false000177191000017719102024-05-062024-05-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): May 6, 2024
ADC Therapeutics SA
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | | | | |
Switzerland (State or Other Jurisdiction of Incorporation) | 001-39071 (Commission File Number) | N/A (IRS Employer Identification Number) |
| | |
Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of Principal Executive Offices) (Zip Code) |
+41 21 653 02 00 (Registrant’s Telephone Number) |
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Exchange Act:
| | | | | | | | |
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered |
| Common Shares, par value CHF 0.08 per share | ADCT | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 C.F.R. §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 C.F.R. §240.12b-2). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
On May 6, 2024, ADC Therapeutics SA (the “Company”) issued a press release announcing the Company’s financial results for the first quarter ended March 31, 2024. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein.
The information contained in this Item 2.02 and Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 7.01. Regulation FD Disclosure.
On May 6, 2024, the Company made publicly available a presentation that includes, among other things, initial data from an investigator-initiated Phase 2 clinical trial of ZYNLONTA patients with relapsed or refractory marginal zone lymphoma. A copy of the presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference herein.
The information contained in this Item 7.01 and Exhibit 99.2 shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| | | | | |
| Exhibit Number | Description |
| 99.1 | |
| 99.2 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | |
| ADC Therapeutics SA |
| Date: May 6, 2024 | |
| By: | /s/ Jose Carmona |
| Name: | Jose Carmona |
| Title: | Chief Financial Officer |
ADC Therapeutics Reports First Quarter 2024 Financial Results and Provides Business Updates
ZYNLONTA® (loncastuximab tesirine-lpyl) net sales of $17.8 million in 1Q 2024; total operating expenses decreased 25% (adjusted total operating expenses decreased 16%)1 compared to 1Q 2023
Successfully completed LOTIS-7 dose escalation and initiated expansion in 2L+ DLBCL
Initial MZL IIT Phase 2 data from 15 patients showed 13 patients with CR and 1 patient with PR; encouraging data compared to current treatments in area with high unmet medical need
Research event featured next-generation ADC platform and the Company’s most advanced ADC targets including promising preclinical data in NaPi2b, Claudin-6, PSMA and ASCT2
Company to host conference call today at 8:30 a.m. EDT
Lausanne, Switzerland, May 6, 2024 – ADC Therapeutics SA (NYSE: ADCT) today reported financial results for the first quarter ended March 31, 2024, and provided business updates.
“During the first quarter of 2024, we were pleased to see continued progress from our corporate and capital allocation strategy focused primarily on hematology with ZYNLONTA® while advancing our emerging solid tumor pipeline,” said Ameet Mallik, Chief Executive Officer of ADC Therapeutics. “In hematology, we delivered sequential ZYNLONTA revenue growth. We were pleased to announce that our LOTIS-7 study of ZYNLONTA in combination with bispecifics has successfully cleared the final dosing cohort and enrollment in Part 2 dose expansion has been initiated. Additionally, we were encouraged by the initial IIT Phase 2 data with ZYNLONTA in MZL which supports potential expansion in MZL and contributes to the overall ZYNLONTA growth strategy in NHL. With multiple potential value-generating catalysts ahead this year including expected completion of enrollment in LOTIS-5, expansion of LOTIS-7 and initial read of ADCT-601 in AXL, I am excited about our prospects for continued progress in 2024.”
Recent Highlights and Developments
ZYNLONTA® (loncastuximab tesirine-lpyl)
•ZYNLONTA generated product net sales of $17.8 million in the first quarter of 2024, representing a 7% increase over the fourth quarter of 2023 and a 6% decrease over the first quarter of 2023. Sequential quarter-over-quarter growth in the first quarter of 2024 continued, with sales volume increasing in both community and academic settings. The year-over-year net sales decline reflected higher gross-to-net deductions and lower volume, partially offset by a higher price.
Hematology Pipeline
•LOTIS-5: The Phase 3 confirmatory trial for ZYNLONTA in combination with rituximab in patients with 2L+ diffuse large B-cell lymphoma (DLBCL) continues to see accelerated enrollment. The Company expects to complete enrollment of this trial in 2024.
•LOTIS-7: On April 4, 2024, The Company announced the completion of dose escalation in LOTIS-7, a Phase 1b open-label clinical trial evaluating ZYNLONTA in combination with bispecific antibodies glofitamab or mosunetuzumab in heavily pre-treated patients with relapsed/refractory B-cell non-Hodgkin lymphoma (r/r B-NHL). In the dose escalation portion (Part 1) of LOTIS-7, no dose-limiting toxicities (DLTs), no or low-grade cytokine release
(1) See reconciliation of GAAP measures to non-GAAP measures in accompanying financial tables
syndrome (CRS) and no immune effector cell-associated neurotoxicity syndrome (ICANS) were observed across all patients when ZYNLONTA was administered in combination with glofitamab or mosunetuzumab. Additionally, after the first investigator assessment, evidence of anti-tumor activity (complete response or partial response) was observed among the majority of patients, with mixed histologies including r/r DLBCL, follicular lymphoma (FL) and marginal zone lymphoma (MZL). In addition, as of April 19, 2024, initial safety findings showed that the majority of CRS events seen were grade 1 (6 out of 18 patients) or grade 2 (2 of 18 patients, 11%), with no CRS greater than grade 2 observed in either combination arm. Furthermore, all grade 2 events responded to Tocilizumab/corticosteroids with no requirement for pressors or ICU management. Based on the data from Part 1, all three dose levels (90, 120 and 150 µg/kg) have now been cleared and enrollment in Part 2 dose expansion has been initiated with ZYNLONTA administered in combination with glofitamab at the 120 µg/kg and 150 µg/kg dose levels in 2L+ DLBCL patients.
•Investigator-Initiated Trial: As announced by the Company today, May 6, 2024, initial data from an investigator-initiated Phase 2 clinical trial evaluating ZYNLONTA for the treatment of relapsed/refractory (r/r) MZL were presented at the Lymphoma Research Foundation’s 2024 Marginal Zone Lymphoma Scientific Workshop by the trial’s lead investigator. The 50-patient single-arm, open-label Phase 2 multicenter study is currently being conducted at the Sylvester Comprehensive Cancer Center at University of Miami and City of Hope, and led by Izidore Lossos, MD, Professor, Director, Lymphoma Program at the Sylvester Comprehensive Cancer Center, University of Miami. This study is evaluating the safety and efficacy of ZYNLONTA in patients with r/r MZL previously treated with ≥1 line of systemic therapy (ClincalTrials.gov identifier: NCT05296070). As of the data cutoff date of March 30, 2024, 15 patients were evaluable. Of these 15 patients evaluated, 13 achieved a complete response (CR) and one patient achieved a partial response (PR). In this study, ZYNLONTA was generally well tolerated and the safety profile was consistent with the known profile, with two patient discontinuations. All patients who achieved responses had maintained them at the time of the data cutoff with the longest responder reaching approximately 20 months.
Solid Tumor Pipeline
•ADCT-601 (targeting AXL): The Phase 1b trial studying ADCT-601 targeting AXL continues enrolling patients in the pancreatic cancer monotherapy arm, optimizing dose and schedule. The ongoing dose-optimization/expansion phase is comprised of a monotherapy arm including patients with sarcoma, pancreatic cancer and AXL-expressing non-small cell lung cancer (NSCLC) and a combination arm with gemcitabine in patients with sarcoma and pancreatic cancer.
•Early-stage pipeline: On April 9, 2024, the Company hosted a virtual Research Investor Event during which details were shared on strategy and recent business updates as well as the Company’s novel exatecan-based ADC platform. The Company provided details on its four lead candidates – targeting Claudin-6, NaPi2b, PSMA and ASCT2 – which have a differentiated profile based on a novel, proprietary linker approach to tracelessly release exatecan and a high therapeutic index. The Company’s NaPi2b and Claudin-6 targeting ADCs are in IND-enabling studies and PSMA and ASCT2 targeting ADCs are in drug candidate selection stage, expected to complete this year. Preclinical data on the Claudin-6 and NaPi2b programs were shared in presentations at the AACR Annual Meeting 2024 which demonstrated that each was well tolerated with potent and specific in vitro and in vivo anti-tumor activity.
Upcoming Expected Milestones
ZYNLONTA
•Achieve commercial brand profitability in 2024
•LOTIS-5: Complete enrollment in 2H 2024
•LOTIS-7: Part 2 enrollment complete with initial efficacy/safety update in 2H 2024; full/mature data in 1H 2025
•Investigator-initiated trial in r/r FL: The study is being expanded to 100 patients in a multicenter clinical trial. Updates are anticipated at medical meetings in 2024/2025.
•Investigator-initiated trial in r/r MZL: The study is designed to enroll 50 patients in a multicenter clinical trial. Further updates are anticipated at medical meetings in 2024/2025.
Pipeline
ADCT-601 (targeting AXL)
•Additional data updates from the Phase 1 study in patients with sarcoma, pancreatic cancer and NSCLC in 2H 2024
ADCT-602 (targeting CD22)
•Additional data from the Phase 1 study in 2H 2024
Preclinical
Advancing a broad portfolio of investigational ADCs for solid tumor indications
First Quarter 2024 Financial Results
Cash and Cash Equivalents
Cash and cash equivalents were $234.3 million as of March 31, 2024, compared to $278.6 million as of December 31, 2023. The Company currently expects its cash runway to extend into the fourth quarter of 2025.
Product Revenues
Net product revenues were $17.8 million for the first quarter 2024, compared to $19.0 million for the first quarter 2023. Net product revenues are for U.S. sales of ZYNLONTA. The decrease was primarily due to higher gross-to-net deductions and lower volume, partially offset by a higher price.
Research and Development (R&D) Expenses
R&D expenses were $25.7 million for the first quarter 2024, compared to $38.4 million for the first quarter 2023. R&D expenses decreased due to less investment in camidanlumab tesirine (Cami), as well as productivity initiatives and focused investment toward prioritized development programs. The decrease in R&D expenses related to Cami was primarily due to our evaluation of FDA feedback and decision to stop the program.
R&D expenses for the first quarter 2024 also decreased due to lower share-based compensation expense resulting from fluctuations in the share price and award forfeitures in connection with terminations.
Selling and Marketing (S&M) Expenses
S&M expenses were $11.4 million for the first quarter 2024, compared to $15.4 million for the first quarter 2023. The decrease in S&M expenses was primarily due to lower spend on marketing and advertising, lower wages and benefits, as well as lower share-based compensation expense resulting from fluctuations in the share price and award forfeitures in connection with terminations.
General & Administrative (G&A) Expenses
G&A expenses were $12.0 million for the first quarter 2024, compared to $15.5 million for the first quarter 2023. The decrease in G&A expenses was primarily due to lower share-based compensation expense resulting from fluctuations in the share price and award forfeitures in connection with terminations, lower wages and benefits and insurance costs, partially offset by higher professional fees including audit and legal fees.
Net Loss and Adjusted Net Loss
Net loss was $46.6 million, or a net loss of $0.56 per basic and diluted share, for the first quarter of 2024 and a net loss of $59.4 million, or a net loss of $0.73 per basic and diluted share for the first quarter of 2023. The decrease in net loss is primarily due to lower operating expenses, partially offset by changes in the fair value of our Deerfield warrant obligation and higher accretion of our deferred royalty obligation.
Adjusted net loss, which is a non-GAAP financial measure, was $31.1 million, or an adjusted net loss of $0.38 per basic and diluted share for the first quarter 2024 and $41.8 million, or an adjusted net loss of $0.52 per basic and diluted share for the first quarter 2023. The decrease in adjusted net loss for the quarter primarily reflects our lower operating expenses.
Conference Call Details
ADC Therapeutics management will host a conference call and live audio webcast to discuss first quarter 2024 financial results and provide a company update today at 8:30 a.m. Eastern Time. To access the conference call, please register here. Registrants will receive the dial-in number and unique PIN. It is recommended that you join 10 minutes before the event, though you may pre-register at any time. A live webcast of the call will be available under “Events & Presentations” in the Investors section of the ADC Therapeutics website at ir.adctherapeutics.com. The archived webcast will be available for 30 days following the call.
About ZYNLONTA® (loncastuximab tesirine-lpyl)
ZYNLONTA® is a CD19-directed antibody drug conjugate (ADC). Once bound to a CD19-expressing cell, ZYNLONTA is internalized by the cell, where enzymes release a pyrrolobenzodiazepine (PBD) payload. The potent payload binds to DNA minor groove with little distortion, remaining less visible to DNA repair mechanisms. This ultimately results in cell cycle arrest and tumor cell death.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved ZYNLONTA (loncastuximab tesirine-lpyl) for the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), DLBCL arising from low-grade lymphoma and also high-grade B-cell lymphoma. The trial included a broad spectrum of heavily pre-treated patients (median three prior lines of therapy) with difficult-to-treat disease, including patients who did not respond to first-line therapy, patients refractory to all prior lines of therapy,
patients with double/triple hit genetics and patients who had stem cell transplant and CAR-T therapy prior to their treatment with ZYNLONTA. This indication is approved by the FDA under accelerated approval and in the European Union under conditional approval based on overall response rate and continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Please see full prescribing information including important safety information about ZYNLONTA at www.ZYNLONTA.com.
ZYNLONTA is also being evaluated as a therapeutic option in combination studies in other B-cell malignancies and earlier lines of therapy.
About ADC Therapeutics
ADC Therapeutics (NYSE: ADCT) is a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs). The Company is advancing its proprietary ADC technology to transform the treatment paradigm for patients with hematologic malignancies and solid tumors.
ADC Therapeutics’ CD19-directed ADC ZYNLONTA (loncastuximab tesirine-lpyl) received accelerated approval by the FDA and conditional approval from the European Commission for the treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy. ZYNLONTA is also in development in combination with other agents and in earlier lines of therapy. In addition to ZYNLONTA, ADC Therapeutics has multiple ADCs in ongoing clinical and preclinical development.
ADC Therapeutics is based in Lausanne (Biopôle), Switzerland and has operations in London and New Jersey. For more information, please visit https://adctherapeutics.com/ and follow the Company on LinkedIn.
ZYNLONTA® is a registered trademark of ADC Therapeutics SA.
Use of Non-GAAP Financial Measures
In addition to financial information prepared in accordance with U.S. Generally Accepted Accounting Principles (GAAP), this document also contains certain non-GAAP financial measures based on management’s view of performance including:
•Adjusted total operating expenses
•Adjusted net loss
•Adjusted net loss per share
Management uses such measures internally when monitoring and evaluating our operational performance, generating future operating plans and making strategic decisions regarding the allocation of capital. We believe that these adjusted financial measures provide useful information to investors and others in understanding and evaluating our operating results in the same manner as our management and facilitate operating performance comparability across both past and future reporting periods. These non-GAAP measures have limitations as financial measures and should be considered in addition to, and not in isolation or as a substitute for, the information prepared in accordance with GAAP. When preparing these supplemental non-GAAP measures, management typically excludes certain GAAP items that management does not believe are indicative of our ongoing operating performance. Furthermore, management does not consider these GAAP items to be normal, recurring cash operating expenses; however, these items may not meet the GAAP definition of unusual or non-recurring items. Since non-GAAP financial measures do not have standardized definitions and meanings, they may differ from the non-GAAP financial measures used by other companies, which reduces their usefulness as comparative financial measures. Because of
these limitations, you should consider these adjusted financial measures alongside other GAAP financial measures.
The following items are excluded from adjusted total operating expenses:
Shared-Based Compensation Expense: We exclude share-based compensation expense from our adjusted financial measures because share-based compensation expense, which is non-cash, fluctuates from period to period based on factors that are not within our control, such as our stock price on the dates share-based grants are issued. Share-based compensation expense has been, and will continue to be for the foreseeable future, a recurring expense in our business and an important part of our compensation strategy.
The following items are excluded from adjusted net loss and adjusted net loss per share:
Shared-Based Compensation Expense: We exclude share-based compensation expense from our adjusted financial measures because share-based compensation expense, which is non-cash, fluctuates from period to period based on factors that are not within our control, such as our stock price on the dates share-based grants are issued. Share-based compensation expense has been, and will continue to be for the foreseeable future, a recurring expense in our business and an important part of our compensation strategy.
Certain Other Items: We exclude certain other significant items that we believe do not represent the performance of our business, from our adjusted financial measures. Such items are evaluated by management on an individual basis based on both quantitative and qualitative aspects of their nature. While not all-inclusive, examples of certain other significant items excluded from our adjusted financial measures would be: changes in the fair value of warrant obligations and the effective interest expense associated with the senior secured term loan facility and the effective interest expense and cumulative catch-up adjustments associated with the deferred royalty obligation under the royalty purchase agreement with HealthCare Royalty Partners.
See the attached Reconciliation of GAAP Measures to Non-GAAP Measures for explanations of the amounts excluded and included to arrive at the non-GAAP financial measures.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the success of the Company’s updated corporate strategy; the expected cash runway into the beginning of Q4 2025, the effectiveness of the new commercial go-to-market strategy, competition from new technologies, the Company’s ability to grow ZYNLONTA® revenue in the United States; Swedish Orphan Biovitrum AB’s (Sobi®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and market acceptance, adequate reimbursement coverage, and future revenue from the same; approval by the NMPA of the BLA for ZYNLONTA® in China submitted by Overland ADCT BioPharma and future revenue from the same, our strategic partners’, including Mitsubishi Tanabe Pharma Corporation, ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing and amount of future revenue and payments to us from such partnerships; the timing and results of the Company’s or its partners’ research and development projects or clinical trials including LOTIS 5 and 7, ADCT 601 and 602 as well as early research in certain solid tumors with different targets, linkers
and payloads; the timing and results of the University of Miami’s investigator-initiated trials in FL and MZL, potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company’s indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company’s activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 10-K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this document.
ADC Therapeutics SA
Condensed Consolidated Statements of Operation (Unaudited)
(in thousands, except for share and per share data)
| | | | | | | | | | | | | | | | | | |
| | | | For the Three Months Ended March 31, |
| | | | | | 2024 | | 2023 |
| Revenue | | | | | | | | |
| Product revenues, net | | | | | | $ | 17,848 | | | $ | 18,953 | |
| License revenues and royalties | | | | | | 205 | | | 39 | |
| Total revenue, net | | | | | | 18,053 | | | 18,992 | |
| Operating expense | | | | | | | | |
| Cost of product sales | | | | | | (2,510) | | | 27 | |
| Research and development | | | | | | (25,735) | | | (38,375) | |
| Selling and marketing | | | | | | (11,390) | | | (15,351) | |
| General and administrative | | | | | | (12,031) | | | (15,503) | |
| Total operating expense | | | | | | (51,666) | | | (69,202) | |
| Loss from operations | | | | | | (33,613) | | | (50,210) | |
| | | | | | | | |
| Other income (expense) | | | | | | | | |
| Interest income | | | | | | 2,948 | | | 2,175 | |
| Interest expense | | | | | | (12,496) | | | (10,291) | |
| Other, net | | | | | | (2,595) | | | 833 | |
| Total other expense | | | | | | (12,143) | | | (7,283) | |
| Loss before income taxes | | | | | | (45,756) | | | (57,493) | |
| Income tax expense | | | | | | (163) | | | (518) | |
| Loss before equity in net losses of joint venture | | | | | | (45,919) | | | (58,011) | |
| Equity in net losses of joint venture | | | | | | (687) | | | (1,363) | |
| Net loss | | | | | | $ | (46,606) | | | $ | (59,374) | |
| | | | | | | | |
| Net loss per share | | | | | | | | |
| Net loss per share, basic and diluted | | | | | | $ | (0.56) | | | $ | (0.73) | |
| Weighted average shares outstanding, basic and diluted | | | | | | 82,552,322 | | | 80,805,770 | |
| | | | | | | | |
ADC Therapeutics SA
Condensed Consolidated Balance Sheet (Unaudited)
(in thousands)
| | | | | | | | | | | | | | |
| | March 31, 2024 | | December 31, 2023 |
| ASSETS | | | | |
| Current assets | | | | |
| Cash and cash equivalents | | $ | 234,285 | | | $ | 278,598 | |
| Accounts receivable, net | | 23,186 | | | 25,182 | |
| Inventory | | 15,997 | | | 16,177 | |
| Prepaid expenses and other current assets | | 16,738 | | | 16,334 | |
| Total current assets | | 290,206 | | | 336,291 | |
| Non-current assets | | | | |
| Property and equipment, net | | 5,785 | | | 5,622 | |
| Operating lease right-of-use assets | | 10,059 | | | 10,511 | |
| Interest in joint venture | | 930 | | | 1,647 | |
| | | | |
| Other long-term assets | | 986 | | | 711 | |
| Total assets | | $ | 307,966 | | | $ | 354,782 | |
| | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | |
| Current liabilities | | | | |
| Accounts payable | | $ | 14,315 | | | $ | 15,569 | |
| Accrued expenses and other current liabilities | | 48,670 | | | 52,101 | |
| | | | |
| Total current liabilities | | 62,985 | | | 67,670 | |
| | | | |
| Deferred royalty obligation | | 310,010 | | | 303,572 | |
| Senior secured term loans | | 113,234 | | | 112,730 | |
| Operating lease liabilities, long-term | | 9,662 | | | 10,180 | |
| Other long-term liabilities | | 6,524 | | | 8,879 | |
| Total liabilities | | 502,415 | | | 503,031 | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| Total shareholders’ (deficit) equity | | (194,449) | | | (148,249) | |
| | | | |
| Total liabilities and shareholders’ equity | | $ | 307,966 | | | $ | 354,782 | |
ADC Therapeutics SA
Reconciliation of GAAP Measures to Non-GAAP Measures (Unaudited)
(in thousands, except for share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| (in thousands) | | | | | | | | | 2024 | | 2023 | | Change | | % Change |
| Total operating expense | | | | | | | | | $ | (51,666) | | | $ | (69,202) | | | $ | 17,536 | | | (25) | % |
| Adjustments: | | | | | | | | | | | | | | | |
| Share-based compensation expense (i) | | | | | | | | | 158 | | | 8,074 | | | (7,916) | | | (98) | % |
| Adjusted total operating expenses | | | | | | | | | $ | (51,508) | | | $ | (61,128) | | | $ | 9,620 | | | (16) | % |
| | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| in thousands (except for share and per share data) | | | | | 2024 | | 2023 |
| Net loss | | | | | $ | (46,606) | | | $ | (59,374) | |
| Adjustments: | | | | | | | |
| Share-based compensation expense (i) | | | | | 158 | | | 8,074 | |
| Deerfield warrants obligation, change in fair value expense (income) (ii) | | | | | 3,068 | | | (616) | |
| Effective interest expense on senior secured term loan facility (iii) | | | | | 4,403 | | | 4,540 | |
| Deferred royalty obligation interest expense (iv) | | | | | 8,093 | | | 5,746 | |
| Deferred royalty obligation cumulative catch-up adjustment income (iv) | | | | | (263) | | | (129) | |
| Adjusted net loss | | | | | $ | (31,147) | | | $ | (41,759) | |
| | | | | | | |
| Net loss per share, basic and diluted | | | | | $ | (0.56) | | | $ | (0.73) | |
| Adjustment to net loss per share, basic and diluted | | | | | 0.18 | | | 0.21 | |
| Adjusted net loss per share, basic and diluted | | | | | $ | (0.38) | | | $ | (0.52) | |
| Weighted average shares outstanding, basic and diluted | | | | | 82,552,322 | | | 80,805,770 | |
(i)Share-based compensation expense represents the cost of equity awards issued to our directors, management and employees. The fair value of awards is computed at the time the award is granted, and is recognized over the requisite service period less actual forfeitures by a charge to the statement of operations and a corresponding increase in additional paid-in capital within equity. These accounting entries have no cash impact.
(ii)Change in the fair value of the Deerfield warrant obligation results from the valuation at the end of each accounting period. There are several inputs to these valuations, but those most likely to result in significant changes to the valuations are changes in the value of the underlying instrument (i.e., changes in the price of our common shares) and changes in expected volatility in that price. These accounting entries have no cash impact.
(iii)Effective interest expense on senior secured term loans relates to the increase in the value of our loans in accordance with the amortized cost method.
(iv)Deferred royalty obligation interest expense relates to the accretion expense on our deferred royalty obligation pursuant to the royalty purchase agreement with HCR and cumulative catch-up adjustments related to changes in the expected payments to HCR based on a periodic assessment of our underlying revenue projections.
CONTACT:
Investors and Media
Nicole Riley
ADC Therapeutics
Nicole.Riley@adctherapeutics.com
+1 862-926-9040

May 2024 ZYNLONTA R/R MZL Phase 2 IIT Initial Data Exhibit 99.2

2 Forward-Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or ADC Therapeutics or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provided in this presentation and any accompanying oral presentation speak only as of the date hereof. This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward-looking statements by terminology such as “may”, “assumes”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the future timing and results of the University of Miami’s investigator initiated trial for r/r MZL and the ability to obtain regulatory approval or NCCN compendia inclusion, our estimate for the MZL market opportunity, the success of the Company’s updated corporate strategy; the expected cash runway into the beginning of Q4 2025, the effectiveness of the new commercial go-to-market strategy, competition from new technologies, the Company’s ability to grow ZYNLONTA® revenue in the United States; Swedish Orphan Biovitrum AB (Sobi®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and market acceptance, adequate reimbursement coverage, and future revenue from the same; approval by the NMPA of the BLA for ZYNLONTA® in China submitted by Overland ADCT BioPharma and future revenue from the same, our strategic partners’, including Mitsubishi Tanabe Pharma Corporation, ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing and amount of future revenue and payments to us from such partnerships; the timing and results of the Company’s or its partners’ research and development projects or clinical trials including LOTIS 5 and 7, ADCT 601 and 602 as well as IITs in FL and MZL and early research in certain solid tumors with different targets, linkers and payloads; the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company’s ability to enter into business development or research collaboration transactions; the Company’s indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company’s activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 10-K and Form 10-Q and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this document. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. No assurance can be given that such future results will be achieved. Such forward- looking statements contained in this presentation speak only as of the date of this presentation. The Company expressly disclaim any obligation or undertaking to update these forward-looking statements contained in this presentation to reflect any change in our expectations or any change in events, conditions, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data derived from third-party sources and our own internal estimates and research. While we believe these third- party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third- party sources. In addition, all of the market data included in this presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is reliable, such research has not been verified by any independent source.

3 Estimated 3 – 4 K 2L+ MZL patients are drug-treated in the US1; despite patients achieving durable responses, high unmet medical need remains with <30% CR for 2L+ NCCN preferred treatments2 Total addressable 2L+ MZL patient population has a potential peak market value of approximately $500 M3; Potential expansion in MZL contributes to the overall ZYNLONTA growth strategy in NHL Initial data^ from ZYNLONTA r/r MZL U of Miami Ph2 IIT in 15 patients (n total = 50) showed 13 achieved a complete response and 1 achieved a partial response; ZYNLONTA was generally well-tolerated, and safety was consistent with the known profile 2 FDA approved regimens (n = 63 – 68; CR 26 – 29%) & 5 additional preferred for 2L+ in NCCN guidelines (n = 43 – 46; CR 13 – 17%)* 2 sites currently enrolling, expanding to 5 sites to accelerate trial enrollment; ADCT plans to potentially pursue regulatory pathway and compendia in parallel as soon as sufficient data is available *Data does not include R-CHOP, R-CVP, or B-R which are included in 2L+ NCCN preferred guidelines based on 1L systemic therapy data only; ^As of the data cutoff date of March 30, 2024. Source: 1. Clarivate DRG (2022), Global Data (2017), Cerner Enviza CancerMPact (2023); 2. MAGNOLIA: ph2, single arm in r/r MZL, AUGMENT: ph3, randomized of R2 vs. R in r/r iNHL, GADOLIN: ph3 randomized of benda vs. benda + obin in r/r iNHL, ACE-LY-003: ph2, part 2 (r/r MZL cohort) of 3 part ph1b/2 study of acalabrutinib alone or in combination in B-cell NHL; 3. Assumes total addressable population and average net price for ZYNLONTA in 2030 with a CAGR of ~2% and ~3% respectively, and average cycles expected in MZL. Key Takeaways – ZYNLONTA R/R MZL Phase 2 IIT Initial Data

4 Overview of MZL Market and ZYNLONTA Study Source: 1. Clarivate DRG (2022), Global Data (2017), Cerner Enviza CancerMPact (2023); 2.NCCN guidelines; 3. MAGNOLIA: ph2, single arm in r/r MZL, AUGMENT: ph3, randomized of R2 vs. R in r/r iNHL, GADOLIN: ph3 randomized of benda vs. benda + obin in r/r iNHL, ACE-LY-003: ph2, part 2 (r/r MZL cohort) of 3 part ph1b/2 study of acalabrutinib alone or in combination in B-cell NHL; 4. Alderuccio et al., J Clin Oncol (2018), Alderuccio et al., Am J Hematol (2019). MZL Treatment and Market Overview An estimated 3 – 4 K r/r MZL patients1 are treated with systemic anti-cancer regimens annually NCCN preferred agents include anti-CD20-based regimens across all lines in addition to BTKis in 2L+2 CR rates are modest for 2L+ FDA approved and NCCN preferred agents (i.e., <30%)3 Clinicians continue to seek novel agents with higher CR rates and fixed treatment duration Achievement of complete response to treatment represents the strongest predictor of positive outcomes in MZL4 The U of Miami IIT is a multi-institutional Phase 2 trial evaluating ZYNLONTA in 50 patients with r/r MZL ZYNLONTA is administered with a fixed duration of 6 cycles; patients will be treated for 18 weeks and followed for up to 3 years with PFS and OS measured at 24 months Based on modest CR rates from currently approved therapies, the futility threshold for ZYNLONTA in this patient population was determined to be a 31% CR rate Initial data of ZYNLONTA in 15 patients showed 13 achieved a complete response and 1 achieved a partial response All patients achieving responses are maintaining them as of the data cutoff on March 30 with the longest responder reaching approximately 20 months ZYNLONTA was generally well-tolerated, and safety was consistent with the known profile Trial Design & Initial Results
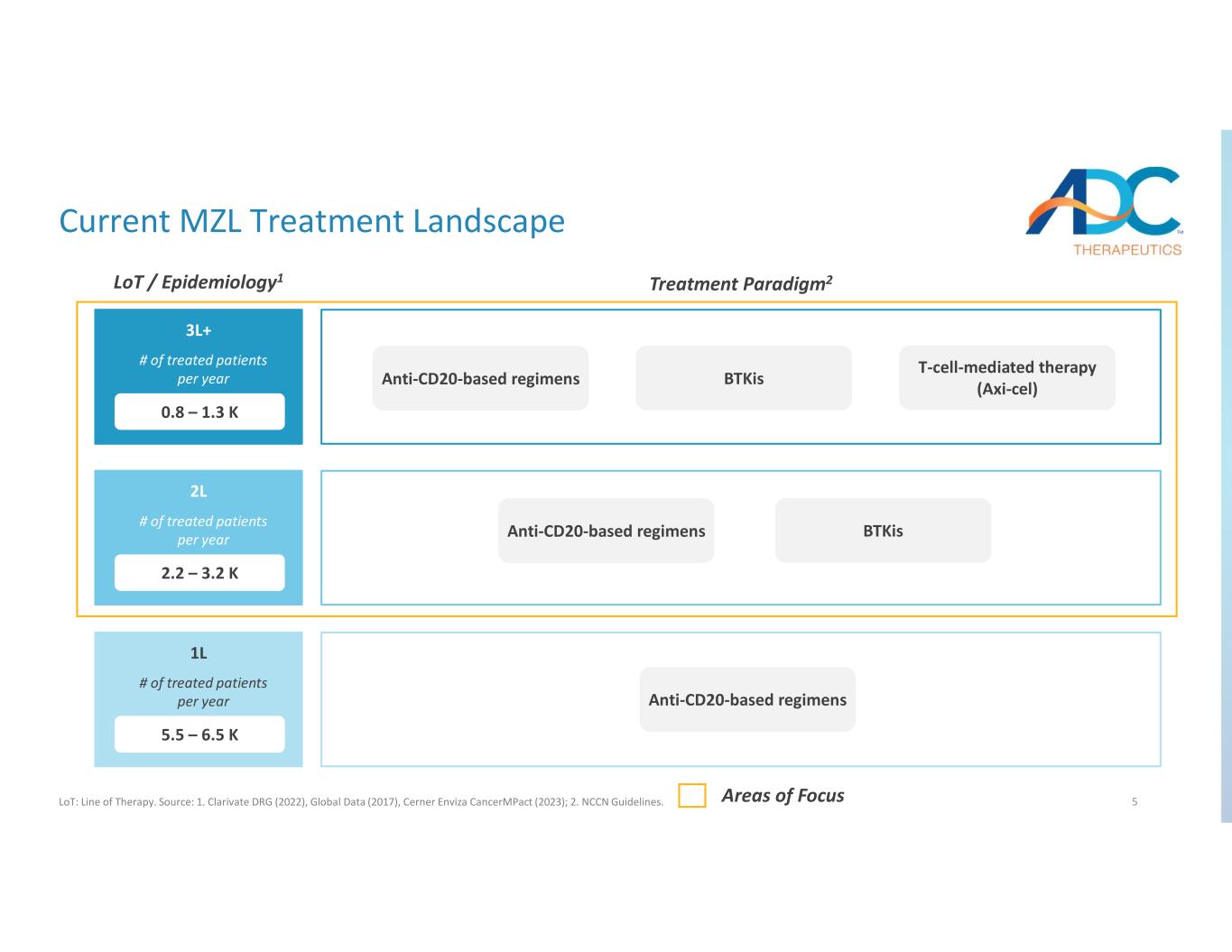
5 3L+ Current MZL Treatment Landscape LoT: Line of Therapy. Source: 1. Clarivate DRG (2022), Global Data (2017), Cerner Enviza CancerMPact (2023); 2. NCCN Guidelines. LoT / Epidemiology1 Treatment Paradigm2 Anti-CD20-based regimens BTKis Anti-CD20-based regimens T-cell-mediated therapy (Axi-cel)Anti-CD20-based regimens BTKis # of treated patients per year 0.8 – 1.3 K 2L # of treated patients per year 2.2 – 3.2 K 1L # of treated patients per year 5.5 – 6.5 K Areas of Focus
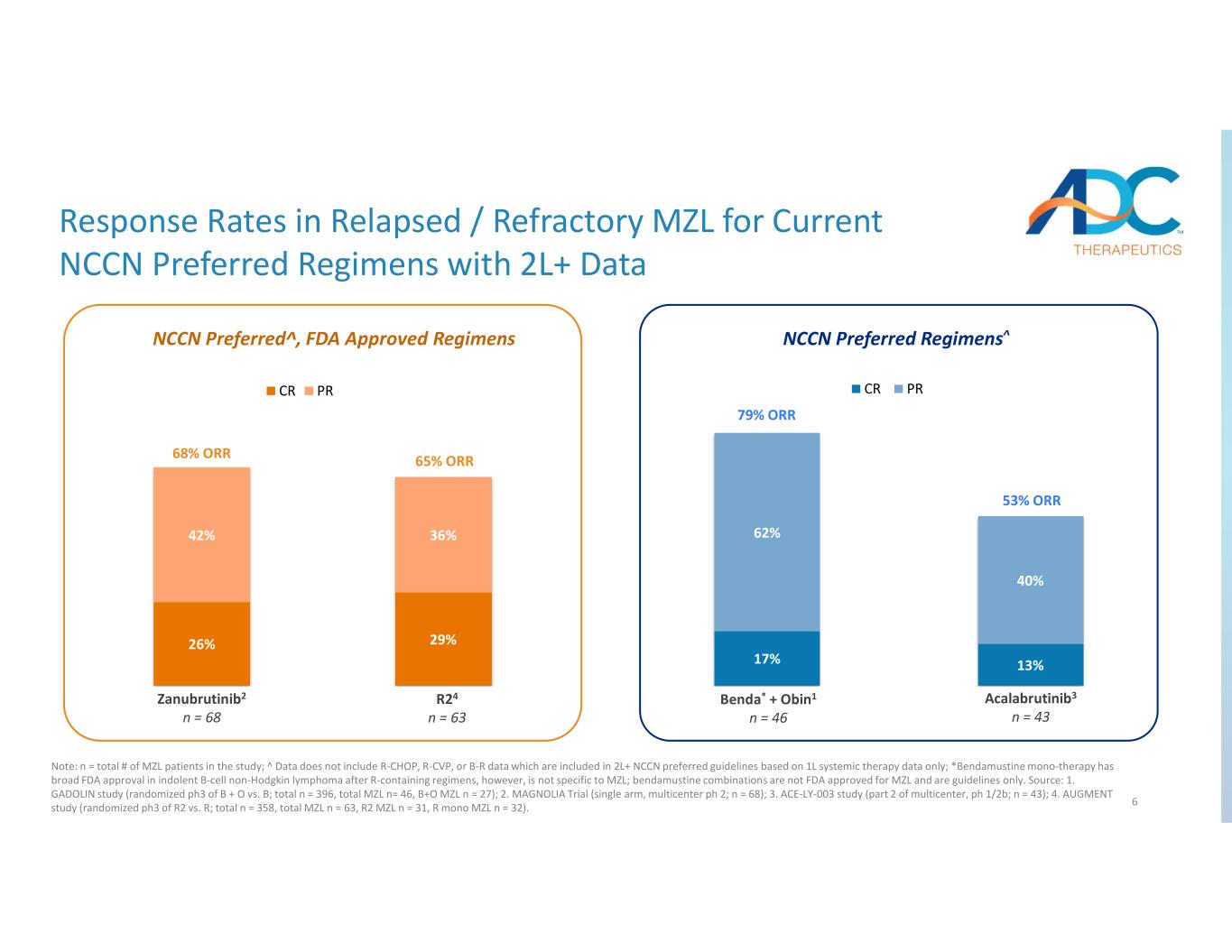
6 17% 13% 62% 40% Benda + Obin1* n=46 Acalabrutinib4 n=43 CR PR 26% 29% 42% 36% Zanubrutinib2 n=68 R25 n=63 CR PR NCCN Preferred^, FDA Approved Regimens NCCN Preferred Regimens^ 68% ORR 65% ORR 79% ORR 53% ORR Zanu r tinib2 n 68 24 n 63 Be * + Obin1 n 46 Acalabrutinib3 n 43 Response Rates in Relapsed / Refractory MZL for Current NCCN Preferred Regimens with 2L+ Data Note: n = total # of MZL patients in the study; ^ Data does not include R-CHOP, R-CVP, or B-R data which are included in 2L+ NCCN preferred guidelines based on 1L systemic therapy data only; *Bendamustine mono-therapy has broad FDA approval in indolent B-cell non-Hodgkin lymphoma after R-containing regimens, however, is not specific to MZL; bendamustine combinations are not FDA approved for MZL and are guidelines only. Source: 1. GADOLIN study (randomized ph3 of B + O vs. B; total n = 396, total MZL n= 46, B+O MZL n = 27); 2. MAGNOLIA Trial (single arm, multicenter ph 2; n = 68); 3. ACE-LY-003 study (part 2 of multicenter, ph 1/2b; n = 43); 4. AUGMENT study (randomized ph3 of R2 vs. R; total n = 358, total MZL n = 63, R2 MZL n = 31, R mono MZL n = 32).

7 A Phase 2, Open-label, Study Evaluating Safety and Efficacy of Loncastuximab in R/R MZL Source: 1. UTX-TGR-205 Study (two single-arm cohorts; total n = 710, total MZL n = 69). Clinical Design R/R MZL Previously Treated with ≥ 1 Line of Systemic Therapy Loncastuximab 150 μg / kg (Day 1, +/- 3) CT / MRI or FDG- PET / CT based on PET Avidity at screening Loncastuximab 75 μg / kg (Day 1 +/-3 q 21 days) CT / MRI or FDG- PET / CT Bone Marrow CT / MRI or FDG- PET / CT based on PET Avidity at screening Cycles 1 – 2 Cycles 3 – 6 30 Days End of Study / Follow-up Confirmation by bone marrow CR by Imaging No CR by Imaging Study Overview The null hypothesis is H0: P≤P0, and the alternative hypothesis is Ha: P≥P1 The best reported CR rate for relapsed MZL using single agent at study initiation was reported with umbralisib - 16% in 69 patients treated1 Based on this estimate, under the null hypothesis, we assumed the CR rate (P) to be 16% (P0) Expected a 15% increase in the CR rate, resulting in CR rate of 31% or greater. Thus, under the alternative hypothesis, the CR rate for the proposed treatment is 31% (P1) A multi-institutional study of 50 patients with r/r MZL after previous systemic treatment (clinicaltrials.gov ID: NCT05296070) 2 sites currently enrolling, expanding to 5 sites Futility analysis after 20 patients Study Rationale
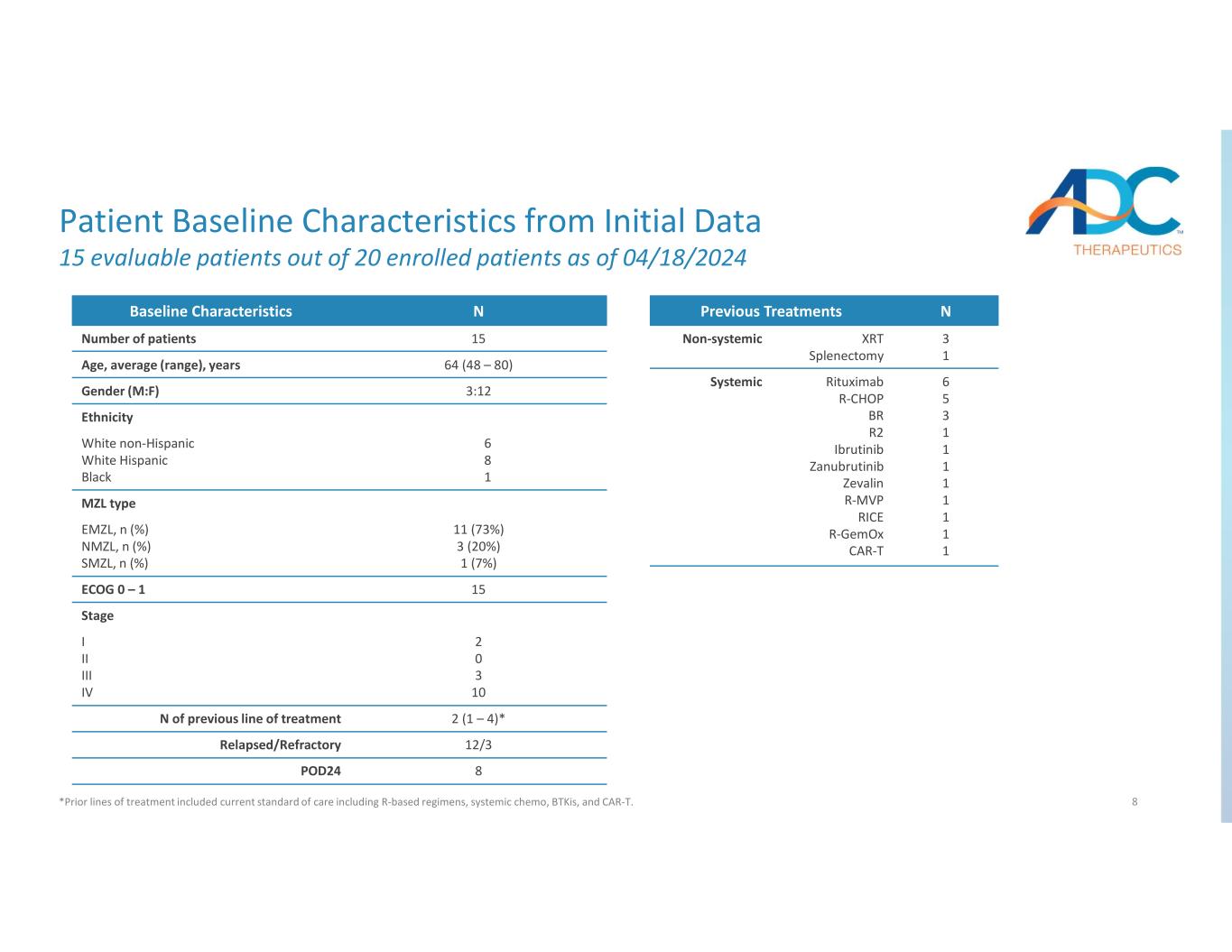
8 Patient Baseline Characteristics from Initial Data 15 evaluable patients out of 20 enrolled patients as of 04/18/2024 *Prior lines of treatment included current standard of care including R-based regimens, systemic chemo, BTKis, and CAR-T. NBaseline Characteristics 15Number of patients 64 (48 – 80)Age, average (range), years 3:12Gender (M:F) Ethnicity 6 8 1 White non-Hispanic White Hispanic Black MZL type 11 (73%) 3 (20%) 1 (7%) EMZL, n (%) NMZL, n (%) SMZL, n (%) 15ECOG 0 – 1 Stage 2 0 3 10 I II III IV 2 (1 – 4)*N of previous line of treatment 12/3Relapsed/Refractory 8POD24 NPrevious Treatments 3 1 XRT Splenectomy Non-systemic 6 5 3 1 1 1 1 1 1 1 1 Rituximab R-CHOP BR R2 Ibrutinib Zanubrutinib Zevalin R-MVP RICE R-GemOx CAR-T Systemic
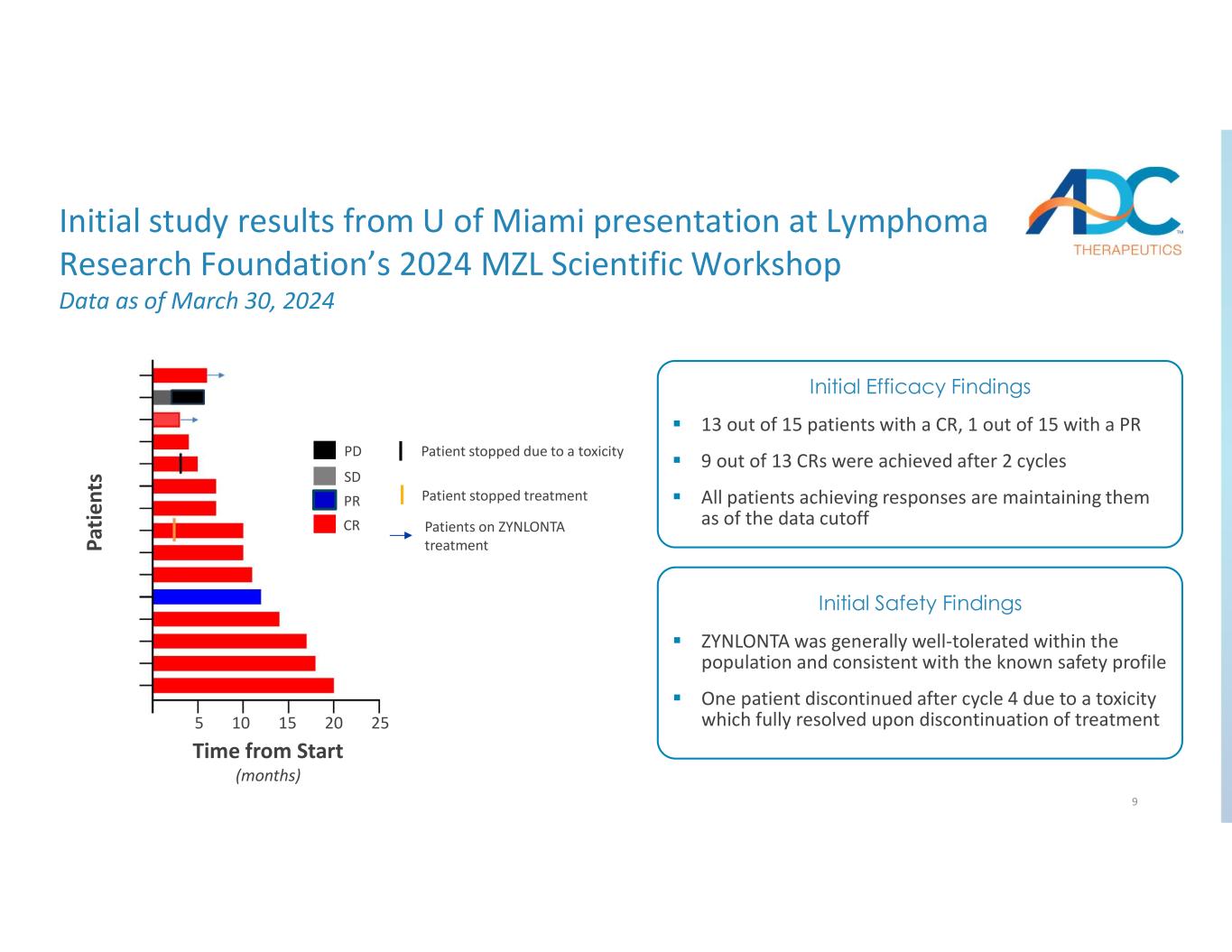
9 PR CR PD SD Patients on ZYNLONTA treatment Patient stopped treatment Patient stopped due to a toxicity 13 out of 15 patients with a CR, 1 out of 15 with a PR 9 out of 13 CRs were achieved after 2 cycles All patients achieving responses are maintaining them as of the data cutoff Initial Efficacy Findings ZYNLONTA was generally well-tolerated within the population and consistent with the known safety profile One patient discontinued after cycle 4 due to a toxicity which fully resolved upon discontinuation of treatment Initial Safety Findings Initial study results from U of Miami presentation at Lymphoma Research Foundation’s 2024 MZL Scientific Workshop Data as of March 30, 2024 Pa tie nt s Time from Start (months) 5 10 15 20 25
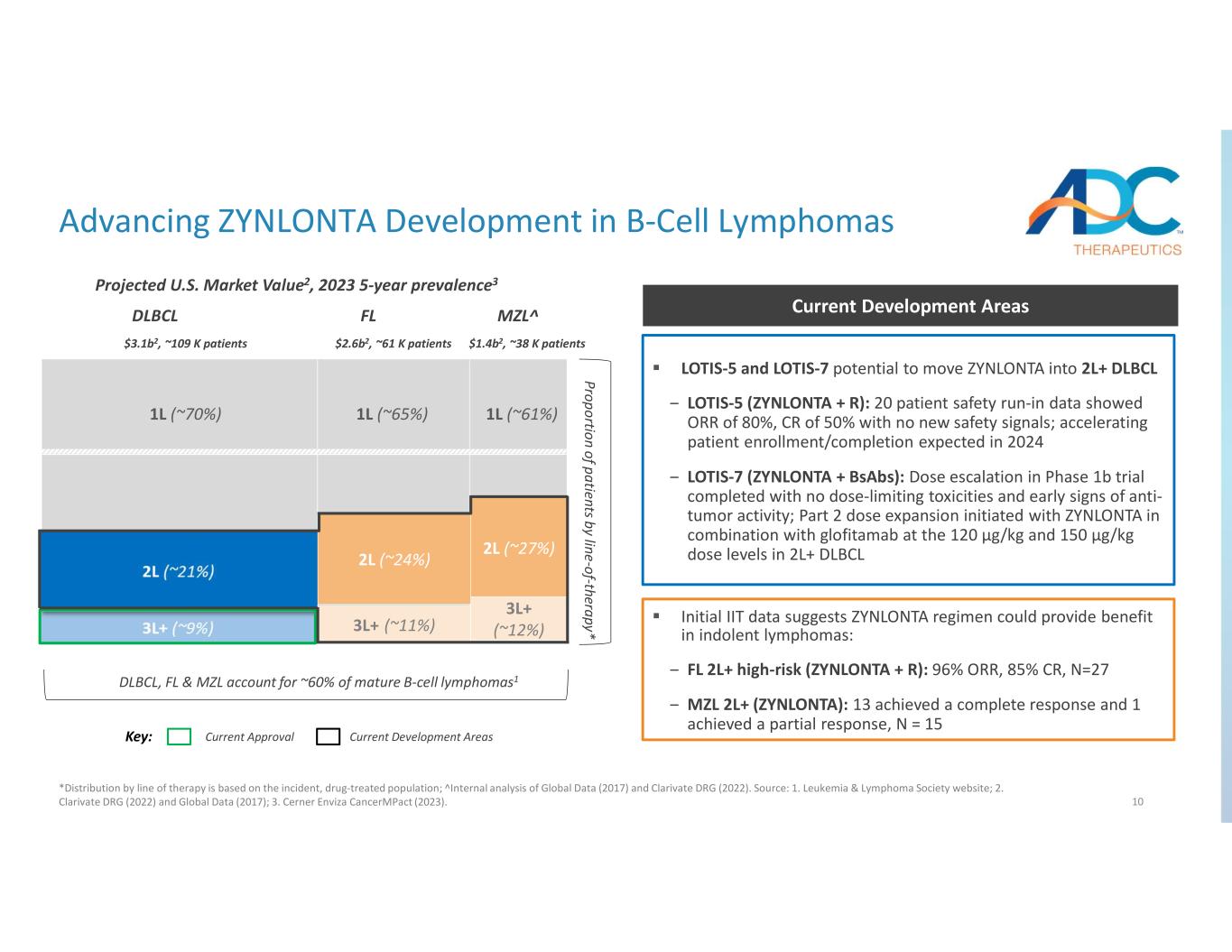
10 Proportion of patients by line-of-therapy* DLBCL, FL & MZL account for ~60% of mature B-cell lymphomas1 1L (~70%) 1L (~65%) 1L (~61%) MZL^FLDLBCL Key: Current Approval Current Development Areas Advancing ZYNLONTA Development in B-Cell Lymphomas *Distribution by line of therapy is based on the incident, drug-treated population; ̂ Internal analysis of Global Data (2017) and Clarivate DRG (2022). Source: 1. Leukemia & Lymphoma Society website; 2. Clarivate DRG (2022) and Global Data (2017); 3. Cerner Enviza CancerMPact (2023). 2L (~24%) 2L (~27%) 3L+ (~11%) 3L+ (~12%) Projected U.S. Market Value2, 2023 5-year prevalence3 LOTIS-5 and LOTIS-7 potential to move ZYNLONTA into 2L+ DLBCL ‒ LOTIS-5 (ZYNLONTA + R): 20 patient safety run-in data showed ORR of 80%, CR of 50% with no new safety signals; accelerating patient enrollment/completion expected in 2024 ‒ LOTIS-7 (ZYNLONTA + BsAbs): Dose escalation in Phase 1b trial completed with no dose-limiting toxicities and early signs of anti- tumor activity; Part 2 dose expansion initiated with ZYNLONTA in combination with glofitamab at the 120 µg/kg and 150 µg/kg dose levels in 2L+ DLBCL Current Development Areas Initial IIT data suggests ZYNLONTA regimen could provide benefit in indolent lymphomas: ‒ FL 2L+ high-risk (ZYNLONTA + R): 96% ORR, 85% CR, N=27 ‒ MZL 2L+ (ZYNLONTA): 13 achieved a complete response and 1 achieved a partial response, N = 15 $3.1b2, ~109 K patients $2.6b2, ~61 K patients $1.4b2, ~38 K patients
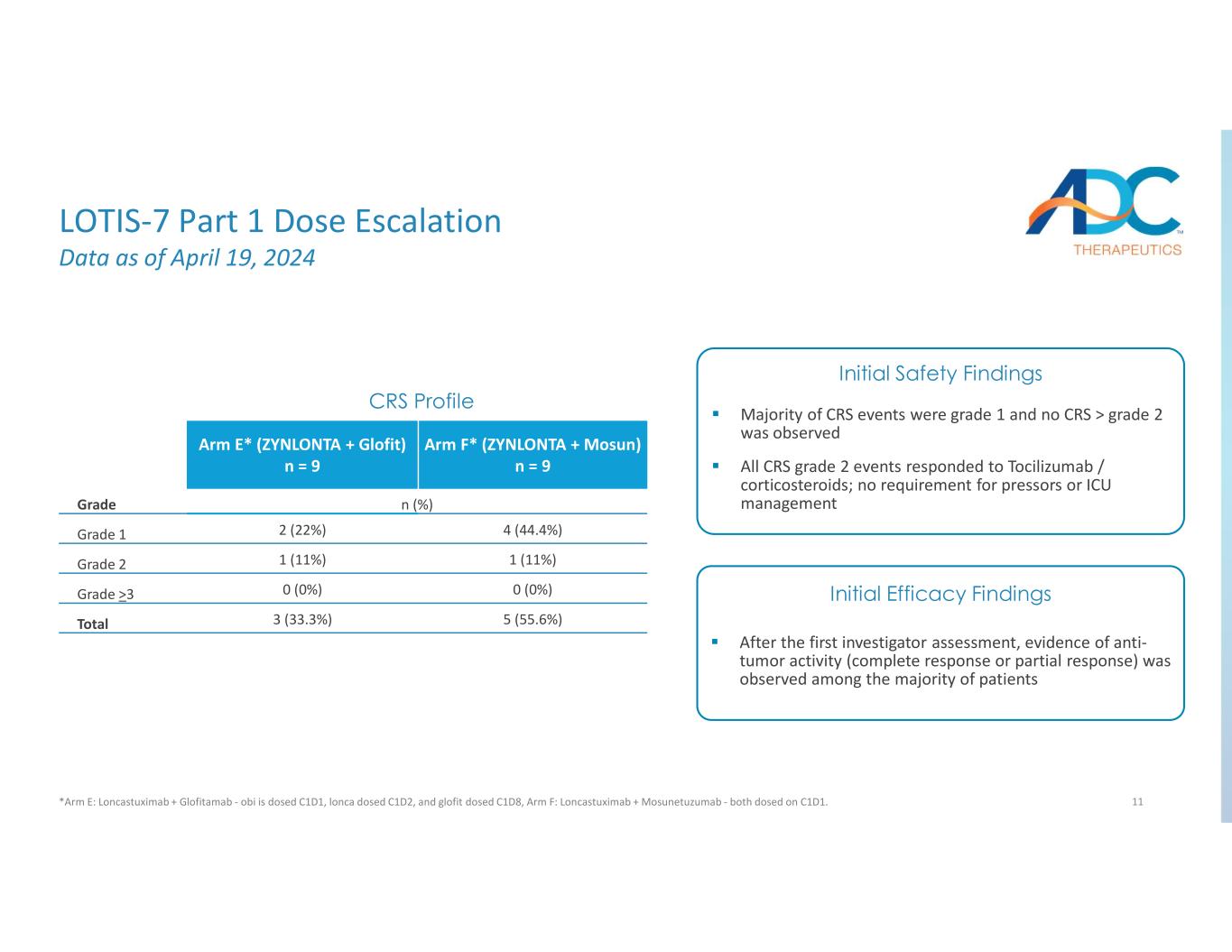
11 Arm F* (ZYNLONTA + Mosun) n = 9 Arm E* (ZYNLONTA + Glofit) n = 9 n (%)Grade 4 (44.4%)2 (22%)Grade 1 1 (11%)1 (11%)Grade 2 0 (0%)0 (0%)Grade >3 5 (55.6%)3 (33.3%)Total CRS Profile After the first investigator assessment, evidence of anti- tumor activity (complete response or partial response) was observed among the majority of patients Majority of CRS events were grade 1 and no CRS > grade 2 was observed All CRS grade 2 events responded to Tocilizumab / corticosteroids; no requirement for pressors or ICU management Initial Safety Findings Initial Efficacy Findings LOTIS-7 Part 1 Dose Escalation Data as of April 19, 2024 *Arm E: Loncastuximab + Glofitamab - obi is dosed C1D1, lonca dosed C1D2, and glofit dosed C1D8, Arm F: Loncastuximab + Mosunetuzumab - both dosed on C1D1.
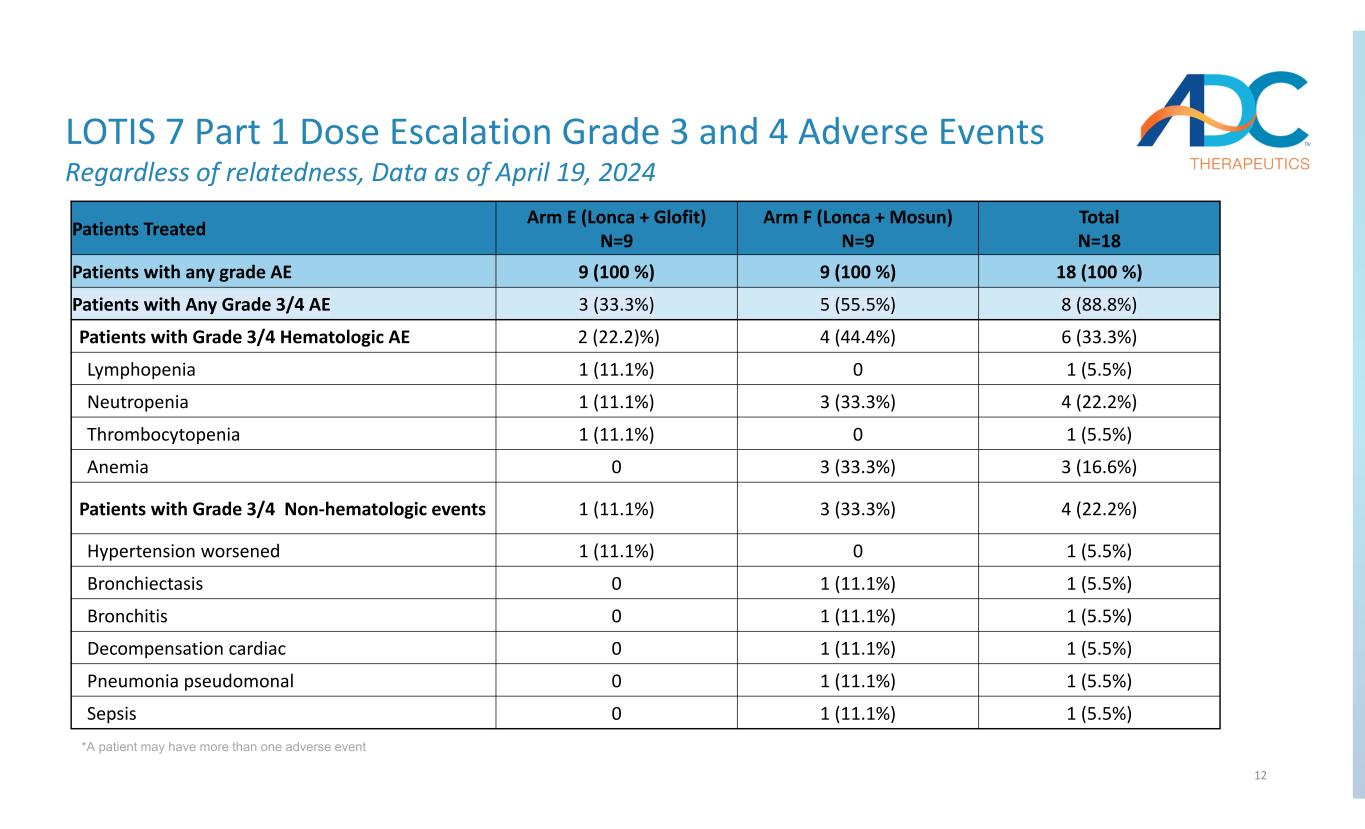
12 LOTIS 7 Part 1 Dose Escalation Grade 3 and 4 Adverse Events Regardless of relatedness, Data as of April 19, 2024 *A patient may have more than one adverse event Total N=18 Arm F (Lonca + Mosun) N=9 Arm E (Lonca + Glofit) N=9 Patients Treated 18 (100 %) 9 (100 %) 9 (100 %) Patients with any grade AE 8 (88.8%)5 (55.5%)3 (33.3%)Patients with Any Grade 3/4 AE 6 (33.3%)4 (44.4%) 2 (22.2)%)Patients with Grade 3/4 Hematologic AE 1 (5.5%)01 (11.1%)Lymphopenia 4 (22.2%)3 (33.3%)1 (11.1%)Neutropenia 1 (5.5%)01 (11.1%)Thrombocytopenia 3 (16.6%)3 (33.3%)0Anemia 4 (22.2%)3 (33.3%)1 (11.1%)Patients with Grade 3/4 Non‐hematologic events 1 (5.5%)01 (11.1%)Hypertension worsened 1 (5.5%)1 (11.1%)0Bronchiectasis 1 (5.5%)1 (11.1%)0Bronchitis 1 (5.5%)1 (11.1%)0Decompensation cardiac 1 (5.5%)1 (11.1%)0Pneumonia pseudomonal 1 (5.5%)1 (11.1%)0Sepsis
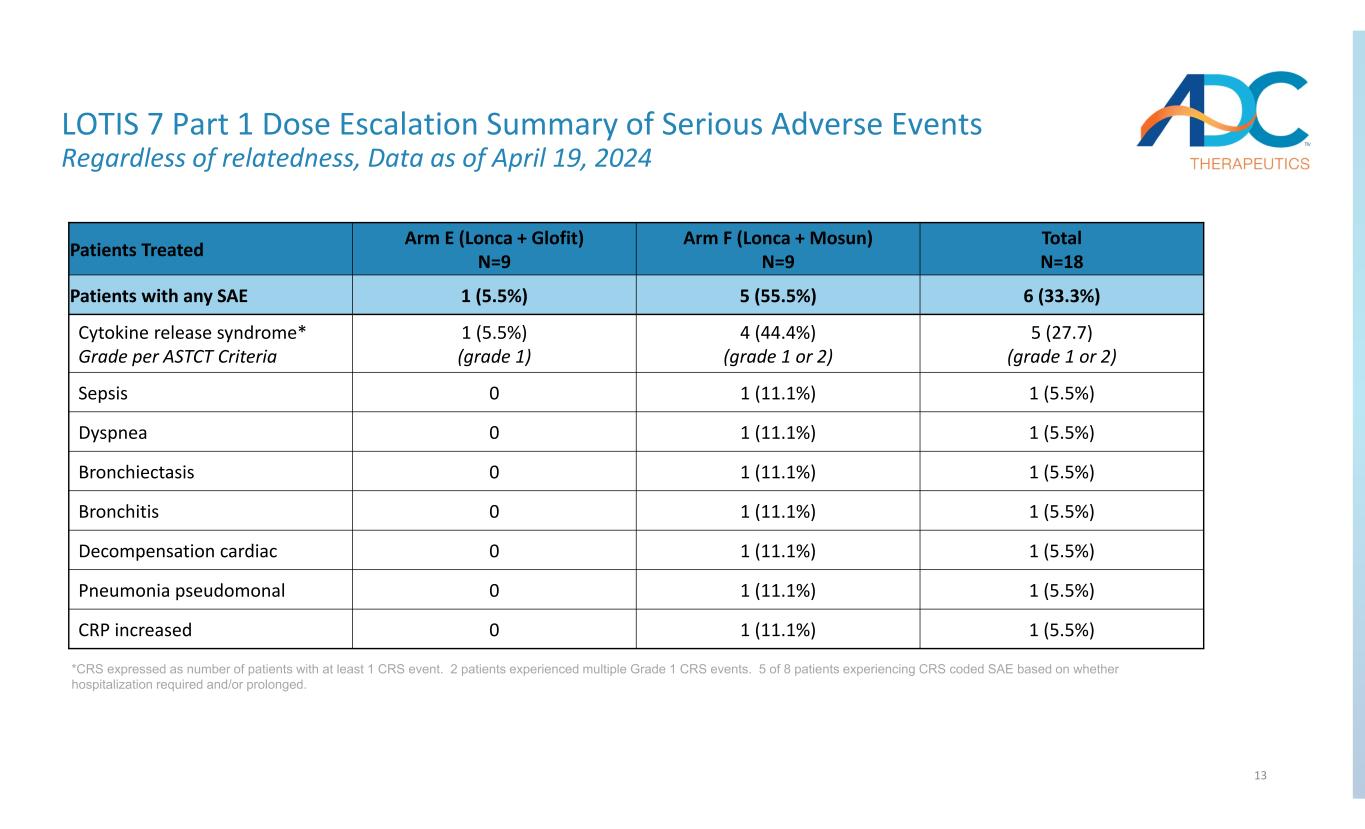
13 LOTIS 7 Part 1 Dose Escalation Summary of Serious Adverse Events Regardless of relatedness, Data as of April 19, 2024 *CRS expressed as number of patients with at least 1 CRS event. 2 patients experienced multiple Grade 1 CRS events. 5 of 8 patients experiencing CRS coded SAE based on whether hospitalization required and/or prolonged. Total N=18 Arm F (Lonca + Mosun) N=9 Arm E (Lonca + Glofit) N=9 Patients Treated 6 (33.3%)5 (55.5%)1 (5.5%)Patients with any SAE 5 (27.7) (grade 1 or 2) 4 (44.4%) (grade 1 or 2) 1 (5.5%) (grade 1) Cytokine release syndrome* Grade per ASTCT Criteria 1 (5.5%)1 (11.1%)0Sepsis 1 (5.5%)1 (11.1%)0Dyspnea 1 (5.5%)1 (11.1%)0Bronchiectasis 1 (5.5%)1 (11.1%)0Bronchitis 1 (5.5%)1 (11.1%)0Decompensation cardiac 1 (5.5%)1 (11.1%)0Pneumonia pseudomonal 1 (5.5%)1 (11.1%)0CRP increased
v3.24.1.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
ADC Therapeutics (NYSE:ADCT)
過去 株価チャート
から 4 2024 まで 5 2024
ADC Therapeutics (NYSE:ADCT)
過去 株価チャート
から 5 2023 まで 5 2024