FALSE000182429300018242932024-05-132024-05-13
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549 FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 13, 2024
GRI BIO, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | | | | |
| Delaware | | 001-40034 | | 82-4369909 |
| (State or other jurisdiction | | (Commission File Number) | | (IRS Employer Identification No.) |
| of incorporation) | | | | |
2223 Avenida de la Playa, #208
La Jolla, CA 92037
(Address of principal executive offices and zip code)
(619) 400-1170
(Registrant’s telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, par value $0.0001 per share | GRI | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging Growth Company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On May 13, 2024, GRI Bio, Inc. (the Company) issued a press release announcing its financial results for the quarter ended March 31, 2024, and provided a business update. A copy of this press release is furnished as Exhibit 99.1 to this report and is incorporated herein by reference.
The information in this report, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained herein and in the accompanying Exhibit 99.1 shall not be deemed incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof regardless of any general incorporation language in such filing.
Item 7.01 Regulation FD Disclosure
The information in this Item 7.01, including Exhibit 99.2, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference into any registration statement or other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| | | | | | | | |
| Exhibit No. | | Description |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | | | | |
| Date: May 13, 2024 | | GRI BIO, INC. | |
| | By: | /s/ Leanne Kelly | |
| | Name: | Leanne Kelly | |
| | Title: | Chief Financial Officer | |
GRI Bio Reports First Quarter 2024 Financial Results and Provides Corporate Update
Interim data readout of Phase 2a biomarker study evaluating lead program GRI-0621 for the treatment of Idiopathic Pulmonary Fibrosis (IPF) expected Q3 2024 and topline data on track for Q4 2024
Ongoing progress of Investigational New Drug (IND) enabling studies in systemic lupus erythematosus (SLE) program for GRI-0803 with IND filing expected in H2 2024
LA JOLLA, CA, May 10, 2024 (GLOBE NEWSWIRE) -- GRI Bio, Inc. (NASDAQ: GRI) (“GRI Bio” or the “Company”), a biotechnology company advancing an innovative pipeline of Natural Killer T (NKT) cell modulators for the treatment of inflammatory, fibrotic and autoimmune diseases, today reported its financial results for the first quarter ended March 31, 2024 and provided a corporate update.
"We continue to execute on both the clinical and corporate fronts. Our team is working diligently to drive our Phase 2a biomarker study in IPF toward interim data readout which we believe will provide valuable insight for our development program moving forward,” commented Marc Hertz, PhD, Chief Executive Officer of GRI Bio. “On the corporate side, we continue to bolster our patent estate as well as actively engage with and present at scientific meetings which we believe underscore the potential of GRI-0621. We are committed to building a growing body of data and believe we are positioned to unlock significant value in 2024.”
Recent Highlights
•Announced oral presentation at the 14th International Congress on Autoimmunity;
•Expanded intellectual property protection for proprietary NKT cell modulators with issuance of Korea patent;
•Announced abstract titled, “Altered NKT Cell Populations in the Airways of Patients with IPF,” has been accepted for poster presentation at the 2024 American Thoracic Society International Conference;
•Received notice of allowance for Canadian patent covering proprietary NKT cell modulators;
•Received authorization of the Company’s Clinical Trial Application (CTA) by the United Kingdom (UK) Medicines and Healthcare Products Regulatory Agency (MHRA) to initiate a Phase 2a biomarker study evaluating GRI-0621 for the treatment of IPF in the UK; and
•Closed a public offering with participation from healthcare focused institutional investors for aggregate gross proceeds of $5.5 million.
GRI-0621: Type 1 invariant NKT (iNKT) antagonist in development for the treatment of IPF
IPF is a rare chronic progressive pulmonary disease with abnormal scarring of the lung blocking the movement of oxygen into the bloodstream. Currently available treatments for IPF are limited
with only two approved drugs that come with significant side-effects, limited compliance and no impact on survival1.
GRI Bio’s lead program, GRI-0621, is a small molecule RAR-βɣ dual agonist that inhibits the activity of human iNKT cells. In preliminary trials to date and previous trials with the oral formulation, GRI-0621 has been shown to improve fibrosis in multiple disease models and improve liver function tests and other markers of inflammation and injury in patients.
The Company plans to leverage the 505(b)(2) regulatory pathway and has launched a Phase 2a biomarker study evaluating GRI-0621 for the treatment of IPF. For more information about the Phase 2a study, please visit clinicaltrials.gov and reference identifier NCT06331624.
Expected GRI-0621 Upcoming Milestones
•Q3 2024: Report interim data from Phase 2a biomarker study
•Q4 2024: Report topline results from Phase 2a biomarker study
GRI-0803: Novel activator of human type 2 NKT cells in development for the treatment of autoimmune disorders, with an initial focus on SLE
SLE is an autoimmune disease in which the immune system attacks its own tissue and organs. SLE is the most common form of lupus. Current treatments are limited, consisting primarily of immunosuppressive therapies.
GRI Bio’s second asset in development, GRI-0803, is a novel activator of human type 2 NKT cells. Activation of type 2 NKT leads to a dendritic cell-mediated inhibition of iNKT cells. In the Company’s preclinical studies, type 2 NKT activating molecules, GRI-0803 and GRI-0124, were observed to inhibit both murine and human iNKT cells. Oral administration of these type 2 NKT activating molecules was observed to inhibit lupus nephritis and to significantly improve overall survival.
Expected GRI-0803 Upcoming Milestones
•Q3 2024: Complete IND-enabling studies
•Q3 2024: File IND and launch Phase 1a/b
•Q4 2024: Report Phase 1a single ascending dose (SAD) study topline results
•Q4 2024: Report Phase 1b multiple ascending dose (MAD) study topline results
Summary of Financial Results for First Quarter 2024
Net loss was $1.9 million for the three months ended March 31, 2024.
Research and development expenses were $0.9 million and $0.1 million for the three months ended March 31, 2024 and 2023, respectively.
General and administrative expenses were $1.0 million and $0.9 million for the three months ended March 31, 2024 and 2023, respectively.
As of March 31, 2024, the Company had cash and cash equivalents of approximately $4.1 million. In February 2024, the Company closed a public offering with participation from healthcare focused institutional investors for aggregate gross proceeds of $5.5 million. Based on
1 T. M. Maher et al., Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res 22, 197 (2021)
the Company’s current operating plan, the Company believes that its existing cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements into the third quarter of 2024.
About GRI Bio, Inc.
GRI Bio is a clinical-stage biopharmaceutical company focused on fundamentally changing the way inflammatory, fibrotic and autoimmune diseases are treated. GRI Bio’s therapies are designed to target the activity of NKT cells, which are key regulators earlier in the inflammatory cascade, to interrupt disease progression and restore the immune system to homeostasis. NKT cells are innate-like T cells that share properties of both NK and T cells and are a functional link between the innate and adaptive immune responses. iNKT cells play a critical role in propagating the injury, inflammatory response, and fibrosis observed in inflammatory and fibrotic indications. GRI Bio’s lead program, GRI-0621, is an inhibitor of iNKT cell activity and is being developed as a novel oral therapeutic for the treatment of IPF, a serious disease with significant unmet need. The Company is also developing a pipeline of novel type 2 NKT agonists for the treatment of SLE. Additionally, with a library of over 500 proprietary compounds, GRI Bio has the ability to fuel a growing pipeline.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions. These forward-looking statements are based on the Company’s current beliefs and expectations. Forward-looking statements include, but are not limited to, statements regarding: the Company’s expectations with respect to development and commercialization of the Company’s product candidates, the timing of initiation or completion of clinical trials and availability of resulting data, the potential benefits and impact of the Company’s clinical trials and product candidates and any implication that the data or results observed in preclinical trials or earlier studies or trials will be indicative of results of later studies or clinical trials, the Company’s beliefs and expectations regarding potential stakeholder value and future financial performance, the Company’s beliefs about the timing and outcome of regulatory approvals and potential regulatory approval pathways, the Company’s expected milestones for 2024, and the Company’s beliefs and expectations regarding the sufficiency of its existing cash and cash equivalents to fund its operating expenses and capital expenditure requirements. Actual results may differ from the forward-looking statements expressed by the Company in this press release and consequently, you should not rely on these forward-looking statements as predictions of future events. These forward-looking statements are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including, without limitation: (1) the inability to maintain the listing of the Company’s common stock on Nasdaq and to comply with applicable listing requirements; (2) changes in applicable laws or regulations; (3) the inability of the Company to raise financing in the future; (4) the success, cost and timing of the Company’s product development activities; (5) the inability of the Company to obtain and maintain regulatory clearance or approval for its respective products, and any related restrictions and limitations of any cleared or approved product; (6) the inability of the Company to identify, in-license or acquire additional technology; (7) the inability of the Company to compete with other companies
currently marketing or engaged in the development of products and services that the Company is currently developing; (8) the size and growth potential of the markets for the Company’s products and services, and their respective ability to serve those markets, either alone or in partnership with others; (9) the failure to achieve any milestones or receive any milestone payments under any agreements; (10) inaccuracy in the Company’s estimates regarding expenses, future revenue, capital requirements and needs for and the ability to obtain additional financing; (11) the Company’s ability to protect and enforce its intellectual property portfolio, including any newly issued patents; and (12) other risks and uncertainties indicated from time to time in the Company’s filings with the U.S. Securities and Exchange Commission (the “SEC”), including the risks and uncertainties described in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K filed with the SEC on March 28, 2024 and subsequently filed reports. Forward-looking statements contained in this announcement are made as of this date, and the Company undertakes no duty to update such information except as required under applicable law.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
GRI@jtcir.com

A New Approach to Inflammatory Diseases NASDAQ: GRI | gribio.com May 2024 Corporate Presentation

Forward Looking Statements This presentation is for informational purposes only and is not an offer to sell or a solicitation of an offer to buy any securities of GRI Bio, Inc. (“GRI” or the “Company”). This presentation contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions. These forward-looking statements are based on the Company’s current beliefs and expectations. Forward-looking statements include, but are not limited to, statements regarding: the Company’s expectations with respect to development and commercialization of the Company’s product candidates, the timing of initiation or completion of clinical trials and availability of resulting data, the potential benefits and impact of the Company’s clinical trials and product candidates and any implication that the data or results observed in preclinical trials or earlier studies or trials will be indicative of results of later studies or clinical trials, the Company’s beliefs and expectations regarding potential stakeholder value and future financial performance, the Company’s beliefs about the timing and outcome of regulatory approvals and potential regulatory approval pathways, the Company’s expected milestones for 2024, and the Company’s beliefs and expectations regarding the sufficiency of its existing cash and cash equivalents to fund its operating expenses and capital expenditure requirements. Actual results may differ from the forward-looking statements expressed by the Company in this press release and consequently, you should not rely on these forward-looking statements as predictions of future events. These forward-looking statements are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including, without limitation: (1) the inability to maintain the listing of the Company’s common stock on Nasdaq and to comply with applicable listing requirements; (2) changes in applicable laws or regulations; (3) the inability of the Company to raise financing in the future; (4) the success, cost and timing of the Company’s product development activities; (5) the inability of the Company to obtain and maintain regulatory clearance or approval for its respective products, and any related restrictions and limitations of any cleared or approved product; (6) the inability of the Company to identify, in-license or acquire additional technology; (7) the inability of the Company to compete with other companies currently marketing or engaged in the development of products and services that the Company is currently developing; (8) the size and growth potential of the markets for the Company’s products and services, and their respective ability to serve those markets, either alone or in partnership with others; (9) the failure to achieve any milestones or receive any milestone payments under any agreements; (10) inaccuracy in the Company’s estimates regarding expenses, future revenue, capital requirements and needs for and the ability to obtain additional financing; (11) the Company’s ability to protect and enforce its intellectual property portfolio, including any newly issued patents; and (12) other risks and uncertainties indicated from time to time in the Company’s filings with the U.S. Securities and Exchange Commission (the “SEC”), including the risks and uncertainties described in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K filed with the SEC on March 28, 2024 and subsequently filed reports. Forward-looking statements contained in this announcement are made as of this date, and the Company undertakes no duty to update such information except as required under applicable law. This presentation includes information and statistics regarding market participants in the sectors in which GRI competes and other industry data which was obtained from third-party sources, including reports by market research firms and company filings. None of the information provided by third-party sources has been independently verified. This presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. The use or display of any third-party’s trademarks, service marks, trade names or products in this presentation is not intended to, and does not imply, a relationship with GRI, or an endorsement or sponsorship by GRI. Solely for convenience, the trademarks, service marks and trade names referred to in this presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that GRI will not assert, to the fullest extent under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade names. 2

Highlights Advancing an Innovative Pipeline of NKT Cell Modulators for the Treatment of Inflammatory, Fibrotic and Autoimmune Diseases Encouraging Preclinical Data Observed to Date on Par with OFEV® (nintedanib), a Leading Tyrosine Kinase Inhibitor with 2025 Projected Sales of $5 Billion3 3 1. Sharif, R. (2017). Overview of Idiopathic Pulmonary Fibrosis (IPF) and Evidence-Based Guidelines. Am J Manag Care, 23(11), 176–182 2. https://www.cdc.gov/lupus/facts/detailed.html 3. Projected sales per Evaluate Consensus NKT Science Innovative Small Molecules High-Value Indications Leveraging Natural Killer T (NKT) regulation to target earlier in the inflammatory cascade to interrupt disease progression Small molecule drugs that act like cell therapy Provides favorable economics in manufacturing and dosing ~100K People in the US1 Idiopathic Pulmonary Fibrosis ~160K People in the US2 Systemic Lupus Erythematosus
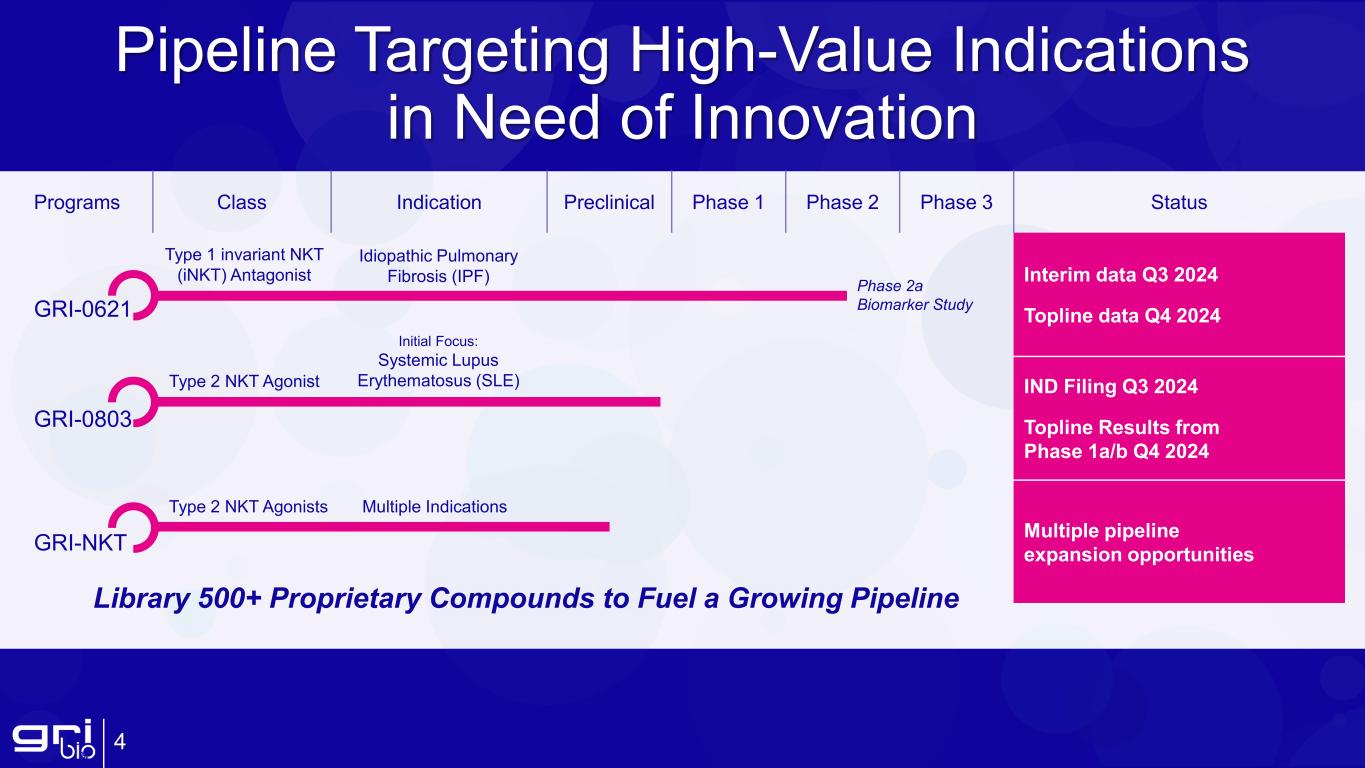
Programs Class Indication Preclinical Phase 1 Phase 2 Phase 3 Status GRI-0621 Interim data Q3 2024 Topline data Q4 2024 GRI-0803 IND Filing Q3 2024 Topline Results from Phase 1a/b Q4 2024 GRI-NKT Multiple pipeline expansion opportunities Pipeline Targeting High-Value Indications in Need of Innovation Phase 2a Biomarker Study Type 1 invariant NKT (iNKT) Antagonist Idiopathic Pulmonary Fibrosis (IPF) Type 2 NKT Agonist Initial Focus: Systemic Lupus Erythematosus (SLE) Type 2 NKT Agonists Library 500+ Proprietary Compounds to Fuel a Growing Pipeline 4 Multiple Indications
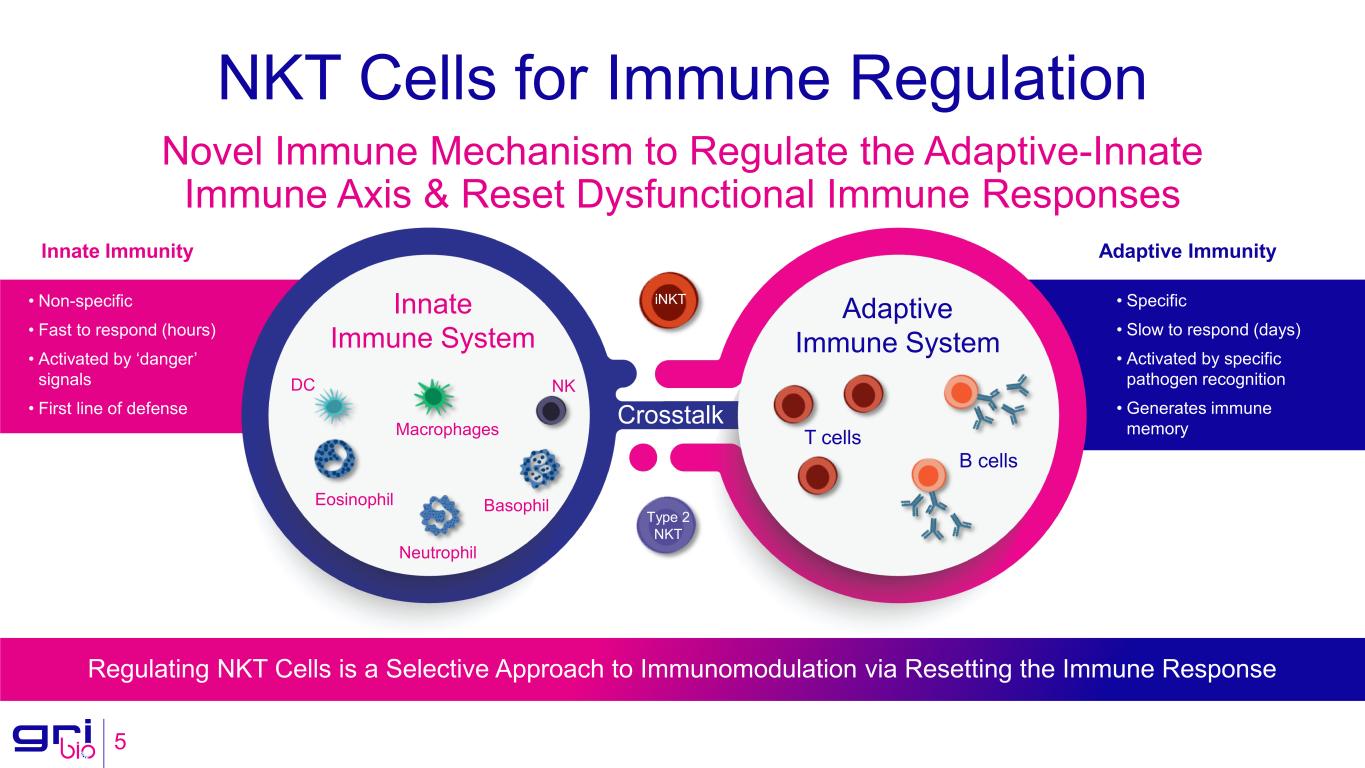
NKT Cells for Immune Regulation 5 Novel Immune Mechanism to Regulate the Adaptive-Innate Immune Axis & Reset Dysfunctional Immune Responses Innate Immune System Adaptive Immune System T cells B cells Type 2 NKT iNKT Macrophages DC Eosinophil Basophil Neutrophil NK Crosstalk Adaptive ImmunityInnate Immunity • Non-specific • Fast to respond (hours) • Activated by ‘danger’ signals • First line of defense • Specific • Slow to respond (days) • Activated by specific pathogen recognition • Generates immune memory Regulating NKT Cells is a Selective Approach to Immunomodulation via Resetting the Immune Response
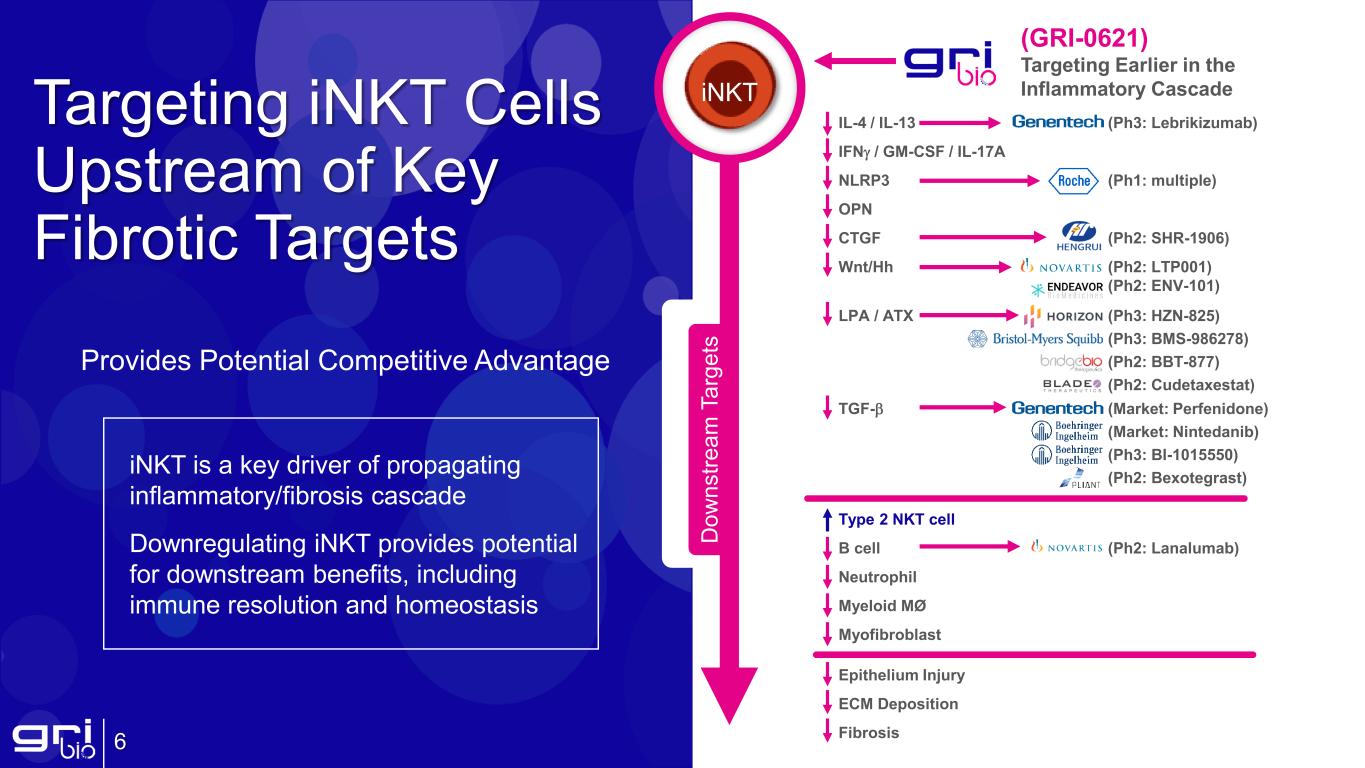
6 Targeting iNKT Cells Upstream of Key Fibrotic Targets iNKT is a key driver of propagating inflammatory/fibrosis cascade Downregulating iNKT provides potential for downstream benefits, including immune resolution and homeostasis Provides Potential Competitive Advantage IL-4 / IL-13 (Ph3: Lebrikizumab) IFNγ / GM-CSF / IL-17A NLRP3 (Ph1: multiple) OPN CTGF (Ph2: SHR-1906) Wnt/Hh (Ph2: LTP001) (Ph2: ENV-101) LPA / ATX (Ph3: HZN-825) (Ph3: BMS-986278) (Ph2: BBT-877) (Ph2: Cudetaxestat) TGF-β (Market: Perfenidone) (Market: Nintedanib) (Ph3: BI-1015550) (Ph2: Bexotegrast) Type 2 NKT cell B cell (Ph2: Lanalumab) Neutrophil Myeloid MØ Myofibroblast Epithelium Injury ECM Deposition Fibrosis (GRI-0621) Targeting Earlier in the Inflammatory Cascade D ow ns tre am T ar ge ts iNKT
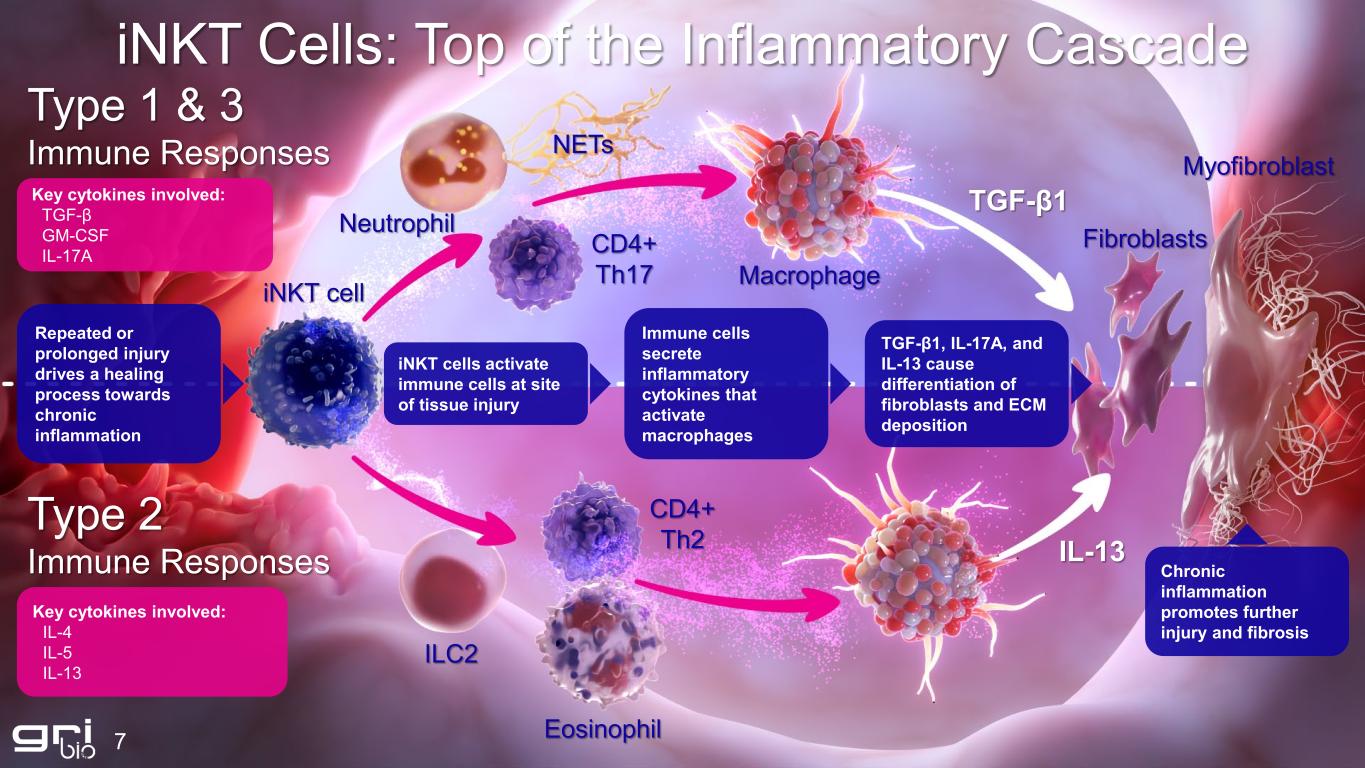
7 iNKT Cells: Top of the Inflammatory Cascade Type 1 & 3 Immune Responses Key cytokines involved: TGF-β GM-CSF IL-17A Type 2 Immune Responses Key cytokines involved: IL-4 IL-5 IL-13 Repeated or prolonged injury drives a healing process towards chronic inflammation iNKT cells activate immune cells at site of tissue injury Immune cells secrete inflammatory cytokines that activate macrophages TGF-β1, IL-17A, and IL-13 cause differentiation of fibroblasts and ECM deposition Chronic inflammation promotes further injury and fibrosis Eosinophil ILC2 CD4+ Th2 iNKT cell Neutrophil NETs CD4+ Th17 Macrophage Fibroblasts Myofibroblast TGF-β1 IL-13
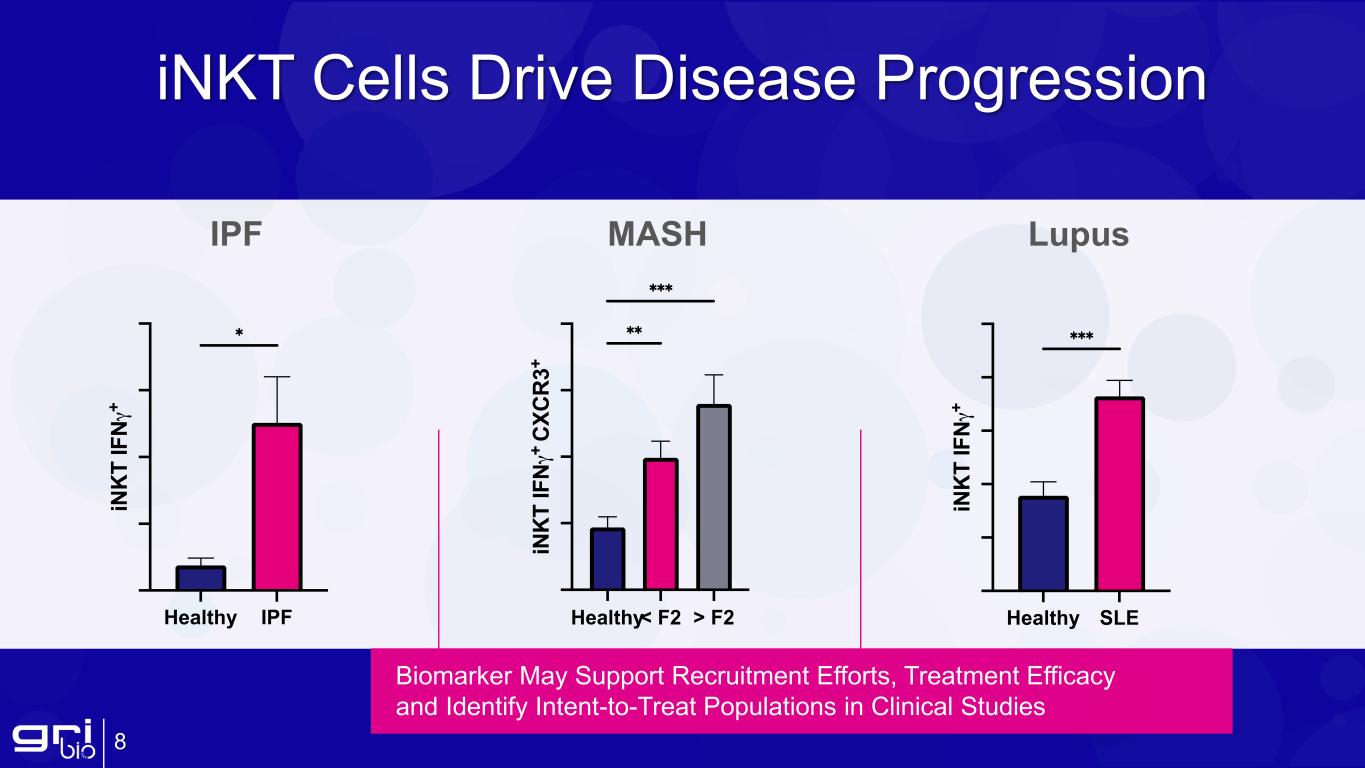
iNKT Cells Drive Disease Progression 8 Biomarker May Support Recruitment Efforts, Treatment Efficacy and Identify Intent-to-Treat Populations in Clinical Studies IPF MASH Lupus
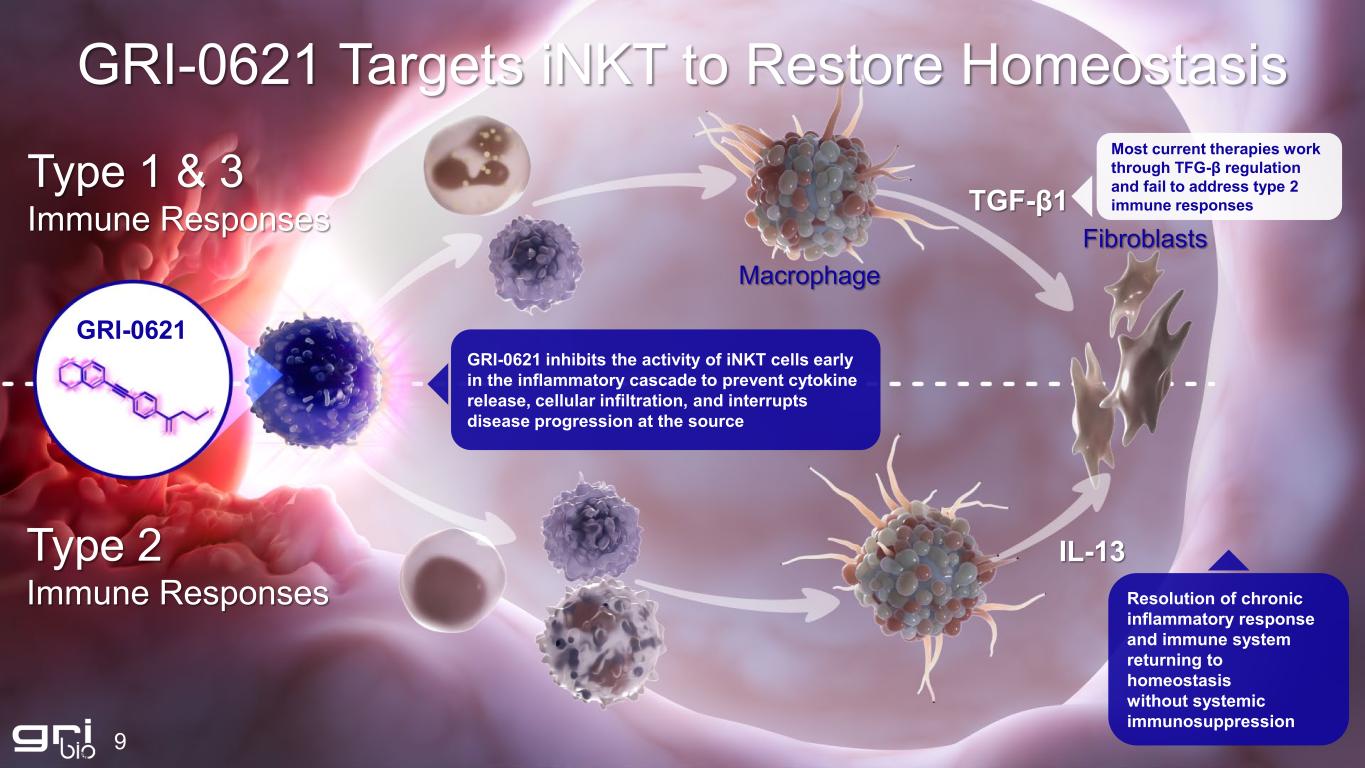
9 GRI-0621 Targets iNKT to Restore Homeostasis Type 1 & 3 Immune Responses Type 2 Immune Responses GRI-0621 inhibits the activity of iNKT cells early in the inflammatory cascade to prevent cytokine release, cellular infiltration, and interrupts disease progression at the source Resolution of chronic inflammatory response and immune system returning to homeostasis without systemic immunosuppression Macrophage Fibroblasts TGF-β1 IL-13 Most current therapies work through TFG-β regulation and fail to address type 2 immune responses GRI-0621

GRI-0621 Idiopathic Pulmonary Fibrosis (IPF) Ongoing Phase 2a biomarker study with interim data expected Q3 2024 and topline data Q4 2024 Leveraging FDA agreed 505(b)(2) regulatory pathway Orphan indication with ~40K newly diagnosed cases annually1 10 1. Sauleda J, Núñez B, Sala E, Soriano JB. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med Sci (Basel). 2018 Nov 29;6(4):110. doi: 10.3390/medsci6040110. PMID: 30501130; PMCID: PMC6313500
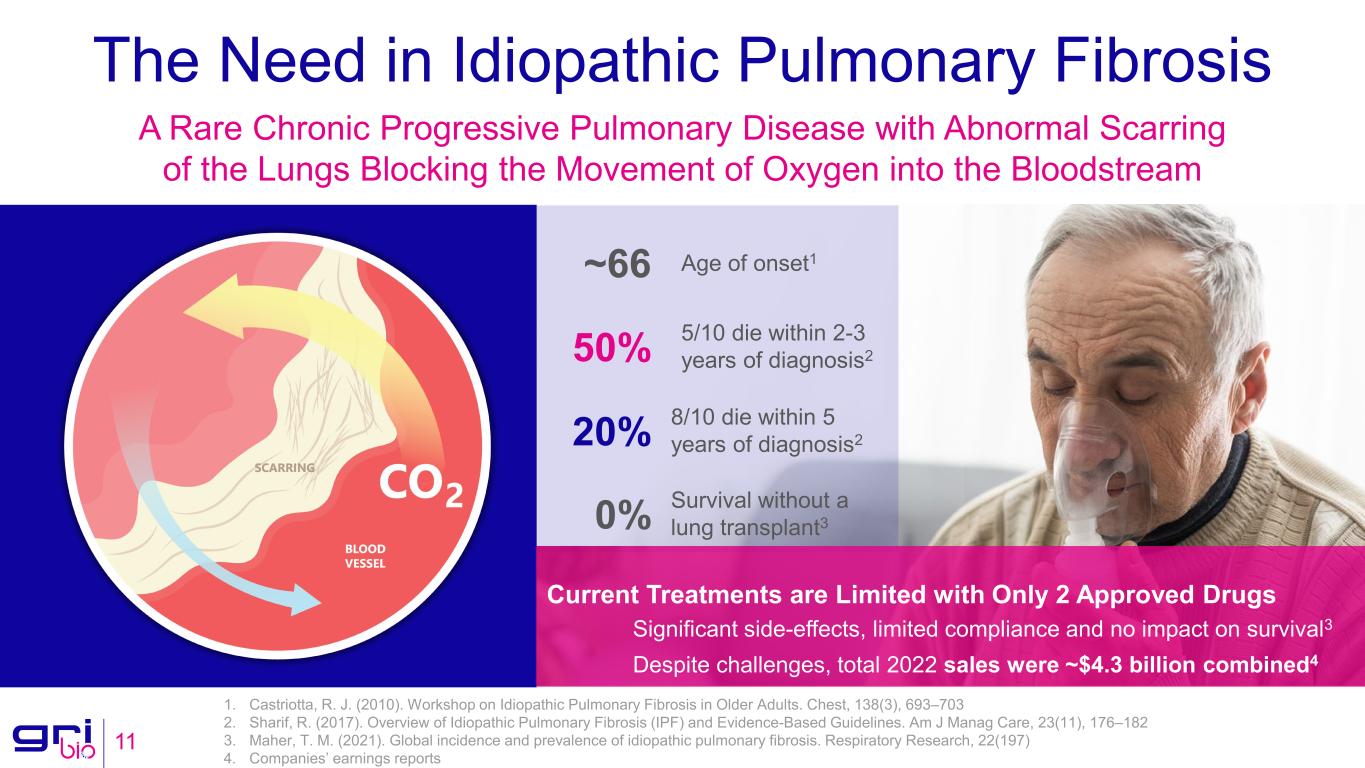
The Need in Idiopathic Pulmonary Fibrosis 11 A Rare Chronic Progressive Pulmonary Disease with Abnormal Scarring of the Lungs Blocking the Movement of Oxygen into the Bloodstream Significant side-effects, limited compliance and no impact on survival3 Despite challenges, total 2022 sales were ~$4.3 billion combined4 Current Treatments are Limited with Only 2 Approved Drugs 8/10 die within 5 years of diagnosis220% Age of onset1~66 5/10 die within 2-3 years of diagnosis250% Survival without a lung transplant30% 1. Castriotta, R. J. (2010). Workshop on Idiopathic Pulmonary Fibrosis in Older Adults. Chest, 138(3), 693–703 2. Sharif, R. (2017). Overview of Idiopathic Pulmonary Fibrosis (IPF) and Evidence-Based Guidelines. Am J Manag Care, 23(11), 176–182 3. Maher, T. M. (2021). Global incidence and prevalence of idiopathic pulmonary fibrosis. Respiratory Research, 22(197) 4. Companies’ earnings reports
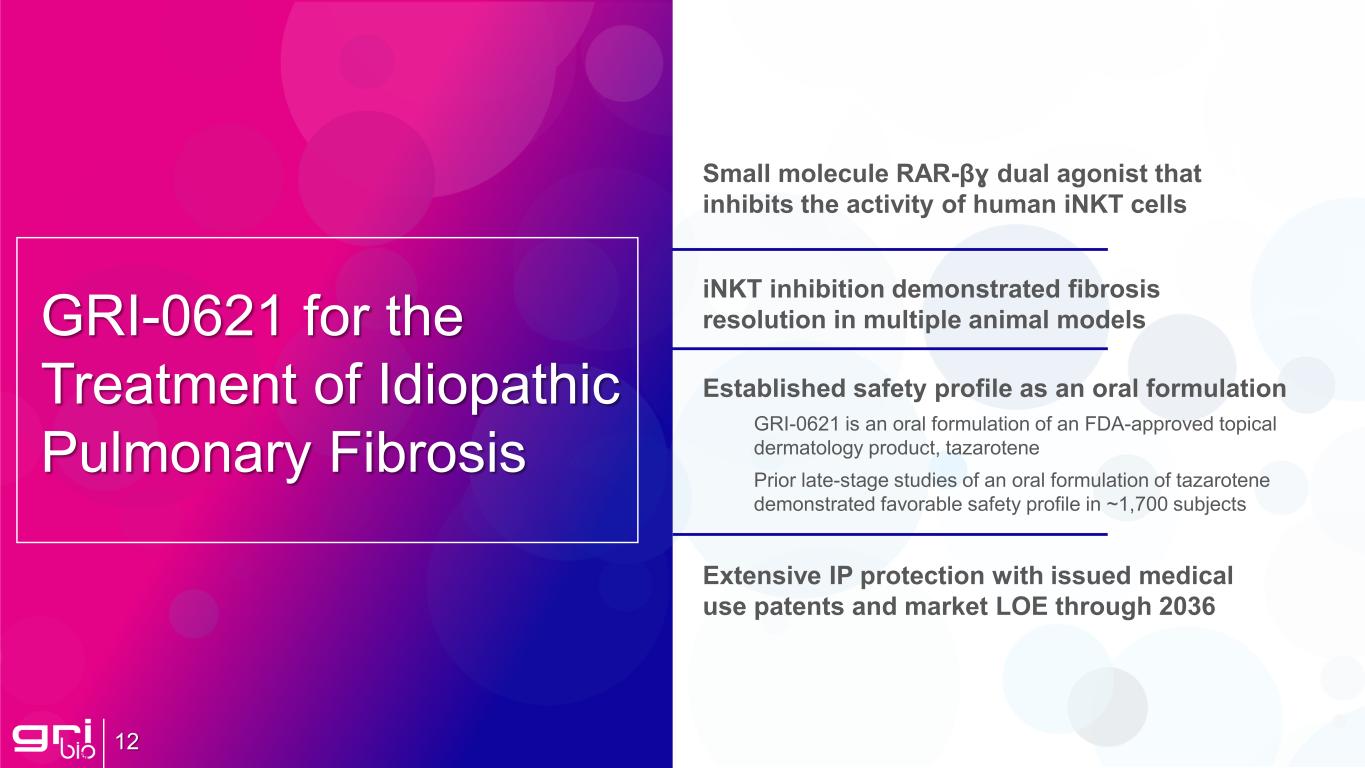
12 GRI-0621 for the Treatment of Idiopathic Pulmonary Fibrosis Established safety profile as an oral formulation GRI-0621 is an oral formulation of an FDA-approved topical dermatology product, tazarotene Prior late-stage studies of an oral formulation of tazarotene demonstrated favorable safety profile in ~1,700 subjects Small molecule RAR-βɣ dual agonist that inhibits the activity of human iNKT cells Extensive IP protection with issued medical use patents and market LOE through 2036 iNKT inhibition demonstrated fibrosis resolution in multiple animal models
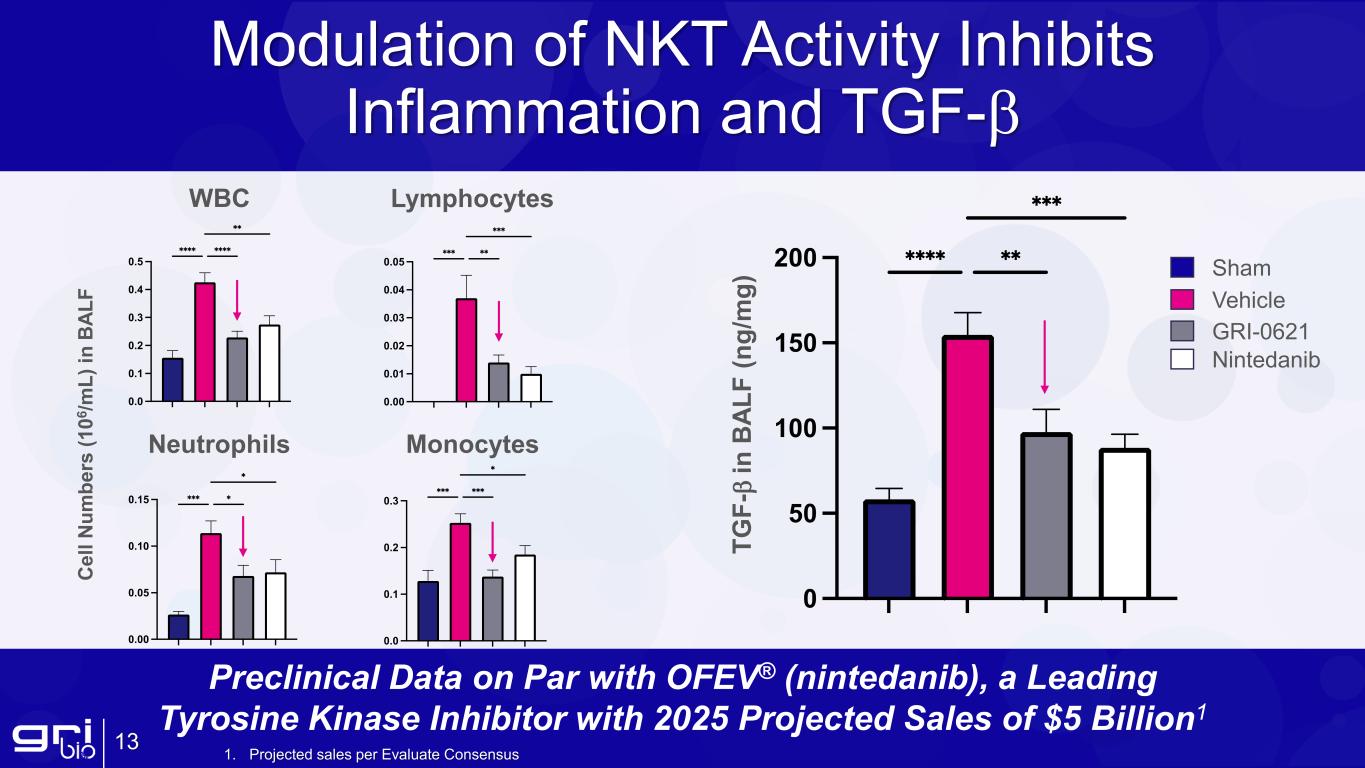
Modulation of NKT Activity Inhibits Inflammation and TGF-β 13 C el l N um be rs (1 06 /m L) in B AL F WBC Neutrophils Monocytes Lymphocytes TG F- β in B AL F (n g/ m g) Sham Vehicle GRI-0621 Nintedanib Preclinical Data on Par with OFEV® (nintedanib), a Leading Tyrosine Kinase Inhibitor with 2025 Projected Sales of $5 Billion1 1. Projected sales per Evaluate Consensus
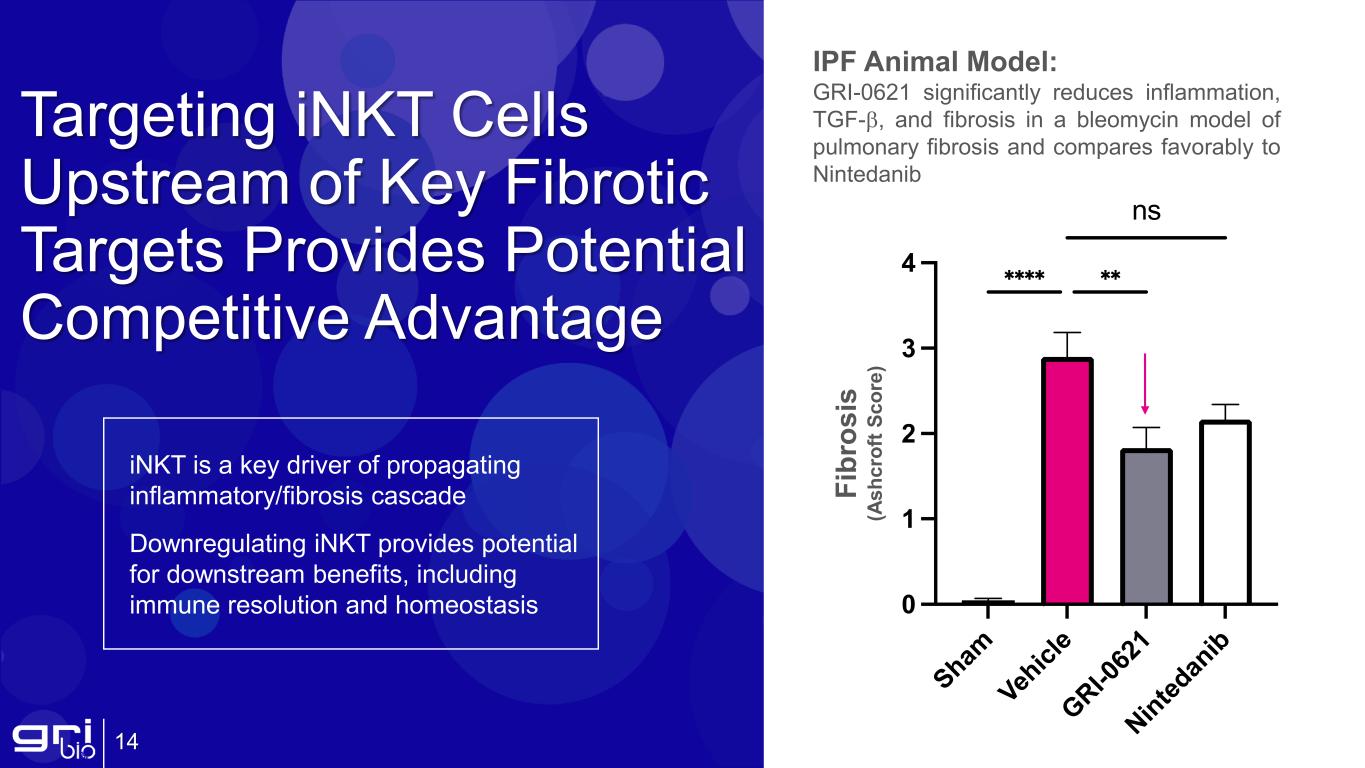
Targeting iNKT Cells Upstream of Key Fibrotic Targets Provides Potential Competitive Advantage 14 IPF Animal Model: GRI-0621 significantly reduces inflammation, TGF-β, and fibrosis in a bleomycin model of pulmonary fibrosis and compares favorably to Nintedanib iNKT is a key driver of propagating inflammatory/fibrosis cascade Downregulating iNKT provides potential for downstream benefits, including immune resolution and homeostasis Fi br os is (A sh cr of t S co re )
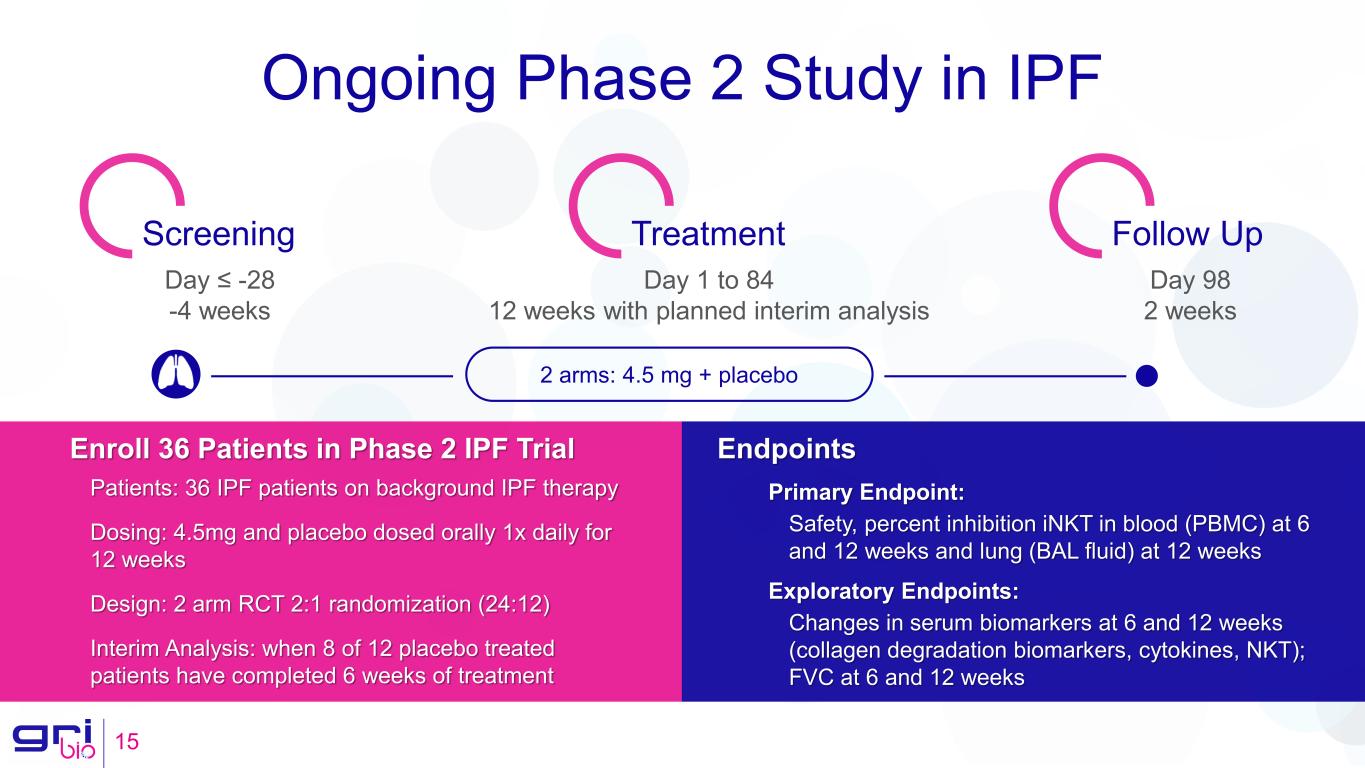
Ongoing Phase 2 Study in IPF 15 Screening Treatment Follow Up Day ≤ -28 -4 weeks Day 1 to 84 12 weeks with planned interim analysis Day 98 2 weeks 2 arms: 4.5 mg + placebo Enroll 36 Patients in Phase 2 IPF Trial Patients: 36 IPF patients on background IPF therapy Dosing: 4.5mg and placebo dosed orally 1x daily for 12 weeks Design: 2 arm RCT 2:1 randomization (24:12) Interim Analysis: when 8 of 12 placebo treated patients have completed 6 weeks of treatment Endpoints Primary Endpoint: Safety, percent inhibition iNKT in blood (PBMC) at 6 and 12 weeks and lung (BAL fluid) at 12 weeks Exploratory Endpoints: Changes in serum biomarkers at 6 and 12 weeks (collagen degradation biomarkers, cytokines, NKT); FVC at 6 and 12 weeks
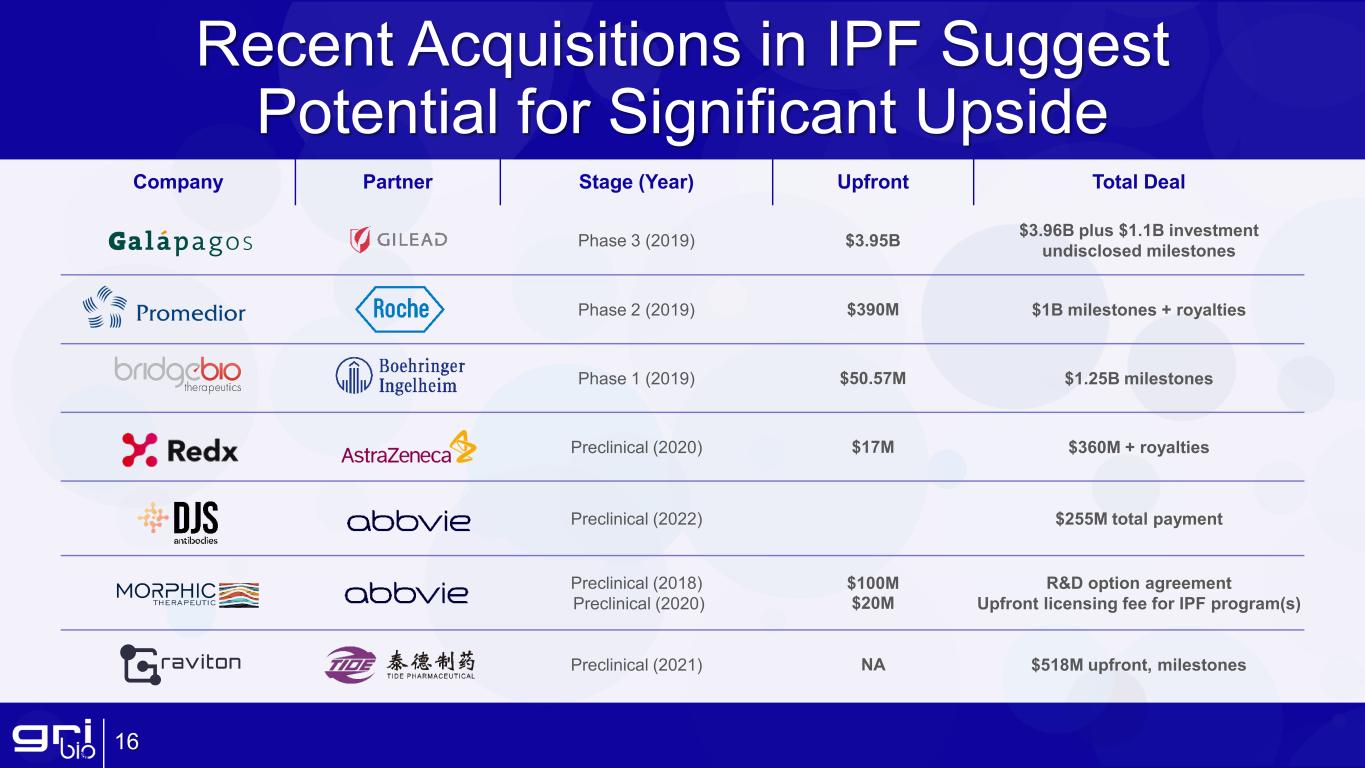
Recent Acquisitions in IPF Suggest Potential for Significant Upside 16 Company Partner Stage (Year) Upfront Total Deal Phase 3 (2019) $3.95B $3.96B plus $1.1B investment undisclosed milestones Phase 2 (2019) $390M $1B milestones + royalties Phase 1 (2019) $50.57M $1.25B milestones Preclinical (2020) $17M $360M + royalties Preclinical (2022) $255M total payment Preclinical (2018) Preclinical (2020) $100M $20M R&D option agreement Upfront licensing fee for IPF program(s) Preclinical (2021) NA $518M upfront, milestones

GRI-0803 Initial Focus on Systemic Lupus Erythematosus (SLE) 17 Target IND filing in Q3 2024 with topline data from Phase 1a/b expected Q4 2024 Extensive IP protection with issued composition of matter and use patents and market LOE through 2038
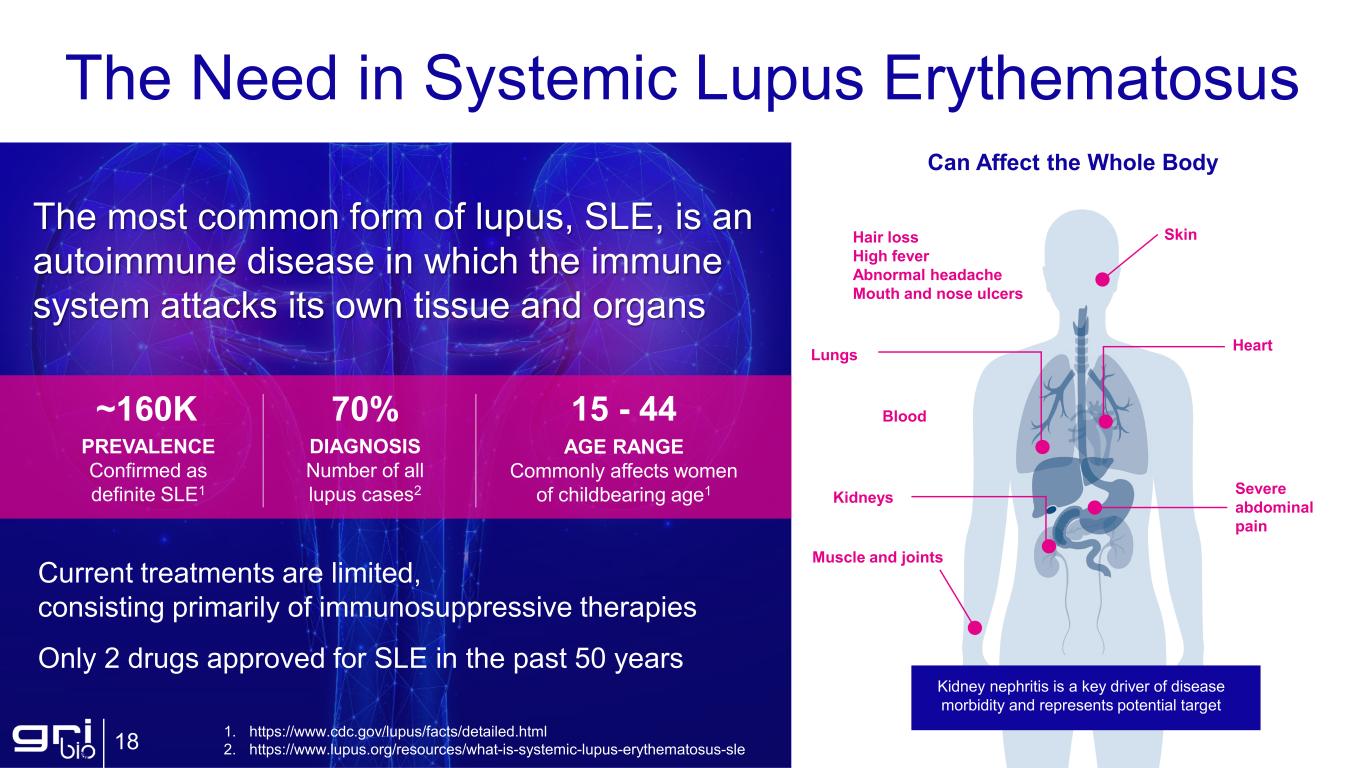
The Need in Systemic Lupus Erythematosus 18 The most common form of lupus, SLE, is an autoimmune disease in which the immune system attacks its own tissue and organs DIAGNOSIS Number of all lupus cases2 70% AGE RANGE Commonly affects women of childbearing age1 15 - 44 PREVALENCE Confirmed as definite SLE1 ~160K Current treatments are limited, consisting primarily of immunosuppressive therapies Only 2 drugs approved for SLE in the past 50 years Can Affect the Whole Body Lungs Kidneys Skin Heart Blood Muscle and joints Severe abdominal pain Hair loss High fever Abnormal headache Mouth and nose ulcers Kidney nephritis is a key driver of disease morbidity and represents potential target 1. https://www.cdc.gov/lupus/facts/detailed.html 2. https://www.lupus.org/resources/what-is-systemic-lupus-erythematosus-sle
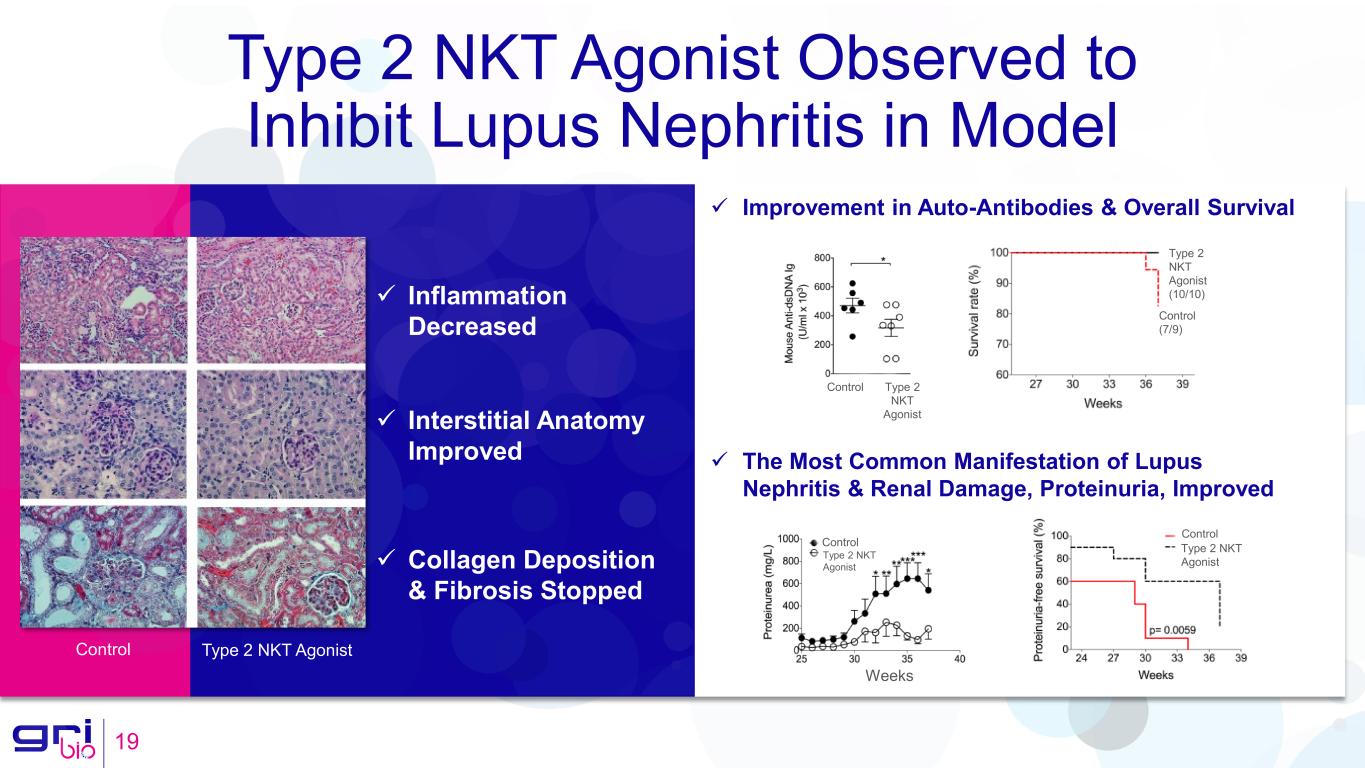
Type 2 NKT Agonist Observed to Inhibit Lupus Nephritis in Model 19 Control Type 2 NKT Agonist Control (7/9) Type 2 NKT Agonist (10/10) The Most Common Manifestation of Lupus Nephritis & Renal Damage, Proteinuria, Improved Improvement in Auto-Antibodies & Overall Survival Weeks Control Type 2 NKT Agonist Control Type 2 NKT Agonist Inflammation Decreased Interstitial Anatomy Improved Collagen Deposition & Fibrosis Stopped Control Type 2 NKT Agonist

20 Rapidly Advancing into the Clinic Target IND Filing in Q3 2024 with Topline Data Expected Q4 2024 Validate bioanalytical methods Complete cGMP manufacturing Complete toxicology studies Steps Toward IND Filing

Pipeline Expansion Opportunities 21 Type 2 NKT Agonists For Autoimmunity Potential Future Indications and Patient Populations (United States) 3M 1.5M 1.6M <200K <20K Multiple Sclerosis Rheumatoid Arthritis Inflammatory Bowel Disease Insulin Dependent Diabetes Mellitus Amyotrophic Lateral Sclerosis

Proven Leadership Team Marc Hertz, PhD Chief Executive Officer Vipin Kumar, PhD Prof. UCSD | CSO Albert Agro, PhD Chief Medical Officer 20+ years immunotherapy experience (fibrosis, inflammation, autoimmunity, allergy and oncology) Drug discovery through Phase 3 C-level & board positions at Pharmexa, Multimeric Biotherapeutics, GemVax & Evozym An internationally recognized leader in NKT cell research, regulation and autoimmunity Academic appointments at UCSD, UCLA, La Jolla Institute for Allergy & Immunology Published more than 130 peer- reviewed articles 20+ plus years in drug development; CEO Sublimity Therapeutics Played an instrumental role as C- level executive in $624 million sale of Cynapsus to Sunovion in 2016 Filed and FDA approval of 7 NDAs, 40 INDs, 30 CTAs Leanne Kelly Chief Financial Officer 20+ years financial executive leading private & public life science, technology and e-commerce companies Strong background in healthcare finance and accounting Audit and advisory experience 22
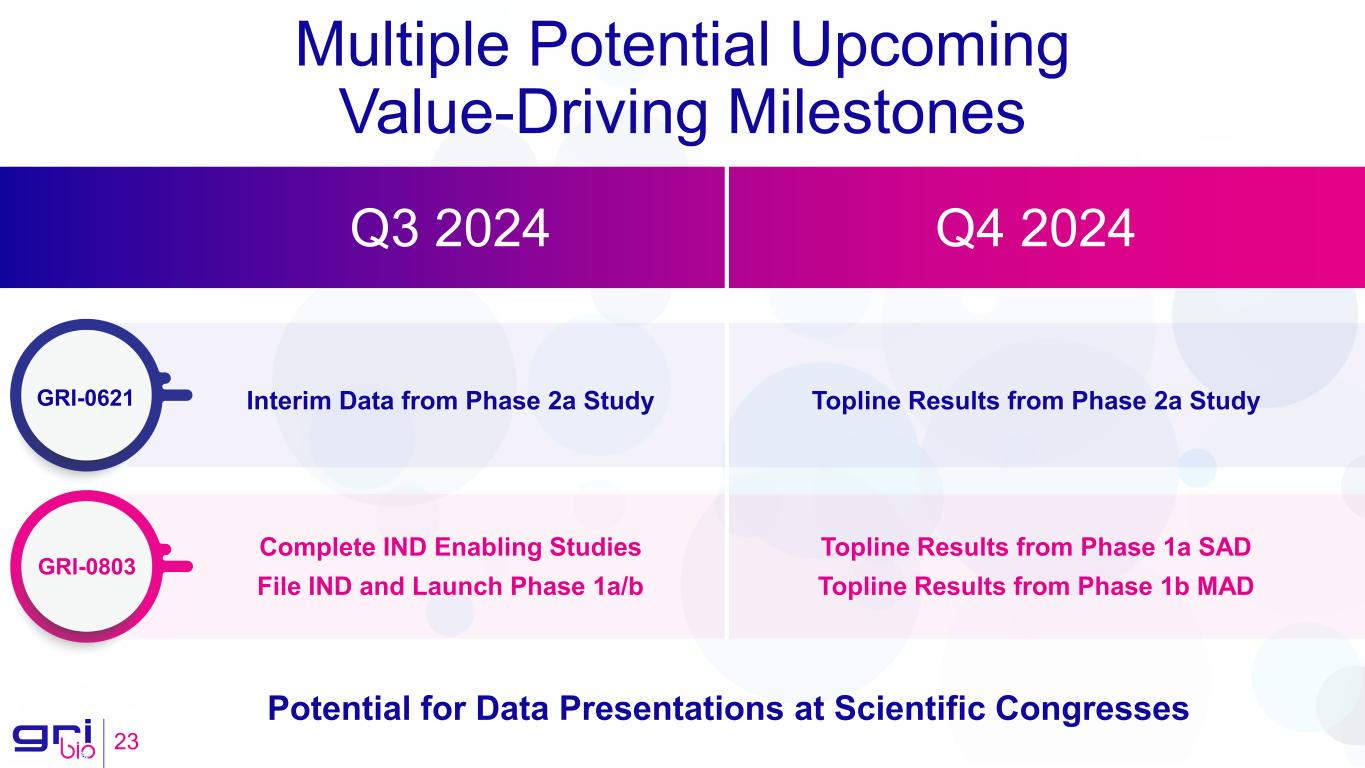
Multiple Potential Upcoming Value-Driving Milestones 23 Potential for Data Presentations at Scientific Congresses Q3 2024 Q4 2024 GRI-0803 GRI-0621 Complete IND Enabling Studies File IND and Launch Phase 1a/b Topline Results from Phase 1a SAD Topline Results from Phase 1b MAD Interim Data from Phase 2a Study Topline Results from Phase 2a Study

Summary 24 Elevating Clinical Stage Biotechnology Company Advancing Innovative Pipeline Across Multiple Orphan and High-Value Inflammatory, Fibrotic and Autoimmune Diseases NKT Science Leading NKT regulation technology targeting earlier in the inflammatory cascade to interrupt disease progression High-Value Indications Clinical pipeline in potential high- value indications with multiple pipeline expansion opportunities Proven Team Team with proven NKT, immunology and drug development experience We Believe NKT Science is Compelling to Fundamental Institutional Investors and Big Pharma Partners

A New Approach to Inflammatory Diseases NASDAQ: GRI | gribio.com Thank You! Investor Relations JTC Team 833-475-8247 gri@jtcir.com
v3.24.1.1.u2
Cover
|
May 13, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
May 13, 2024
|
| Entity Registrant Name |
GRI BIO, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-40034
|
| Entity Tax Identification Number |
82-4369909
|
| Entity Address, Address Line One |
2223 Avenida de la Playa
|
| Entity Address, Address Line Two |
#208
|
| Entity Address, City or Town |
La Jolla
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92037
|
| City Area Code |
619
|
| Local Phone Number |
400-1170
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
GRI
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| Entity Central Index Key |
0001824293
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Vallon Pharmaceuticals (NASDAQ:VLON)
過去 株価チャート
から 4 2024 まで 5 2024

Vallon Pharmaceuticals (NASDAQ:VLON)
過去 株価チャート
から 5 2023 まで 5 2024
