictedCashIncludedInOtherCurrentAssets |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as current. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477220/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.2.u1
Marketable Securities - Amortized Cost and Fair Value of Debt Securities (Details) - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Fair Value to Amortized Cost |
|
|
| Amortized Cost |
|
$ 167,316
|
| Allowance for Credit Losses |
|
0
|
| Net Amortized Cost |
|
167,316
|
| Gross Unrealized Gains |
|
34
|
| Gross Unrealized Losses |
|
(31)
|
| Fair Value |
$ 234,745
|
167,319
|
| Cash and cash equivalents |
|
|
| Fair Value to Amortized Cost |
|
|
| Fair Value |
36,944
|
33,902
|
| Marketable securities |
|
|
| Fair Value to Amortized Cost |
|
|
| Fair Value |
197,801
|
133,417
|
| U.S. corporate bonds |
|
|
| Fair Value to Amortized Cost |
|
|
| Amortized Cost |
|
46,228
|
| Allowance for Credit Losses |
|
0
|
| Net Amortized Cost |
|
46,228
|
| Gross Unrealized Gains |
|
7
|
| Gross Unrealized Losses |
|
(24)
|
| Fair Value |
|
46,211
|
| Foreign corporate bonds |
|
|
| Fair Value to Amortized Cost |
|
|
| Amortized Cost |
|
7,180
|
| Allowance for Credit Losses |
|
0
|
| Net Amortized Cost |
|
7,180
|
| Gross Unrealized Gains |
|
0
|
| Gross Unrealized Losses |
|
(7)
|
| Fair Value |
|
7,173
|
| U.S. treasury bills |
|
|
| Fair Value to Amortized Cost |
|
|
| Amortized Cost |
234,748
|
113,908
|
| Allowance for Credit Losses |
0
|
0
|
| Net Amortized Cost |
234,748
|
113,908
|
| Gross Unrealized Gains |
2
|
27
|
| Gross Unrealized Losses |
(5)
|
0
|
| Fair Value |
$ 234,745
|
$ 113,935
|
| X |
- DefinitionAmount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 103
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-103
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481830/320-10-45-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AvailableForSaleSecuritiesFairValueToAmortizedCostBasisAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of allowance for credit loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479130/326-30-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost, after allowance for credit loss, of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAmortizedCostAfterAllowanceForCreditLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_CashAndCashEquivalentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=bhvn_MarketableSecuritiesCurrentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_DomesticCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_ForeignCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- References
+ Details
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtMaturitiesAmortizedCostBasisRollingMaturityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtMaturitiesFairValueRollingMaturityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing in next rolling fiscal year following latest fiscal year. For interim and annual periods when interim period is reported on rolling approach, from latest statement of financial position date. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-3
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtMaturitiesNextRollingTwelveMonthsAmortizedCostBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing in next rolling fiscal year following latest fiscal year. For interim and annual periods when interim period is reported on rolling approach, from latest statement of financial position date. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-3
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtMaturitiesNextRollingTwelveMonthsFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.2.u1
Marketable Securities - Debt Securities Available-for-Sale in Unrealized Loss Position (Details)
$ in Thousands |
Jun. 30, 2024
USD ($)
investment
|
Dec. 31, 2023
USD ($)
investment
|
| Debt Securities, Available-for-sale, Unrealized Loss Position [Line Items] |
|
|
| Number of Securities, unrealized loss position less than 12 months | investment |
|
7
|
| Fair Value, unrealized loss position less than 12 months |
|
$ 36,710
|
| Unrealized Losses, unrealized loss position less than 12 months |
|
$ (31)
|
| U.S. treasury bills |
|
|
| Debt Securities, Available-for-sale, Unrealized Loss Position [Line Items] |
|
|
| Number of Securities, unrealized loss position less than 12 months | investment |
11
|
|
| Fair Value, unrealized loss position less than 12 months |
$ 133,504
|
|
| Unrealized Losses, unrealized loss position less than 12 months |
$ (5)
|
|
| U.S. corporate bonds |
|
|
| Debt Securities, Available-for-sale, Unrealized Loss Position [Line Items] |
|
|
| Number of Securities, unrealized loss position less than 12 months | investment |
|
6
|
| Fair Value, unrealized loss position less than 12 months |
|
$ 29,537
|
| Unrealized Losses, unrealized loss position less than 12 months |
|
$ (24)
|
| Foreign corporate bonds |
|
|
| Debt Securities, Available-for-sale, Unrealized Loss Position [Line Items] |
|
|
| Number of Securities, unrealized loss position less than 12 months | investment |
|
1
|
| Fair Value, unrealized loss position less than 12 months |
|
$ 7,173
|
| Unrealized Losses, unrealized loss position less than 12 months |
|
$ (7)
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479081/326-30-55-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-4
| Name: |
us-gaap_AvailableForSaleSecuritiesContinuousUnrealizedLossPositionFairValueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-7
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-7
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of investments in debt securities measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without an allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsNumberOfPositions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_DomesticCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_ForeignCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- DefinitionNumber of investments in debt securities measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for 12 months or longer, without an allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerNumberOfPositions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Marketable Securities - Net Investment Income (Details) - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Net investment income |
|
|
|
|
| Debt securities (including realized losses) |
$ 3,000
|
$ 2,225
|
$ 5,069
|
$ 5,634
|
| Other investments |
2,140
|
1,941
|
4,402
|
2,727
|
| Gross investment income (including realized losses) |
5,140
|
4,166
|
9,471
|
8,361
|
| Investment expenses |
(40)
|
(68)
|
(70)
|
(138)
|
| Net investment income |
$ 5,100
|
$ 4,098
|
$ 9,401
|
$ 8,223
|
| X |
- DefinitionAmount of interest income, amortization of premium and accretion of discount, on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), investment in debt security measured at amortized cost (held-to-maturity) and investment in debt security measured at fair value with change in fair value recognized in net income (trading); classified as operating.
| Name: |
us-gaap_InterestIncomeDebtSecuritiesOperating |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income and dividend income on nonoperating securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeInterestAndDividend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses related to the generation of investment income. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(2)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
| Name: |
us-gaap_InvestmentIncomeInvestmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accretion (amortization) of discount (premium), and investment expense, of interest income and dividend income on nonoperating securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentIncomeNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
| X |
- DefinitionAmount of realized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleRealizedLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481830/320-10-45-11
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-9
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-11
| Name: |
us-gaap_ProceedsFromSaleOfAvailableForSaleSecuritiesDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.2.u1
Fair Value of Financial Assets and Liabilities - Financial Assets and Liabilities Measured at Fair Value on Recurring Basis (Details) - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Liabilities: |
|
|
| Derivative liability, non-current |
$ 12,180
|
$ 0
|
| Recurring |
|
|
| Assets: |
|
|
| Total assets |
350,639
|
228,418
|
| Liabilities: |
|
|
| Forward contract and derivative liabilities |
81,220
|
|
| Total liabilities |
93,400
|
|
| Recurring | Put Option |
|
|
| Liabilities: |
|
|
| Derivative liability, non-current |
12,180
|
|
| Recurring | Money market funds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
113,379
|
59,199
|
| Other non-current assets |
2,515
|
1,900
|
| Recurring | U.S. treasury bills |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
36,944
|
27,901
|
| Marketable securities |
197,801
|
86,034
|
| Recurring | U.S. corporate bonds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
|
6,001
|
| Marketable securities |
|
40,210
|
| Recurring | Foreign corporate bonds |
|
|
| Assets: |
|
|
| Marketable securities |
|
7,173
|
| Recurring | Level 1 |
|
|
| Assets: |
|
|
| Total assets |
145,595
|
70,973
|
| Liabilities: |
|
|
| Forward contract and derivative liabilities |
0
|
|
| Total liabilities |
0
|
|
| Recurring | Level 1 | Put Option |
|
|
| Liabilities: |
|
|
| Derivative liability, non-current |
0
|
|
| Recurring | Level 1 | Money market funds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
113,379
|
59,199
|
| Other non-current assets |
2,515
|
1,900
|
| Recurring | Level 1 | U.S. treasury bills |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
0
|
0
|
| Marketable securities |
29,701
|
9,874
|
| Recurring | Level 1 | U.S. corporate bonds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
|
0
|
| Marketable securities |
|
0
|
| Recurring | Level 1 | Foreign corporate bonds |
|
|
| Assets: |
|
|
| Marketable securities |
|
0
|
| Recurring | Level 2 |
|
|
| Assets: |
|
|
| Total assets |
205,044
|
157,445
|
| Liabilities: |
|
|
| Forward contract and derivative liabilities |
0
|
|
| Total liabilities |
0
|
|
| Recurring | Level 2 | Put Option |
|
|
| Liabilities: |
|
|
| Derivative liability, non-current |
0
|
|
| Recurring | Level 2 | Money market funds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
0
|
0
|
| Other non-current assets |
0
|
0
|
| Recurring | Level 2 | U.S. treasury bills |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
36,944
|
27,901
|
| Marketable securities |
168,100
|
76,160
|
| Recurring | Level 2 | U.S. corporate bonds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
|
6,001
|
| Marketable securities |
|
40,210
|
| Recurring | Level 2 | Foreign corporate bonds |
|
|
| Assets: |
|
|
| Marketable securities |
|
7,173
|
| Recurring | Level 3 |
|
|
| Assets: |
|
|
| Total assets |
0
|
0
|
| Liabilities: |
|
|
| Forward contract and derivative liabilities |
81,220
|
|
| Total liabilities |
93,400
|
|
| Recurring | Level 3 | Put Option |
|
|
| Liabilities: |
|
|
| Derivative liability, non-current |
12,180
|
|
| Recurring | Level 3 | Money market funds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
0
|
0
|
| Other non-current assets |
0
|
0
|
| Recurring | Level 3 | U.S. treasury bills |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
0
|
0
|
| Marketable securities |
$ 0
|
0
|
| Recurring | Level 3 | U.S. corporate bonds |
|
|
| Assets: |
|
|
| Cash and cash equivalents |
|
0
|
| Marketable securities |
|
0
|
| Recurring | Level 3 | Foreign corporate bonds |
|
|
| Assets: |
|
|
| Marketable securities |
|
$ 0
|
| X |
- DefinitionFair value portion of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_AssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_CashAndCashEquivalentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset, expected to be settled within one year or normal operating cycle, if longer. Includes assets not subject to a master netting arrangement and not elected to be offset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483466/210-20-50-3
| Name: |
us-gaap_DerivativeLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset, expected to be settled after one year or the normal operating cycle, if longer. Includes assets not subject to a master netting arrangement and not elected to be offset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483466/210-20-50-3
| Name: |
us-gaap_DerivativeLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value of financial obligations, including, but not limited to, debt instruments, derivative liabilities, federal funds purchased and sold under agreements to repurchase, securities loaned or sold under agreements to repurchase, financial instruments sold not yet purchased, guarantees, line of credit, loans and notes payable, servicing liability, and trading liabilities. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FinancialLiabilitiesFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value portion of investment securities, including, but not limited to, marketable securities, derivative financial instruments, and investments accounted for under the equity method. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_InvestmentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value portion of other assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_OtherAssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByLiabilityClassAxis=us-gaap_PutOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_DomesticCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_ForeignCorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_ForwardContractLiability2025AddtionalConsiderationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_ForwardContractAndDerivativeLiabilitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_ForwardContractAndDerivativeLiabilitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 103
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-103
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_ForwardContractAndDerivativeLiabilitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Balance Sheet Components - Property and Equipment, Net (Details) - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Property, Plant and Equipment [Line Items] |
|
|
| Accumulated depreciation |
$ (10,201)
|
$ (8,283)
|
| Property and equipment, net |
18,665
|
17,191
|
| Building and land |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
14,078
|
11,728
|
| Leasehold improvements |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
824
|
802
|
| Computer hardware and software |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
875
|
875
|
| Office and lab equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
11,027
|
9,961
|
| Furniture and fixtures |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
1,793
|
1,550
|
| Depreciable Property, Plant and Equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
28,597
|
24,916
|
| Property and equipment, net |
18,396
|
16,633
|
| Equipment not yet in service |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property and equipment, gross |
$ 269
|
$ 558
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LandAndBuildingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=bhvn_DepreciablePropertyPlantAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ConstructionInProgressMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of other miscellaneous assets expected to be realized or consumed after one year or normal operating cycle, if longer.
| Name: |
us-gaap_OtherAssetsMiscellaneousNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PrepaidExpenseCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Balance Sheet Components - Accrued Expenses and Other Current Liabilities (Details) - USD ($)
$ in Thousands |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Accrued Expense and Other Current Liabilities |
|
|
| Accrued employee compensation and benefits |
$ 10,845
|
$ 837
|
| Accrued clinical trial costs |
36,446
|
29,501
|
| Operating lease liabilities - current portion |
3,550
|
3,308
|
| Other accrued expenses and other current liabilities |
6,887
|
6,200
|
| Accrued expenses and other current liabilities |
$ 57,728
|
$ 39,846
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as clinical trial costs, due within one year or the normal operating cycle, if longer.
| Name: |
bhvn_AccruedClinicalTrialCostsCurrent |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccruedLiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.2.u1
Shareholders' Equity - Changes in Shareholders' Deficit (Details) - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Mar. 31, 2024 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Net loss |
$ (319,771)
|
|
$ (90,346)
|
|
$ (499,275)
|
$ (160,838)
|
| Other comprehensive (loss) income |
28
|
|
(146)
|
|
(13)
|
(264)
|
| Total Shareholders' Equity |
|
|
|
|
|
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Beginning balance |
301,447
|
$ 427,975
|
472,258
|
$ 538,771
|
427,975
|
538,771
|
| Net loss |
(319,771)
|
(179,504)
|
(90,346)
|
(70,492)
|
|
|
| Issuance of common shares, net of offering costs |
317,720
|
|
|
|
|
|
| Issuance of common shares as payment for assets |
446
|
10,347
|
|
|
|
|
| Issuance of common shares as payment under license and other agreements |
65,981
|
5,637
|
|
|
|
|
| Issuance of common shares under 2022 Equity Incentive Plan |
|
2,156
|
|
332
|
|
|
| Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan |
2,317
|
|
794
|
|
|
|
| Issuance of warrant as payment under license agreement |
3,340
|
|
|
|
|
|
| Non-cash share-based compensation expense |
12,232
|
34,877
|
4,695
|
3,765
|
|
|
| Other comprehensive (loss) income |
28
|
(41)
|
(146)
|
(118)
|
|
|
| Ending balance |
$ 383,740
|
$ 301,447
|
$ 387,255
|
$ 472,258
|
$ 383,740
|
$ 387,255
|
| Common Shares |
|
|
|
|
|
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Beginning balance (in shares) |
81,807,221
|
81,115,723
|
68,212,479
|
68,190,479
|
81,115,723
|
68,190,479
|
| Beginning balance |
$ 910,964
|
$ 887,528
|
$ 616,246
|
$ 615,742
|
$ 887,528
|
$ 615,742
|
| Issuance of common shares, net of offering costs(in shares) |
8,544,951
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
$ 317,720
|
|
|
|
|
|
| Issuance of common shares as payment for assets (in shares) |
10,452
|
242,958
|
|
|
|
|
| Issuance of common shares as payment for assets |
$ 446
|
$ 10,347
|
|
|
|
|
| Issuance of common shares as payment under license and other agreements (in shares) |
1,872,874
|
97,233
|
|
|
|
|
| Issuance of common shares as payment under license and other agreements |
$ 65,981
|
$ 5,637
|
|
|
|
|
| Issuance of common shares under equity incentive plan (shares) |
|
351,307
|
|
22,000
|
|
|
| Issuance of common shares under 2022 Equity Incentive Plan |
|
$ 7,452
|
|
$ 504
|
|
|
| Issuance of common shares under equity incentive plan and employee share purchase plan (in shares) |
110,834
|
|
104,474
|
|
|
|
| Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan |
$ 3,442
|
|
$ 1,264
|
|
|
|
| Ending balance (in shares) |
92,346,332
|
81,807,221
|
68,316,953
|
68,212,479
|
92,346,332
|
68,316,953
|
| Ending balance |
$ 1,298,553
|
$ 910,964
|
$ 617,510
|
$ 616,246
|
$ 1,298,553
|
$ 617,510
|
| Additional Paid-in Capital |
|
|
|
|
|
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Beginning balance |
69,385
|
39,804
|
17,462
|
13,869
|
39,804
|
13,869
|
| Issuance of common shares under 2022 Equity Incentive Plan |
|
(5,296)
|
|
(172)
|
|
|
| Issuance of common shares under 2022 Equity Incentive Plan and 2022 Employee Share Purchase Plan |
(1,125)
|
|
(470)
|
|
|
|
| Issuance of warrant as payment under license agreement |
3,340
|
|
|
|
|
|
| Non-cash share-based compensation expense |
12,232
|
34,877
|
4,695
|
3,765
|
|
|
| Ending balance |
83,832
|
69,385
|
21,687
|
17,462
|
83,832
|
21,687
|
| Accumulated Deficit |
|
|
|
|
|
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Beginning balance |
(678,796)
|
(499,292)
|
(161,616)
|
(91,124)
|
(499,292)
|
(91,124)
|
| Net loss |
(319,771)
|
(179,504)
|
(90,346)
|
(70,492)
|
|
|
| Ending balance |
(998,567)
|
(678,796)
|
(251,962)
|
(161,616)
|
(998,567)
|
(251,962)
|
| Accumulated Other Comprehensive Loss |
|
|
|
|
|
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
| Beginning balance |
(106)
|
(65)
|
166
|
284
|
(65)
|
284
|
| Other comprehensive (loss) income |
28
|
(41)
|
(146)
|
(118)
|
|
|
| Ending balance |
$ (78)
|
$ (106)
|
$ 20
|
$ 166
|
$ (78)
|
$ 20
|
| X |
- DefinitionStock Issued During Period, Shares, Collaborative Arrangement
| Name: |
bhvn_StockIssuedDuringPeriodSharesCollaborativeArrangement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock Issued During Period, Shares, Share Based Compensation And Employee Stock Purchase Plan
| Name: |
bhvn_StockIssuedDuringPeriodSharesShareBasedCompensationAndEmployeeStockPurchasePlan |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock Issued During Period, Value, Collaborative Arrangement
| Name: |
bhvn_StockIssuedDuringPeriodValueCollaborativeArrangement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionStock Issued During Period, Value, Share Based Compensation And Employee Stock Purchase Plan
| Name: |
bhvn_StockIssuedDuringPeriodValueShareBasedCompensationAndEmployeeStockPurchasePlan |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in additional paid in capital (APIC) resulting from the issuance of warrants. Includes allocation of proceeds of debt securities issued with detachable stock purchase warrants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 20
-Section 25
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481284/470-20-25-2
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalWarrantIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_IncreaseDecreaseInStockholdersEquityRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of other comprehensive income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-4
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482739/220-10-55-15
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period as part of a transaction to acquire assets that do not qualify as a business combination.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesPurchaseOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber, after forfeiture, of shares or units issued under share-based payment arrangement. Excludes shares or units issued under employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued during the period as part of a transaction to acquire assets that do not qualify as a business combination.
| Name: |
us-gaap_StockIssuedDuringPeriodValuePurchaseOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483550/848-10-65-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479832/842-10-65-8
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-24
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-23
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483421/250-10-45-5
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-5
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480528/815-20-65-6
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479343/105-10-65-6
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482615/740-10-65-8
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479654/326-10-65-4
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-5
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-17
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481694/830-30-45-20
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 43: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 44: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 45: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 46: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-15
Reference 47: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-16
Reference 48: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4I
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476166/350-60-65-1
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_ParentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_AdditionalPaidInCapitalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_RetainedEarningsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_AccumulatedOtherComprehensiveIncomeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Shareholders' Equity - Narrative (Details) - USD ($)
$ / shares in Units, $ in Thousands |
|
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
9 Months Ended |
10 Months Ended |
|
|
Apr. 22, 2024 |
Jan. 07, 2024 |
Aug. 09, 2024 |
May 31, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Jun. 30, 2024 |
Jun. 30, 2024 |
Jun. 30, 2024 |
Aug. 09, 2024 |
May 01, 2024 |
Oct. 31, 2023 |
| Purchase Agreement, Knopp Amendment |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock issued (shares) |
|
|
|
1,872,874
|
|
|
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
|
|
$ 65,981
|
|
|
|
|
|
|
|
|
| Pyramid Biosciences, Inc. |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Upfront payment of common shares, shares (in shares) |
|
255,794,000
|
|
|
|
|
|
|
|
|
|
|
| Upfront payment of common shares, value |
|
$ 10,894
|
|
|
|
|
|
|
|
|
|
|
| Upfront payment of common shares, shares issued (in shares) |
|
|
|
|
|
|
253,410,000
|
|
|
|
|
|
| Research and development |
|
|
|
|
|
$ 5,689
|
|
|
|
|
|
|
| Common shares issuable (in shares) |
|
|
|
|
|
98,129
|
|
|
|
|
|
|
| Common shares issued (in shares) |
|
|
|
|
|
|
|
97,233
|
|
|
|
|
| Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock issued (shares) |
|
|
|
|
8,544,951
|
|
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
|
|
|
$ 317,720
|
|
|
|
|
|
|
|
| Number of shares sold in transaction (in shares) |
6,451,220
|
|
|
|
|
|
|
|
|
|
|
|
| Issuance price per share (in dollars per share) |
$ 41.00
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from sale of stock |
$ 247,830
|
|
|
|
|
|
|
|
|
|
|
|
| Equity Distribution Agreement aggregate amount |
|
|
|
|
|
|
|
|
|
|
|
$ 150,000
|
| Purchase Agreement, Knopp Amendment |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Number of securities called by warrants (shares) |
|
|
|
|
|
|
|
|
|
|
294,195
|
|
| Exercise price of warrants (in dollars per share) |
|
|
|
|
|
|
|
|
|
|
$ 67.98
|
|
| Warrants, fair value disclosure |
|
|
|
|
|
|
|
|
|
|
$ 3,340
|
|
| Equity Distribution Agreement | Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock issued (shares) |
|
|
|
|
|
|
|
|
2,093,731
|
|
|
|
| Proceeds from sale of stock |
|
|
|
|
|
|
|
|
$ 69,890
|
|
|
|
| Equity Distribution Agreement | Common Shares | Subsequent event |
|
|
|
|
|
|
|
|
|
|
|
|
| Nature of business [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock issued (shares) |
|
|
2,154,857
|
|
|
|
|
|
|
4,248,588
|
|
|
| Proceeds from sale of stock |
|
|
$ 76,360
|
|
|
|
|
|
|
$ 146,250
|
|
|
| X |
- DefinitionAcquisition, Consideration Transferred, Equity Interest Issued and Issuable
| Name: |
bhvn_AcquisitionConsiderationTransferredEquityInterestIssuedAndIssuable |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAcquisition, Consideration Transferred, Equity Interest Issued and Issuable, Shares
| Name: |
bhvn_AcquisitionConsiderationTransferredEquityInterestIssuedAndIssuableShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAcquisition, Consideration Transferred, Equity Interest Issued, Shares
| Name: |
bhvn_AcquisitionConsiderationTransferredEquityInterestIssuedShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDevelopmental Milestone, Equity Interest Issuable, Shares
| Name: |
bhvn_DevelopmentalMilestoneEquityInterestIssuableShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDevelopmental Milestone, Equity Interest Issued, Shares
| Name: |
bhvn_DevelopmentalMilestoneEquityInterestIssuedShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity Distribution Agreement, Maximum Aggregate Available, Amount
| Name: |
bhvn_EquityDistributionAgreementMaximumAggregateAvailableAmount |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWarrants, Fair Value Disclosure
| Name: |
bhvn_WarrantsFairValueDisclosure |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for research and development. Excludes cost for computer software product to be sold, leased, or otherwise marketed, writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both, and write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_PurchaseAgreementKnoppAmendmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
bhvn_AcquisitionAxis=bhvn_PyramidBiosciencesInc.Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bhvn_PurchaseAgreementKnoppAmendmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=bhvn_EquityDistributionAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Accumulated Other Comprehensive (Loss) Income (Details) - USD ($)
|
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| AOCI [Roll Forward] |
|
|
|
|
| Beginning of period balance |
|
|
$ 427,975,000
|
|
| End of period balance |
$ 383,740,000
|
|
383,740,000
|
|
| Accumulated other comprehensive income |
|
|
|
|
| AOCI [Roll Forward] |
|
|
|
|
| Beginning of period balance |
(106,000)
|
$ 166,000
|
(65,000)
|
$ 284,000
|
| Total other comprehensive income (loss) |
28,000
|
(146,000)
|
(13,000)
|
(264,000)
|
| End of period balance |
(78,000)
|
20,000
|
(78,000)
|
20,000
|
| Tax on other comprehensive (loss) income |
0
|
0
|
0
|
0
|
| Net unrealized investment gains (losses): |
|
|
|
|
| AOCI [Roll Forward] |
|
|
|
|
| Beginning of period balance |
(32,000)
|
(278,000)
|
3,000
|
(145,000)
|
| Other comprehensive loss |
|
(1,000)
|
|
(156,000)
|
| Amounts reclassified from accumulated other comprehensive loss |
|
17,000
|
|
39,000
|
| Total other comprehensive income (loss) |
29,000
|
16,000
|
(6,000)
|
(117,000)
|
| End of period balance |
(3,000)
|
(262,000)
|
(3,000)
|
(262,000)
|
| Foreign currency translation adjustments: |
|
|
|
|
| AOCI [Roll Forward] |
|
|
|
|
| Beginning of period balance |
(74,000)
|
444,000
|
(68,000)
|
429,000
|
| Other comprehensive loss |
|
(162,000)
|
|
(147,000)
|
| Total other comprehensive income (loss) |
(1,000)
|
|
(7,000)
|
|
| End of period balance |
$ (75,000)
|
$ 282,000
|
$ (75,000)
|
$ 282,000
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_AOCIAttributableToParentNetOfTaxRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax, before reclassification adjustments, of other comprehensive income (loss), attributable to parent. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482739/220-10-55-15
| Name: |
us-gaap_OciBeforeReclassificationsNetOfTaxAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of tax expense (benefit) allocated to other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossTaxPortionAttributableToParent1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of reclassification adjustments of other comprehensive income (loss) attributable to parent. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482739/220-10-55-15
| Name: |
us-gaap_ReclassificationFromAociCurrentPeriodNetOfTaxAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_AccumulatedOtherComprehensiveIncomeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_AccumulatedNetUnrealizedInvestmentGainLossMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_AccumulatedTranslationAdjustmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Non-Cash Share-Based Compensation - Narrative (Details) - USD ($)
$ / shares in Units, $ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Share-Based Compensation |
|
|
|
| Requisite service period of award (in years) |
|
3 years
|
|
| Total unrecognized compensation cost |
$ 90,206
|
$ 90,206
|
|
| Weighted average period for recognition of compensation cost |
|
2 years 3 months 29 days
|
|
| Granted (in dollars per share) |
|
$ 30.76
|
$ 10.16
|
| Unvested options expected to vest (in shares) |
7,978,678
|
7,978,678
|
|
| Options to purchase common shares |
|
|
|
| Share-Based Compensation |
|
|
|
| Share-based award term (in years) |
|
10 years
|
|
| RSUs |
|
|
|
| Share-Based Compensation |
|
|
|
| Common shares received (in shares) |
1
|
1
|
|
| Total unrecognized compensation cost |
$ 9,405
|
$ 9,405
|
|
| Weighted average period for recognition of compensation cost |
|
2 years 6 months 10 days
|
|
| Fair value of RSUs vested |
$ 3,770
|
$ 3,770
|
|
| X |
- DefinitionShare-based Compensation Arrangement by Share-based Payment Award, Common Stock Receivable Upon Vesting
| Name: |
bhvn_ShareBasedCompensationArrangementByShareBasedPaymentAwardCommonStockReceivableUponVesting |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionShare-Based Compensation Arrangement By Share-Based Payment Award, Options, Nonvested Expected To Vest, Number of Shares
| Name: |
bhvn_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsNonvestedExpectedToVestNumberOfShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEstimated period over which an employee is required to provide service in exchange for the equity-based payment award, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardRequisiteServicePeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based awards for which the grantee gained the right by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodTotalFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Non-Cash Share-Based Compensation - Non-Cash Expense (Details) - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Share-Based Compensation |
|
|
|
|
| Total non-cash share-based compensation expense |
$ 12,232
|
$ 4,695
|
$ 47,109
|
$ 8,460
|
| Research and development expenses |
|
|
|
|
| Share-Based Compensation |
|
|
|
|
| Total non-cash share-based compensation expense |
7,071
|
2,457
|
28,362
|
4,698
|
| General and administrative expenses |
|
|
|
|
| Share-Based Compensation |
|
|
|
|
| Total non-cash share-based compensation expense |
$ 5,161
|
$ 2,238
|
$ 18,747
|
$ 3,762
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Non-Cash Share-Based Compensation - Stock Options (Details)
$ / shares in Units, $ in Thousands |
6 Months Ended |
|
Jun. 30, 2024
USD ($)
$ / shares
shares
|
|---|
| Number of Shares |
|
| Outstanding at beginning of period (in shares) | shares |
11,379,429
|
| Granted (in shares) | shares |
2,500,161
|
| Exercised (in shares) | shares |
(295,890)
|
| Forfeited (in shares) | shares |
(38,684)
|
| Outstanding at end of period (in shares) | shares |
13,545,016
|
| Options exercisable (in shares) | shares |
5,566,338
|
| Options vested and expected to vest (in shares) | shares |
13,545,016
|
| Weighted Average Exercise Price |
|
| Outstanding at beginning of period (in dollars per share) | $ / shares |
$ 11.48
|
| Granted (in dollars per share) | $ / shares |
42.05
|
| Exercised (in dollars per share) | $ / shares |
9.00
|
| Forfeited (in dollars per share) | $ / shares |
20.38
|
| Outstanding at end of period (in dollars per share) | $ / shares |
17.15
|
| Options exercisable (in dollars per share) | $ / shares |
13.44
|
| Options vested and expected to vest (in dollars per share) | $ / shares |
$ 17.15
|
| Weighted Average Contractual Term and Aggregate Intrinsic Value |
|
| Weighted average remaining contractual term of options outstanding (in years) |
8 years 8 months 4 days
|
| Weighted average remaining contractual term of options exercisable (in years) |
8 years 6 months 14 days
|
| Weighted average remaining contractual term of options vested and expected to vest (in years) |
8 years 8 months 4 days
|
| Aggregate intrinsic value of options outstanding | $ |
$ 256,022
|
| Aggregate intrinsic value of options exercisable | $ |
122,803
|
| Aggregate intrinsic value of options vested and expected to vest | $ |
$ 256,022
|
| X |
- DefinitionShare-Based Awards, Weighted Average Contractual Term And Aggregate Intrinsic Value
| Name: |
bhvn_ShareBasedAwardsWeightedAverageContractualTermAndAggregateIntrinsicValueAbstract |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which current fair value of underlying stock exceeds exercise price of fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableAggregateIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest exercisable or convertible options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that were forfeited during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValueRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Net Loss Per Share - Calculation of Basic and Diluted Net Loss per Share (Details) - USD ($)
$ / shares in Units, $ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Numerator: |
|
|
|
|
| Net loss |
$ (319,771)
|
$ (90,346)
|
$ (499,275)
|
$ (160,838)
|
| Denominator: |
|
|
|
|
| Common shares outstanding—basic (shares) |
87,766,069
|
68,248,023
|
84,174,099
|
68,227,564
|
| Common shares outstanding—diluted (shares) |
87,766,069
|
68,248,023
|
84,174,099
|
68,227,564
|
| Net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. - basic (in dollars per share) |
$ (3.64)
|
$ (1.32)
|
$ (5.93)
|
$ (2.36)
|
| Net loss per share attributable to common shareholders of Biohaven Pharmaceutical Holding Company Ltd. - diluted (in dollars per share) |
$ (3.64)
|
$ (1.32)
|
$ (5.93)
|
$ (2.36)
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersDilutedAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingDilutedDisclosureItemsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
Net Loss Per Share - Antidilutive Securities Excluded from Computation of Net Loss per Share (Details) - shares
|
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Securities excluded from computation of diluted net loss per share |
|
|
| Anti-dilutive securities excluded from calculation of diluted net loss per share |
14,102,883
|
9,639,557
|
| Options to purchase common shares |
|
|
| Securities excluded from computation of diluted net loss per share |
|
|
| Anti-dilutive securities excluded from calculation of diluted net loss per share |
13,545,016
|
9,639,557
|
| Warrants to purchase common shares |
|
|
| Securities excluded from computation of diluted net loss per share |
|
|
| Anti-dilutive securities excluded from calculation of diluted net loss per share |
294,195
|
0
|
| Restricted share units |
|
|
| Securities excluded from computation of diluted net loss per share |
|
|
| Anti-dilutive securities excluded from calculation of diluted net loss per share |
263,672
|
0
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified commercial milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedCommercialMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMilestone Payment To Be Paid By Company Upon Specified Development Milestone Achievement
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedDevelopmentMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.2.u1
License, Acquisitions and Other Agreements - Yale University Agreement (Details) - USD ($)
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
Jan. 31, 2021 |
May 31, 2019 |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| License and other agreements |
|
|
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
|
|
$ 641,975,000
|
|
$ 641,975,000
|
|
| Research and development |
|
|
314,819,000
|
$ 79,490,000
|
470,791,000
|
$ 142,951,000
|
| Development milestone payment to be paid |
|
|
140,650,000
|
|
140,650,000
|
|
| Commercial milestone payment to be paid |
|
|
2,150,450,000
|
|
2,150,450,000
|
|
| Yale University | Yale Arrangement |
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
|
|
2,000,000
|
|
2,000,000
|
|
| Minimum annual royalty payment to be paid upon sale of product |
|
$ 1,000,000
|
|
|
|
|
| Research and development |
|
|
0
|
0
|
0
|
0
|
| Yale University | Yale MoDE Agreement |
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
| Minimum annual royalty payment to be paid upon sale of product |
$ 1,000,000
|
|
|
|
|
|
| Research and development |
|
|
0
|
$ 0
|
150,000
|
$ 0
|
| Development milestone payment to be paid |
|
|
650,000
|
|
650,000
|
|
| Commercial milestone payment to be paid |
|
|
$ 2,950,000
|
|
$ 2,950,000
|
|
| Initial term of agreement (in years) |
20 years
|
|
|
|
|
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified commercial milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedCommercialMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMilestone Payment To Be Paid By Company Upon Specified Development Milestone Achievement
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedDevelopmentMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMinimum Annual Royalty Payment To Be Paid By Company Upon Sale Of Product
| Name: |
bhvn_MinimumAnnualRoyaltyPaymentToBePaidByCompanyUponSaleOfProduct |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_YaleUniversityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_YaleCollaborativeArrangementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_YaleMoDEAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
License, Acquisitions and Other Agreements - ALS Biopharma Agreement (Details)
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
|
Aug. 31, 2015
prodrug
claim
|
Jun. 30, 2024
USD ($)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2024
USD ($)
|
Jun. 30, 2023
USD ($)
|
| License and other agreements |
|
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
|
$ 641,975,000
|
|
$ 641,975,000
|
|
| Research and development |
|
314,819,000
|
$ 79,490,000
|
470,791,000
|
$ 142,951,000
|
| ALS Biopharma Agreement | Collaborative arrangement |
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
| Number of prodrugs of glutamate modulating agents | prodrug |
300
|
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
|
4,000,000
|
|
4,000,000
|
|
| Number of claims | claim |
1
|
|
|
|
|
| Research and development |
|
$ 0
|
$ 0
|
$ 0
|
$ 0
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the number of claims if the Company abandons the sale of all products that will cause the reassignment of the applicable patent rights back to ALS Biopharma.
| Name: |
bhvn_NumberOfClaimsOfPatentsToReassign |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRepresents the number of prodrugs of glutamate modulating agents.
| Name: |
bhvn_NumberOfProdrugsOfGlutamateModulatingAgents |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_ALSBiopharmaAndFoxChaseChemicalDiversityCenterIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=us-gaap_CollaborativeArrangementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
License, Acquisitions and Other Agreements - Taldefgrobep Alfa License Agreement (Details) - USD ($)
|
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| License and other agreements |
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
$ 641,975,000
|
|
$ 641,975,000
|
|
| Research and development |
314,819,000
|
$ 79,490,000
|
470,791,000
|
$ 142,951,000
|
| BMS |
|
|
|
|
| License and other agreements |
|
|
|
|
| Research and development |
0
|
$ 0
|
0
|
$ 0
|
| BMS | Taldefgrobep Alfa License Agreement |
|
|
|
|
| License and other agreements |
|
|
|
|
| Milestone payment to be paid upon regulatory achievement |
$ 200,000,000
|
|
$ 200,000,000
|
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_BMSMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_TaldefgrobepAlfaLicenseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
License Agreements - Hangzhou Highlightll Pharmaceutical Agreement (Details) - USD ($)
|
3 Months Ended |
6 Months Ended |
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
Mar. 31, 2023 |
| License and other agreements |
|
|
|
|
|
|
| Development milestone payment to be paid |
$ 140,650,000
|
|
|
$ 140,650,000
|
|
|
| Milestone payment to be paid upon regulatory achievement |
641,975,000
|
|
|
641,975,000
|
|
|
| Commercial milestone payment to be paid |
2,150,450,000
|
|
|
2,150,450,000
|
|
|
| Research and development |
314,819,000
|
|
$ 79,490,000
|
470,791,000
|
$ 142,951,000
|
|
| Highlightll Pharmaceutical Co, Ltd. |
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
| Research and development |
0
|
|
$ 0
|
0
|
$ 0
|
|
| Highlightll Pharmaceutical Co, Ltd. | Collaborative arrangement |
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
$ 21,814,000
|
|
|
|
|
| Development milestone payment to be paid |
75,000,000
|
|
|
75,000,000
|
|
|
| Milestone payment to be paid upon regulatory achievement |
37,500,000
|
|
|
37,500,000
|
|
|
| Commercial milestone payment to be paid |
$ 837,500,000
|
|
|
$ 837,500,000
|
|
|
| Highlightll Pharmaceutical Co, Ltd. | License agreement | Collaborative arrangement |
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
| Payment obligation for license agreement |
|
|
|
|
|
$ 10,000,000
|
| Obligation for issuance of common shares as payment for assets (in shares) |
|
|
|
|
|
721,136
|
| X |
- DefinitionCollaborative Arrangement, Obligation for Payments to Acquire Intangible Assets
| Name: |
bhvn_CollaborativeArrangementObligationForPaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCollaborative Arrangement, Obligation for Stock Issuable, Shares, Purchase of Assets
| Name: |
bhvn_CollaborativeArrangementObligationForStockIssuableSharesPurchaseOfAssets |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified commercial milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedCommercialMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMilestone Payment To Be Paid By Company Upon Specified Development Milestone Achievement
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedDevelopmentMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_HighlightllPharmaceuticalCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=us-gaap_CollaborativeArrangementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_LicensingAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
License, Acquisitions and Other Agreements - Other Agreements (Details) - USD ($)
$ in Thousands |
3 Months Ended |
6 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| License and other agreements |
|
|
|
|
| Development milestone payment to be paid |
$ 140,650
|
|
$ 140,650
|
|
| Regulatory milestone paymnet to be paid |
641,975
|
|
641,975
|
|
| Commercial milestone payment to be paid |
2,150,450
|
|
2,150,450
|
|
| Other Agreements |
|
|
|
|
| License and other agreements |
|
|
|
|
| Milestone payments |
$ 375
|
$ 0
|
$ 1,875
|
$ 0
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified commercial milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedCommercialMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMilestone Payment To Be Paid By Company Upon Specified Development Milestone Achievement
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedDevelopmentMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the amount of milestone payment to be paid by the company upon the achievement of specified regulatory milestones and annual royalty payments based on net sales of products from patents.
| Name: |
bhvn_MilestonePaymentToBePaidByCompanyUponSpecifiedRegulatoryMilestoneAchievement |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_OtherAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
License, Acquisitions and Other Agreements - Acquisitions (Details) - USD ($)
$ / shares in Units, $ in Thousands |
|
|
|
1 Months Ended |
2 Months Ended |
3 Months Ended |
6 Months Ended |
|
May 03, 2024 |
May 01, 2024 |
Jan. 07, 2024 |
May 31, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Jun. 30, 2024 |
Dec. 31, 2023 |
| License and other agreements |
|
|
|
|
|
|
|
|
| Success-based milestone payments, based on regulatory approval |
|
|
|
|
$ 185,000
|
|
$ 185,000
|
|
| Additional regulatory milestone payments |
|
|
|
|
60,000
|
|
60,000
|
|
| Forward contract and derivative liabilities |
|
|
|
|
81,220
|
|
81,220
|
$ 0
|
| Purchase Agreement, Knopp Amendment |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Common stock issued (shares) |
|
|
|
1,872,874
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
|
|
$ 65,981
|
|
|
|
|
| Purchase Agreement, Knopp Amendment |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Number of securities called by warrants (shares) |
|
294,195
|
|
|
|
|
|
|
| Exercise price of warrants (in dollars per share) |
|
$ 67.98
|
|
|
|
|
|
|
| Purchase Agreement, Knopp Amendment |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
$ 75,000
|
|
|
|
|
|
|
| Gain on derivative |
|
|
|
$ 9,239
|
|
|
|
|
| Purchase Agreement, Knopp Amendment, 2025 Additional Consideration |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Common shares issuable, value |
$ 75,000
|
|
|
|
|
|
|
|
| Forward contract, current and written put option, current |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Forward contract and derivative liabilities |
75,220
|
|
|
|
|
|
|
|
| Derivative Liability, 2024 Additional Consideration | Level 3 |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Fair value |
15,540
|
|
|
|
|
|
|
|
| 2024 Additional Consideration True-up | Level 3 |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Change in fair value of derivative in other income (expense), net |
|
|
|
|
(590)
|
|
|
|
| Forward Contract Liability, 2025 Addtional Consideration |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Issuance of common shares, net of offering costs |
|
|
|
|
|
|
75,000
|
|
| Forward Contract Liability, 2025 Addtional Consideration | Level 3 |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Fair value |
|
$ 63,940
|
|
|
|
|
|
|
| Change in fair value of derivative in other income (expense), net |
|
|
|
|
(1,150)
|
|
|
|
| 2025 Additional Consideration True-up | Level 3 |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Fair value |
$ 13,810
|
|
|
|
|
|
|
|
| Change in fair value of derivative in other income (expense), net |
|
|
|
|
1,630
|
|
|
|
| Pyramid Biosciences, Inc. |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Upfront payment of common shares, shares (in shares) |
|
|
255,794,000
|
|
|
|
|
|
| Upfront payment of common shares, value |
|
|
$ 10,894
|
|
|
|
|
|
| Research and development |
|
|
|
|
|
$ 5,689
|
|
|
| Common shares issuable (in shares) |
|
|
|
|
|
98,129
|
|
|
| Pyramid Biosciences, Inc. | Developmental And Regulatory Milestones, First Asset | Lead Asset |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Success-based payments |
|
|
|
|
5,000
|
|
5,000
|
|
| Pyramid Biosciences, Inc. | Developmental And Regulatory Milestones, Second Asset | Second Asset |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Success-based payments |
|
|
|
|
30,000
|
|
30,000
|
|
| Pyramid Biosciences, Inc. | Commercial Sales-Based Milestones |
|
|
|
|
|
|
|
|
| License and other agreements |
|
|
|
|
|
|
|
|
| Success-based payments |
|
|
|
|
$ 40,000
|
|
$ 40,000
|
|
| X |
- DefinitionAcquisition, Consideration Transferred, Equity Interest Issued and Issuable
| Name: |
bhvn_AcquisitionConsiderationTransferredEquityInterestIssuedAndIssuable |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAcquisition, Consideration Transferred, Equity Interest Issued and Issuable, Shares
| Name: |
bhvn_AcquisitionConsiderationTransferredEquityInterestIssuedAndIssuableShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAcquisition, Contingent Consideration, Liability
| Name: |
bhvn_AcquisitionContingentConsiderationLiability |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAdditional Success-Based Milestone Payments to be Paid, Based On Regulatory Approval
| Name: |
bhvn_AdditionalSuccessBasedMilestonePaymentsToBePaidBasedOnRegulatoryApproval |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionDevelopmental Milestone, Equity Interest Issuable, Shares
| Name: |
bhvn_DevelopmentalMilestoneEquityInterestIssuableShares |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionForward Contract and Derivative Liability, Current
| Name: |
bhvn_ForwardContractAndDerivativeLiabilityCurrent |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionForward Contract, Obligation for Stock Issueable, Value
| Name: |
bhvn_ForwardContractObligationForStockIssueableValue |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGain on Forward Contract and Derivative Liability
| Name: |
bhvn_GainOnForwardContractAndDerivativeLiability |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionSuccess-Based Milestone Payments to be Paid, Based On Regulatory Approval
| Name: |
bhvn_SuccessBasedMilestonePaymentsToBePaidBasedOnRegulatoryApproval |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Excludes cost for computer software product to be sold, leased, or otherwise marketed, writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both, and write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_PurchaseAgreementKnoppAmendmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bhvn_PurchaseAgreementKnoppAmendmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=bhvn_PurchaseAgreementKnoppAmendmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=bhvn_PurchaseAgreementKnoppAmendment2025AdditionalConsiderationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_ForwardContractsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_DerivativeLiability2024AdditionalConsiderationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_A2024AdditionalConsiderationTrueUpMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_ForwardContractLiability2025AddtionalConsiderationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bhvn_A2025AdditionalConsiderationTrueUpMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
bhvn_AcquisitionAxis=bhvn_PyramidBiosciencesInc.Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ContingentConsiderationByTypeAxis=bhvn_DevelopmentalAndRegulatoryMilestonesFirstAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=bhvn_LeadAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ContingentConsiderationByTypeAxis=bhvn_DevelopmentalAndRegulatoryMilestonesSecondAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=bhvn_SecondAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ContingentConsiderationByTypeAxis=bhvn_CommercialSalesBasedMilestonesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
Commitments and Contingencies - Narrative (Details)
|
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
|
|
Jan. 01, 2021
USD ($)
shares
|
Aug. 31, 2023
USD ($)
|
Jun. 30, 2024
USD ($)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2024
USD ($)
|
Jun. 30, 2023
USD ($)
|
Mar. 31, 2024
term
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Research and development |
|
|
$ 314,819,000
|
$ 79,490,000
|
$ 470,791,000
|
$ 142,951,000
|
|
| Pittsburgh Centre Avenue Lease Agreement |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Lease agreement, term (in months) |
|
|
|
|
|
|
122 months
|
| Option to extend, term | term |
|
|
|
|
|
|
1
|
| Extension term (in months) |
|
|
|
|
|
|
60 months
|
| Pittsburgh Centre Avenue Lease Agreement | Minimum |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Annual commitments |
|
|
1,859,000
|
|
1,859,000
|
|
|
| Pittsburgh Centre Avenue Lease Agreement | Maximum |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Annual commitments |
|
|
$ 2,373,000
|
|
2,373,000
|
|
|
| Moda Agreement |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Eligible commercial milestone payments |
|
$ 23,000,000
|
|
|
|
|
|
| Eligible develoment and regulatory milestone payments |
|
$ 25,200,000
|
|
|
|
|
|
| Moda Pharmaceuticals LLC |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Research and development |
|
|
|
$ 0
|
850,000
|
$ 0
|
|
| Moda Pharmaceuticals LLC | Moda Agreement |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Eligible development milestone payments |
$ 81,612,000
|
|
|
|
|
|
|
| Eligible commercial milestone payments |
$ 30,171,000
|
|
|
|
|
|
|
| Initial term of agreement (in years) |
4 years
|
|
|
|
|
|
|
| Moda Pharmaceuticals LLC | License agreement | Moda Agreement |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Payment for license agreement |
$ 2,700,000
|
|
|
|
|
|
|
| Issuance of common shares as payment for assets (in shares) | shares |
37,836
|
|
|
|
|
|
|
| Issuance of common shares as payment for assets |
$ 3,243,000
|
|
|
|
|
|
|
| Research and Development Arrangement |
|
|
|
|
|
|
|
| Liability For Sale of Future Royalties [Line Items] |
|
|
|
|
|
|
|
| Research commitments |
|
|
|
|
$ 0
|
|
|
| X |
- DefinitionEligible Milestone Payments, Commercial
| Name: |
bhvn_EligibleMilestonePaymentsCommercial |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEligible Milestone Payments, Development and Regulatory
| Name: |
bhvn_EligibleMilestonePaymentsDevelopmentAndRegulatory |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLessee, Operating Lease, Annual Commitments
| Name: |
bhvn_LesseeOperatingLeaseAnnualCommitments |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLessee, Operating Lease, Number of Option Renewal Terms
| Name: |
bhvn_LesseeOperatingLeaseNumberOfOptionRenewalTerms |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPayments to Acquire Consulting Agreements
| Name: |
bhvn_PaymentsToAcquireConsultingAgreements |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69E
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69F
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1F
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1F
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1F
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1F
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 26: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482900/835-30-50-1
| Name: |
us-gaap_DebtInstrumentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTerm of lessee's operating lease renewal, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRenewalTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe minimum amount the entity agreed to spend under the long-term purchase commitment.
| Name: |
us-gaap_LongTermPurchaseCommitmentAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued during the period as part of a transaction to acquire assets that do not qualify as a business combination.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesPurchaseOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued during the period as part of a transaction to acquire assets that do not qualify as a business combination.
| Name: |
us-gaap_StockIssuedDuringPeriodValuePurchaseOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_LeaseContractualTermAxis=bhvn_PittsburghCentreAvenueLeaseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_ModaAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_ModaPharmaceuticalsLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_LicensingAgreementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LongTermPurchaseCommitmentByCategoryOfItemPurchasedAxis=us-gaap_ResearchAndDevelopmentArrangementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.2.u1
v3.24.2.u1
Related Party Transactions (Details) - USD ($)
|
1 Months Ended |
3 Months Ended |
6 Months Ended |
May 31, 2023 |
Jan. 31, 2021 |
Jun. 30, 2024 |
Jun. 30, 2023 |
Jun. 30, 2024 |
Jun. 30, 2023 |
| Related Party Transactions |
|
|
|
|
|
|
| Revenue from former parent |
|
|
$ 0
|
$ 1,674,000
|
$ 0
|
$ 5,559,000
|
| Research and development |
|
|
314,819,000
|
79,490,000
|
470,791,000
|
142,951,000
|
| Yale University | Related Party |
|
|
|
|
|
|
| Related Party Transactions |
|
|
|
|
|
|
| Research and development |
|
|
582,000
|
947,000
|
1,027,000
|
1,699,000
|
| Due to related party |
|
|
0
|
|
0
|
|
| Yale University | Yale MoDE Agreement |
|
|
|
|
|
|
| Related Party Transactions |
|
|
|
|
|
|
| Amount of funding |
|
$ 4,000,000
|
|
|
|
|
| Research and development |
|
|
$ 0
|
$ 0
|
$ 150,000
|
$ 0
|
| Yale University | 2023 Yale SRA |
|
|
|
|
|
|
| Related Party Transactions |
|
|
|
|
|
|
| Amount of funding |
$ 612,000
|
|
|
|
|
|
| X |
- DefinitionCollaborative Arrangement, Rights and Obligations, Maximum Aggregate Milestone Payments
| Name: |
bhvn_CollaborativeArrangementRightsAndObligationsMaximumAggregateMilestonePayments |
| Namespace Prefix: |
bhvn_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bhvn_YaleUniversityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=bhvn_YaleMoDEAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Biohaven (NYSE:BHVN)
過去 株価チャート
から 8 2024 まで 9 2024

Biohaven (NYSE:BHVN)
過去 株価チャート
から 9 2023 まで 9 2024
