UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
For the month of September 2023
Commission File Number: 001-37643
PURPLE BIOTECH LTD.
(Translation of registrant’s name into English)
4 Oppenheimer Street, Science Park, Rehovot
7670104, Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Purple Biotech Ltd. (the “Company”
or the “Registrant”) is announcing that it has made available an updated Company Presentation on its website. A copy of the
updated Company Presentation is attached hereto as Exhibit 99.1 and may be viewed at the Company’s website at www.purple-biotech.com.
Incorporation by Reference
This Report on Form 6-K,
including all exhibits attached hereto, is hereby incorporated by reference into each of the Registrant’s Registration Statement
on Form S-8 filed with the Securities and Exchange Commission on May 20, 2016 (Registration file number 333-211478), the Registrant’s
Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 6, 2017 (Registration file number 333-218538),
the Registrant’s Registration Statement on Form F-3, as amended, originally filed with the Securities and Exchange Commission
on July 16, 2018 (Registration file number 333-226195), the Registrant’s Registration Statement on Form S-8 filed with the Securities
and Exchange Commission on March 28, 2019 (Registration file number 333-230584), the Registrant’s Registration Statement on Form F-3 filed with the Securities and Exchange Commission on September 16, 2019 (Registration file number 333-233795), the Registrant’s
Registration Statement on Form F-3 filed with the Securities and Exchange Commission on December 2, 2019 (Registration file number 333-235327),
the Registrant’s Registration Statement on Form F-1 filed with the Securities and Exchange Commission on December 27,
2019 (Registration file number 333-235729), the Registrant’s Registration Statement on Form F-3 filed with the Securities and Exchange
Commission on May 13, 2020 (Registration file number 333- 238229), the Registrant’s Registration Statement on Form S-8 filed with
the Securities and Exchange Commission on May 18, 2020 (Registration file number 333-238481), each of the Registrant’s Registration
Statements on Form F-3 filed with the Securities and Exchange Commission on July 10, 2020 (Registration file numbers 333-239807 and 333-233793),
the Registrant’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on April 4, 2022 (Registration
file number 333-264107) and the Registrant’s Registration Statement on Form F-3 filed with the Securities and Exchange Commission
on March 23, 2023 (Registration file number 333-270769) and the Registrant’s Registration Statement on Form F-3, as amended,
originally filed with the Securities and Exchange Commission on December 8, 2022 (Registration file number 333-268710), to be a part thereof
from the date on which this report is submitted, to the extent not superseded by documents or reports subsequently filed or furnished.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| September 13, 2023 |
PURPLE BIOTECH LTD. |
| |
|
| |
By: |
/s/ Lior Fhima |
| |
|
Lior Fhima |
| |
|
Chief Financial Officer |
2
Exhibit
99.1

| 1 NASDAQ/TASE: PPBT September 2023 CORPORATE PRESENTATION

Forward - looking Statements and Safe Harbor | 2 Certain statements in this press release that are forward - looking and not statements of historical fact are forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . Such forward - looking statements include, but are not limited to, statements that are not statements of historical fact, and may be identified by words such as “believe”, “expect”, “intend”, “plan”, “may”, “should”, “could”, “might”, “seek”, “target”, “will”, “project”, “forecast”, “continue” or “anticipate” or their negatives or variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical matters . You should not place undue reliance on these forward - looking statements, which are not guarantees of future performance . Forward - looking statements reflect our current views, expectations, beliefs or intentions with respect to future events, and are subject to a number of assumptions, involve known and unknown risks, many of which are beyond our control, as well as uncertainties and other factors that may cause our actual results, performance or achievements to be significantly different from any future results, performance or achievements expressed or implied by the forward - looking statements . Important factors that could cause or contribute to such differences include, among others, risks relating to : the expected benefits, synergies and costs of the transaction ; management plans relating to the transaction ; the potential future financial impact of the transaction ; and any assumptions underlying any of the foregoing ; the expected timing of the completion of the transaction and the parties’ ability to complete the transaction ; the plans, strategies and objectives of management for future operations ; product development for NT 219 and CM 24 as well as Immunorizon Ltd . ’s portfolio of investigational tri - specific antibody compounds to be acquired ; the process by which such early stage therapeutic candidates could potentially lead to an approved drug product is long and subject to highly significant risks, particularly with respect to a joint development collaboration ; the fact that drug development and commercialization involves a lengthy and expensive process with uncertain outcomes ; our ability to successfully develop and commercialize our pharmaceutical products ; the expense, length, progress and results of any clinical trials ; the impact of any changes in regulation and legislation that could affect the pharmaceutical industry ; the difficulty in receiving the regulatory approvals necessary in order to commercialize our products ; the difficulty of predicting actions of the U . S . Food and Drug Administration or any other applicable regulator of pharmaceutical products ; the regulatory environment and changes in the health policies and regimes in the countries in which we operate ; the uncertainty surrounding the actual market reception to our pharmaceutical products once cleared for marketing in a particular market ; the introduction of competing products ; patents obtained by competitors ; dependence on the effectiveness of our patents and other protections for innovative products ; our ability to obtain, maintain and defend issued patents ; the commencement of any patent interference or infringement action against our patents, and our ability to prevail, obtain a favorable decision or recover damages in any such action ; and the exposure to litigation, including patent litigation, and/or regulatory actions, and other factors that are discussed in our Annual Report on Form 20 - F for the year ended December 31 , 2022 and in our other filings with the U . S . Securities and Exchange Commission (“SEC”), including our cautionary discussion of risks and uncertainties under “Risk Factors” in our Registration Statements and Annual Reports . These are factors that we believe could cause our actual results to differ materially from expected results . Other factors besides those we have listed could also adversely affect us . Any forward - looking statement in this press release speaks only as of the date which it is made . We disclaim any intention or obligation to publicly update or revise any forward - looking statement or other information contained herein, whether as a result of new information, future events or otherwise, except as required by applicable law . You are advised, however, to consult any additional disclosures we make in our reports to the SEC, which are available on the SEC’s website, https : //www . sec . gov .
Corporate Highlights Purple Biotech (NASDAQ/TASE: PPBT) As of June 30, 2023 • ADS Outstanding: 21.4 M • Cash Balance : $18 M Strong position to reach short and mid term value creating clinical data catalysts | 3 • Multiple data read - outs expected in 2023 & 2024 • Two First - in - Class clinical stage drugs • A preclinical tri - specific immuno - engagers platform • Lean & global operation • Cash runway into 1H25 Purple Biotech identifies promising first - in - class drug candidates to treat cancers with high unmet medical need

Leadership Team Hadas Reuveni, PhD VP Research & Development Formerly at Keryx (NASDAQ:KERX) Michael Schickler, PhD Head of Clinical and Regulatory Affairs Formerly at Hoffmann - La Roche, CEO at CureTech Gil Efron Chief Executive Officer Former Deputy CEO & CFO at Kamada (NASDAQ:KMDA) Fabien Sebille, PhD Chief Business Officer Formerly at Debiopharm Lior Fhima Chief Financial Officer Formerly at Kamada (NASDAQ:KMDA) Eric K. Rowinsky, MD Chairman of the Board Former CMO at ImClone, Stemline, Board member at Biogen Inc. | 4

A Pipeline Dedicated to Advancing Oncology Therapies *Clinical collaboration and supply agreement with: Multiple data read - outs expected in the next 12 months Value Drivers Development Stage Indications Target Project Phase III Phase II Phase I Pre - Clinical □ Phase 2 interim analysis 2 H 23 & 1 H 24 □ Phase 2 top line results 2 H 24 Pancreatic Cancer (+nivolumab+SoC) CEACAM1 mAb CM24 * □ Phase 1 results 2H23 □ Initiation of Phase 2 1H24 Solid tumors (monotherapy) Head and Neck & Colorectal Cancer (+Cetuximab) STAT3xIRS1/2 Dual Inhibitor NT219 Solid Tumors CD3x5T4xNKG2A Tri - specific Ab IM1240 | 5

Advancing First - in - Class Oncology Therapies CM24: an α - CEACAM1* mAb Lead indication: Pancreatic Ductal Adenocarcinoma (PDAC) *Carcinoembryonic Antigen Cell Adhesion Molecule
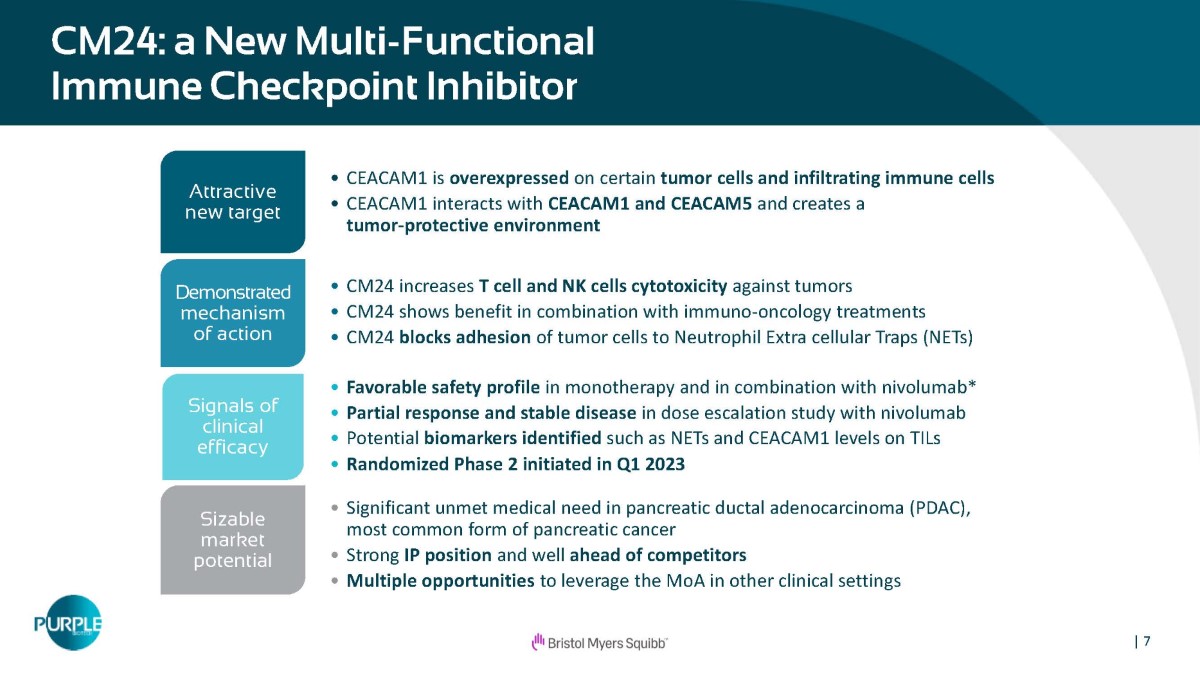
| 7 CM24: a New Multi - Functional Immune Checkpoint Inhibitor • CEACAM1 is overexpressed on certain tumor cells and infiltrating immune cells • CEACAM1 interacts with CEACAM1 and CEACAM5 and creates a tumor - protective environment Attractive new target • CM24 increases T cell and NK cells cytotoxicity against tumors • CM24 shows benefit in combination with immuno - oncology treatments • CM24 blocks adhesion of tumor cells to Neutrophil Extra cellular Traps (NETs) Demonstrated mechanism of action • Favorable safety profile in monotherapy and in combination with nivolumab* • Partial response and stable disease in dose escalation study with nivolumab • Potential biomarkers identified such as NETs and CEACAM1 levels on TILs • Randomized Phase 2 initiated in Q1 2023 Signals of clinical efficacy • Significant unmet medical need in pancreatic ductal adenocarcinoma (PDAC), most common form of pancreatic cancer • Strong IP position and well ahead of competitors • Multiple opportunities to leverage the MoA in other clinical settings Sizable market potential
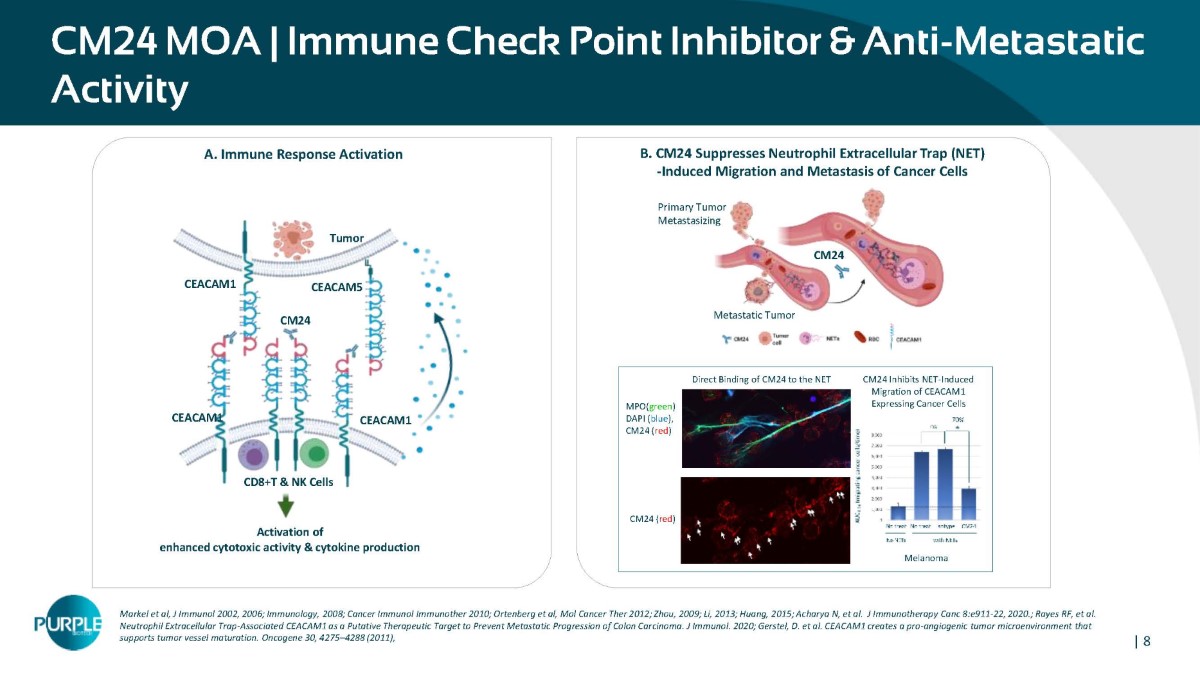
| 8 CM24 MOA | Immune Check Point Inhibitor & Anti - Metastatic Activity Markel et al, J Immunol 2002, 2006; Immunology, 2008; Cancer Immunol Immunother 2010; Ortenberg et al, Mol Cancer Ther 2012; Zhou, 2009; Li, 2013; Huang, 2015; Acharya N, et al. J Immunotherapy Canc 8:e911 - 22, 2020.; Rayes RF, et al. Neutrophil Extracellular Trap - Associated CEACAM1 as a Putative Therapeutic Target to Prevent Metastatic Progression of Colon Carcinoma. J Immunol. 2020; Gerstel, D. et al. CEACAM1 creates a pro - angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene 30, 4275 – 4288 (2011), Tumor CD8+T & NK Cells CEACAM1 CEACAM1 CEACAM1 CEACAM5 Activation of enhanced cytotoxic activity & cytokine production CM24 A. Immune Response Activation B. CM24 Suppresses Neutrophil Extracellular Trap (NET) - Induced Migration and Metastasis of Cancer Cells Primary Tumor Metastasizing CM24 Metastatic Tumor CM24 Inhibits NET - Induced Migration of CEACAM1 Expressing Cancer Cells Direct Binding of CM24 to the NET Structure MPO( green ) DAPI ( blue ), CM 24 ( red ) CM24 ( red ) Melanoma
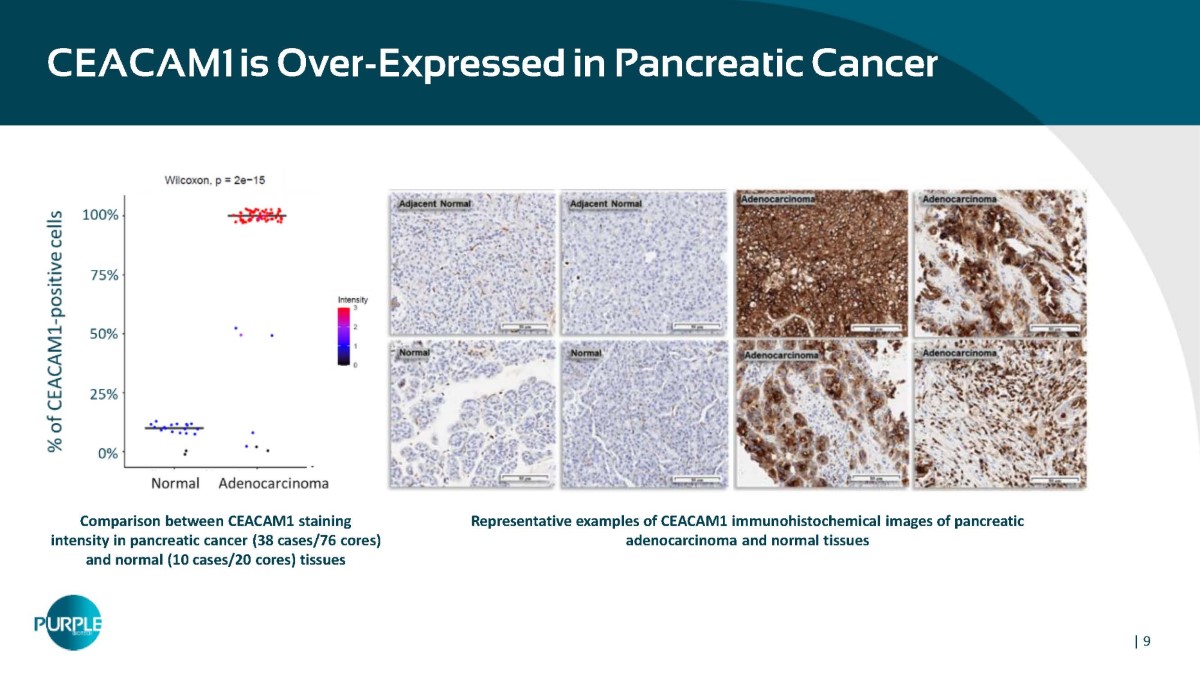
| 9 Representative examples of CEACAM1 immunohistochemical images of pancreatic adenocarcinoma and normal tissues Comparison between CEACAM1 staining intensity in pancreatic cancer (38 cases/76 cores) and normal (10 cases/20 cores) tissues CEACAM1 is Over - Expressed in Pancreatic Cancer
| 10 Large Market Opportunity in Pancreatic Cancer • Pancreatic Cancer accounts for ~60K new cases/year in the US alone; with a 5 - year relative survival rate of 11.5% 1 • Immuno - oncology approaches have been limited to patients with high microsatellite instability (MSI - H) or high tumor mutational burden (TMB - H) • 5 - year overall survival rate with chemotherapy in 2 nd line patients is 3% 1 • 3L standard of care regimens efficacy data: patients treated without chemotherapy: Overall Survival (OS) 2 months, OS of 3 - 4 months with chemotherapy • 2L standard of care regimens efficacy data: Gemcitabine/Nab - paclitaxel 3 : OS 7.9 months, Progression Free Survival (PFS) 4.3 months or Nal - IRI/5FU/LV 4 : OS 6.2 months, PFS 3.1 months • CEACAM1 expression correlates with poor prognosis in Pancreatic cancer 2 • Preclinical data support significant synergy of CM24 with currently marketed immuno - oncology therapies Combining nivolumab with CM24 in a clinical collaboration with Bristol Myers Squibb 1. American Cancer Society, Cancer Facts & Figures 2019, and the ACS website,https://seer.cancer.gov/statfacts/html/pancreas.html 2. Calinescu et al, Journal of Immunology Research 2018: 7169081; Carcinoembryonic antigen - related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer, DOI:10.1371/journal.pone.0113023 3. De Jesus VHF, Camandaroba MPG, Calsavara VF, Riechelmann RP. Systematic review and meta - analysis of gemcitabine - based chemotherapy after FOLFIRINOX in advanced pancreatic cancer. Therapeutic Advances in Medical Oncology. 2020;12. doi:10.1177/1758835920905408 4. Wang - Gillam A, Hubner RA, Siveke JT, et al. NAPOLI - 1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long - term survivors. Eur J Cancer. 2019;108:78 - 87. doi:10.1016/j.ejca.2018.12.007
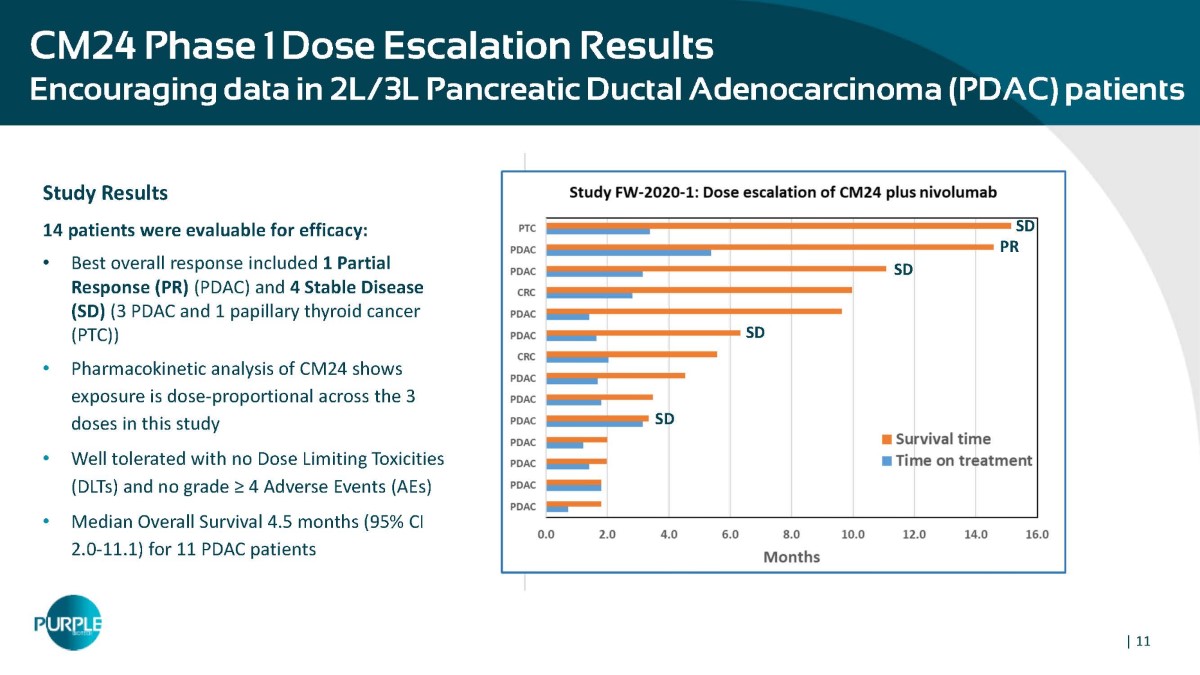
| 11 CM24 Phase 1 Dose Escalation Results Encouraging data in 2L/3L Pancreatic Ductal Adenocarcinoma (PDAC) patients Study Results 14 patients were evaluable for efficacy: • Best overall response included 1 Partial Response (PR) (PDAC) and 4 Stable Disease (SD) (3 PDAC and 1 papillary thyroid cancer (PTC)) • Pharmacokinetic analysis of CM24 shows exposure is dose - proportional across the 3 doses in this study • Well tolerated with no Dose Limiting Toxicities (DLTs) and no grade ≥ 4 Adverse Events (AEs) • Median Overall Survival 4.5 months (95% CI 2.0 - 11.1) for 11 PDAC patients SD SD PR SD SD

| 12 A study of CM24 in combination with nivolumab plus chemotherapy in PDAC patients in 2L treatment 18 centers are currently active in US, EU & Israel Measurement of CEACAM1 and other bio - markers is ongoing 2023 2024 Primary endpoint : OS Secondary endpoints: PFS, OS rate @ 6 & 12 months, PFS rate @ 3 & 6 months, ORR n=60 PDAC patients progressing on or after 1L SoC Chemotherapy Ongoing Experimental arms (n=24) CM24+nivolumab and Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Control arms (n=24) Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Phase 2 Combination Study Design (NCT04731467) Interim analysis: - PFS 2H23 - OS 1H24 Top line data: - PFS 1H24 - OS 1H25 Safety Study (n=16) CM24+nivolumab & Nal - IRI/5FU/LV OR Gemcitabine/nab - paclitaxel Completed Experimental arms (n=6) CM24+nivolumab and Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Control arms (n=6) Gemcitabine/nab - paclitaxel OR Nal - IRI/5FU/LV Interim PFS analysis Topline (OS)

Advancing First - in - Class Oncology Therapies NT219: A Small Molecule Dual Inhibitor of IRS 1/2 and STAT3 Lead indication: Recurrent/Metastatic Head & Neck Cancer (SCCHN)

| 14 NT219 - A Novel Approach to Overcome Resistance to EGFRi and Beyond • NT219 is a First - in - Class small molecule • Dual inhibitor of IRS1/2 and STAT3, two major drivers of resistance to cancer therapies Novel MOA • Significant efficacy in multiple in - vivo models • Modulation of the tumor microenvironment • Uniquely suited to inhibit development of drug resistance (EGFRi and KRASi) Robust preclinical package • Early signs of clinical efficacy as single agent • No dose limiting toxicity observed to date, recommended dose for Phase 2 has not been determined yet Clinical Stage • Opportunity to establish a 2 nd line standard of care • Multiple market upsides in combination with multiple approved therapies Broad Market Potential
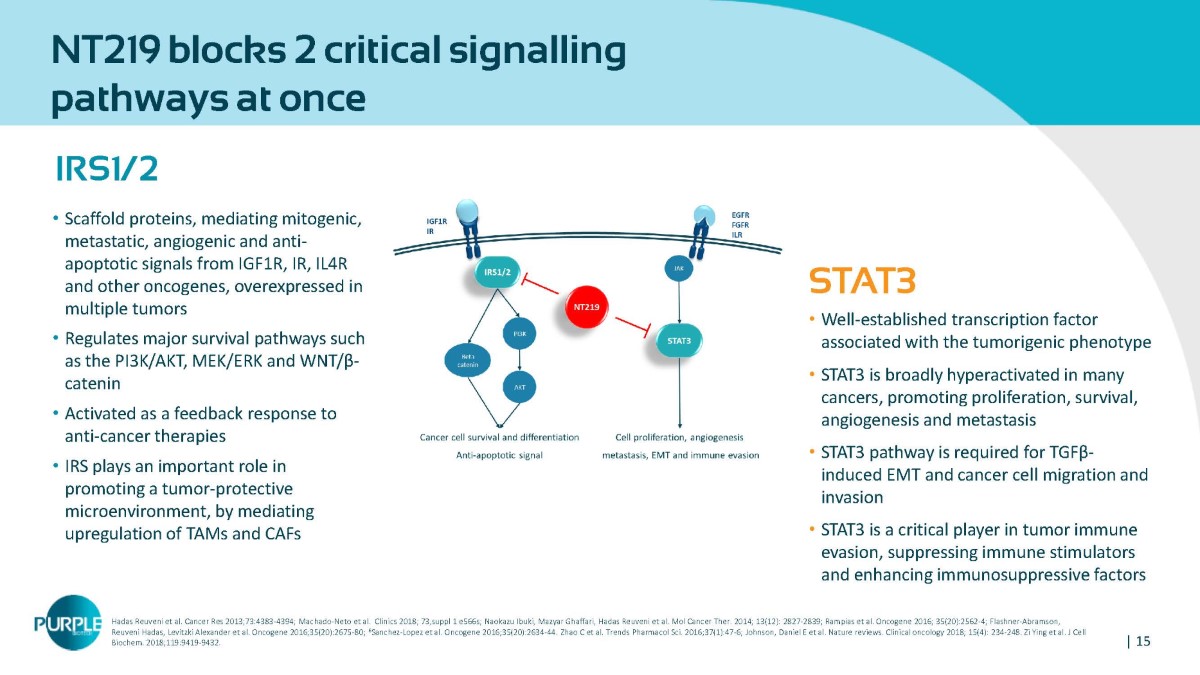
| 15 NT219 blocks 2 critical signalling pathways at once IRS1/2 • Scaffold proteins, mediating mitogenic, metastatic, angiogenic and anti - apoptotic signals from IGF1R, IR, IL4R and other oncogenes, overexpressed in multiple tumors • Regulates major survival pathways such as the PI 3 K/AKT, MEK/ERK and WNT/β - catenin • Activated as a feedback response to anti - cancer therapies • IRS plays an important role in promoting a tumor - protective microenvironment, by mediating upregulation of TAMs and CAFs STAT3 • Well - established transcription factor associated with the tumorigenic phenotype • STAT3 is broadly hyperactivated in many cancers, promoting proliferation, survival, angiogenesis and metastasis • STAT3 pathway is required for TGFβ - induced EMT and cancer cell migration and invasion • STAT 3 is a critical player in tumor immune evasion, suppressing immune stimulators and enhancing immunosuppressive factors Hadas Reuveni et al. Cancer Res 2013;73:4383 - 4394; Machado - Neto et al. Clinics 2018; 73,suppl 1 e566s; Naokazu Ibuki, Mazyar Ghaffari, Hadas Reuveni et al. Mol Cancer Ther. 2014; 13(12): 2827 - 2839; Rampias et al. Oncogene 2016; 35(20):2562 - 4; Flashner - Abramson, Reuveni Hadas, Levitzki Alexander et al. Oncogene 2016;35(20):2675 - 80; 6 Sanchez - Lopez et al. Oncogene 2016;35(20):2634 - 44. Zhao C et al. Trends Pharmacol Sci. 2016;37(1):47 - 6; Johnson, Daniel E et al. Nature reviews. Clinical oncology 2018; 15(4): 234 - 248. Zi Ying et al. J Cell Biochem. 2018;119:9419 - 9432.
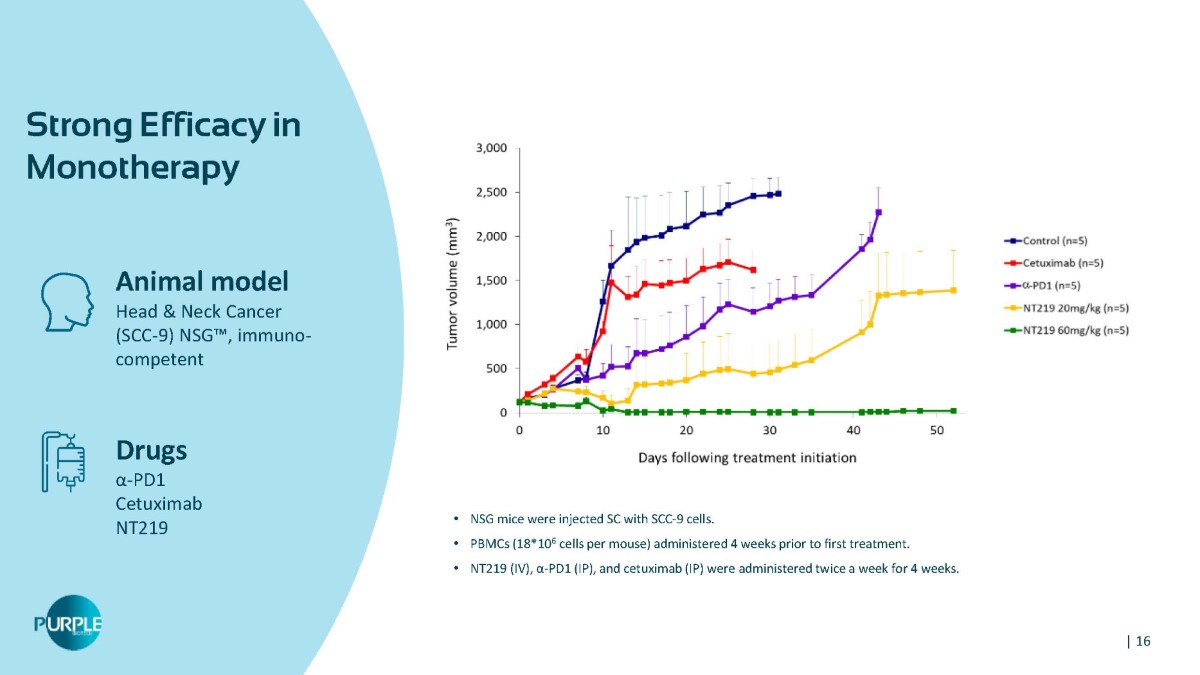
Animal model Head & Neck Cancer (SCC - 9) NSG Ρ , immuno - competent Drugs α - PD1 Cetuximab NT219 Strong Efficacy in Monotherapy • NSG mice were injected SC with SCC - 9 cells. • PBMCs (18*10 6 cells per mouse) administered 4 weeks prior to first treatment. • NT219 (IV), α - PD1 (IP), and cetuximab (IP) were administered twice a week for 4 weeks. | 16
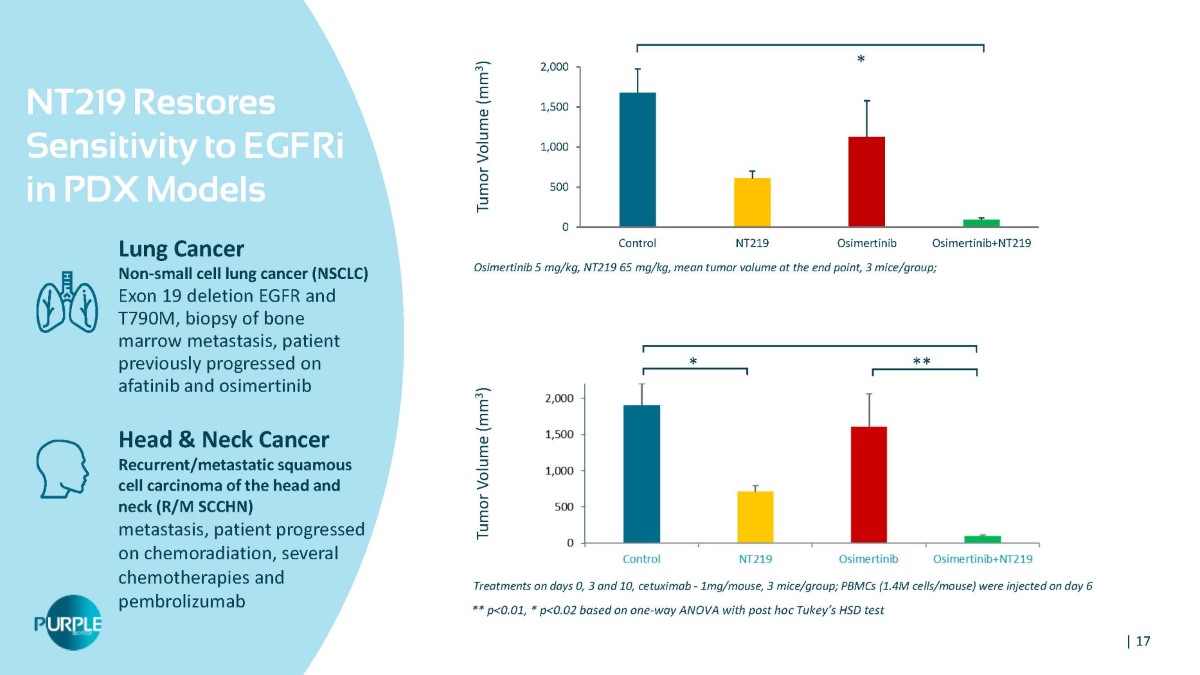
NT219 Restores Sensitivity to EGFRi in PDX Models Lung Cancer Non - small cell lung cancer (NSCLC) Exon 19 deletion EGFR and T790M, biopsy of bone marrow metastasis, patient previously progressed on afatinib and osimertinib Head & Neck Cancer Recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) metastasis, patient progressed on chemoradiation, several chemotherapies and pembrolizumab Treatments on days 0, 3 and 10, cetuximab - 1mg/mouse, 3 mice/group; PBMCs (1.4M cells/mouse) were injected on day 6 ** p<0.01, * p<0.02 based on one - way ANOVA with post hoc Tukey’s HSD test 0 Control NT219 Osimertinib Osimertinib+NT219 Osimertinib 5 mg/kg, NT219 65 mg/kg, mean tumor volume at the end point, 3 mice/group; 500 1,000 1,500 2,000 Tumor Volume (mm 3 ) Tumor Volume (mm 3 ) * ** * | 17
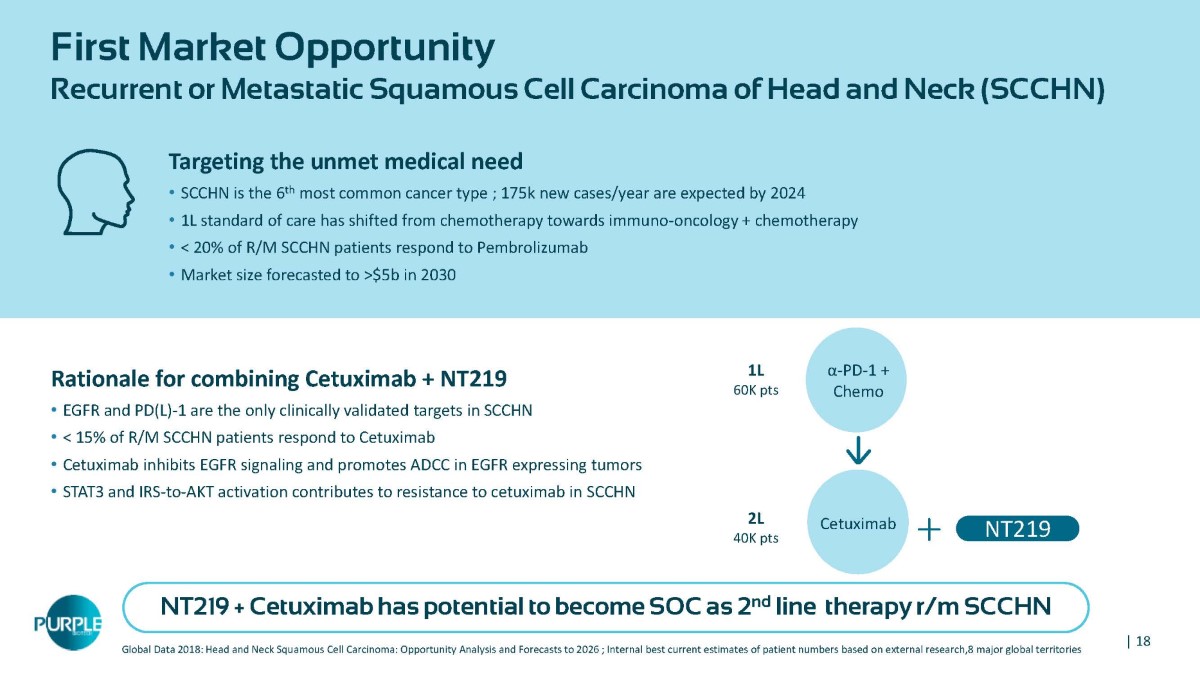
| 18 First Market Opportunity Recurrent or Metastatic Squamous Cell Carcinoma of Head and Neck (SCCHN) Global Data 2018: Head and Neck Squamous Cell Carcinoma: Opportunity Analysis and Forecasts to 2026 ; Internal best current estimates of patient numbers based on external research,8 major global territories Rationale for combining Cetuximab + NT219 • EGFR and PD(L) - 1 are the only clinically validated targets in SCCHN • < 15% of R/M SCCHN patients respond to Cetuximab • Cetuximab inhibits EGFR signaling and promotes ADCC in EGFR expressing tumors • STAT3 and IRS - to - AKT activation contributes to resistance to cetuximab in SCCHN Targeting the unmet medical need • SCCHN is the 6 th most common cancer type ; 175k new cases/year are expected by 2024 • 1L standard of care has shifted from chemotherapy towards immuno - oncology + chemotherapy • < 20% of R/M SCCHN patients respond to Pembrolizumab • Market size forecasted to >$5b in 2030 α - PD - 1 + Chemo 1L 60K pts Cetuximab 2L 40K pts NT219 NT219 + Cetuximab has potential to become SOC as 2 nd line therapy r/m SCCHN
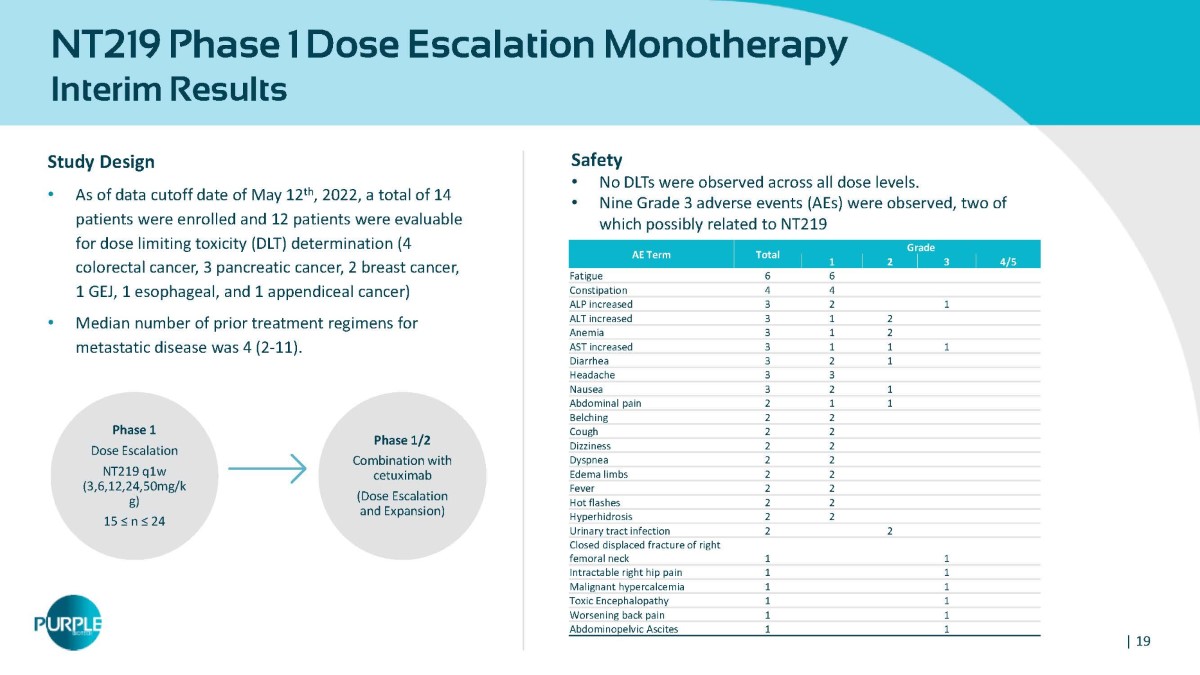
NT219 Phase 1 Dose Escalation Monotherapy Interim Results Study Design • As of data cutoff date of May 12 th , 2022, a total of 14 patients were enrolled and 12 patients were evaluable for dose limiting toxicity (DLT) determination (4 colorectal cancer, 3 pancreatic cancer, 2 breast cancer, 1 GEJ, 1 esophageal, and 1 appendiceal cancer) • Median number of prior treatment regimens for metastatic disease was 4 (2 - 11). Grade AE Term 4/5 3 2 1 Total 6 6 Fatigue 4 4 Constipation 1 2 3 ALP increased 2 1 3 ALT increased 2 1 3 Anemia 1 1 1 3 AST increased 1 2 3 Diarrhea 3 3 Headache 1 2 3 Nausea 1 1 2 Abdominal pain 2 2 Belching 2 2 Cough 2 2 Dizziness 2 2 Dyspnea 2 2 Edema limbs 2 2 Fever 2 2 Hot flashes 2 2 Hyperhidrosis 2 2 Urinary tract infection 1 1 Closed displaced fracture of right femoral neck 1 1 Intractable right hip pain 1 1 Malignant hypercalcemia 1 1 Toxic Encephalopathy 1 1 Worsening back pain 1 1 Abdominopelvic Ascites Phase 1 Dose Escalation NT219 q1w (3,6,12,24,50mg/k g) 15 ≤ n ≤ 24 Phase 1/2 Combination with cetuximab (Dose Escalation and Expansion) Safety • No DLTs were observed across all dose levels. • Nine Grade 3 adverse events (AEs) were observed, two of which possibly related to NT219 | 19
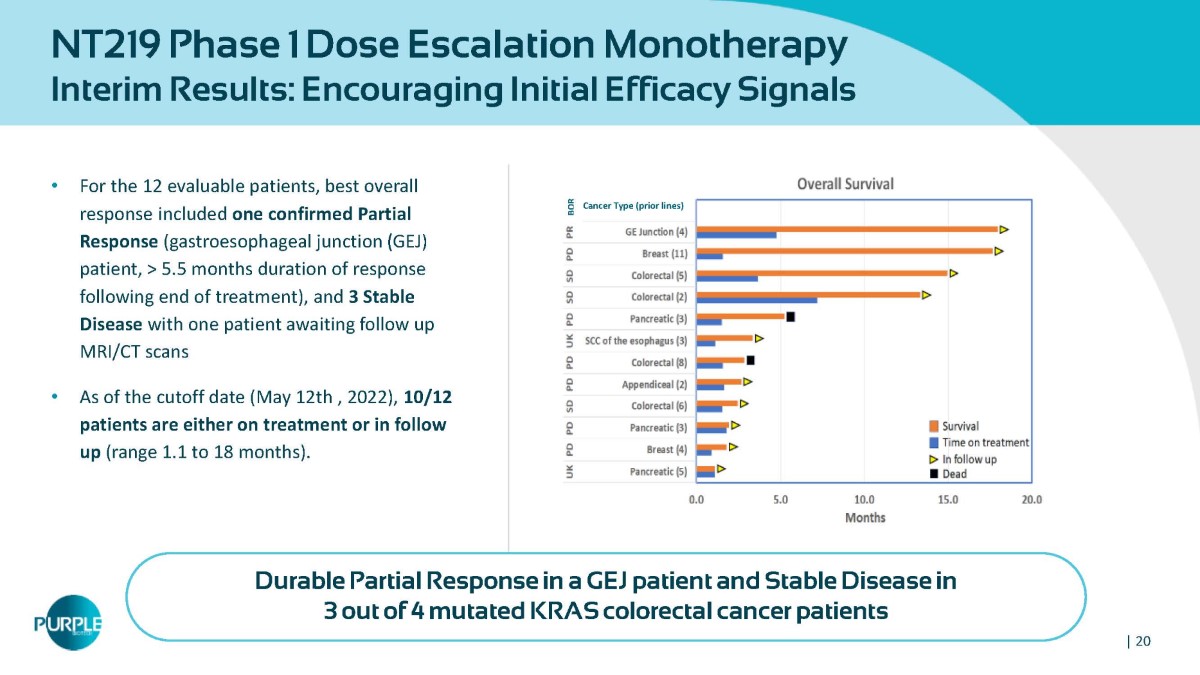
NT219 Phase 1 Dose Escalation Monotherapy Interim Results: Encouraging Initial Efficacy Signals • For the 12 evaluable patients, best overall response included one confirmed Partial Response (gastroesophageal junction ( GEJ) patient, > 5.5 months duration of response following end of treatment), and 3 Stable Disease with one patient awaiting follow up MRI/CT scans • As of the cutoff date (May 12 th , 2022 ), 10 / 12 patients are either on treatment or in follow up (range 1 . 1 to 18 months) . BOR Cancer Type (prior lines) Durable Partial Response in a GEJ patient and Stable Disease in 3 out of 4 mutated KRAS colorectal cancer patients | 20
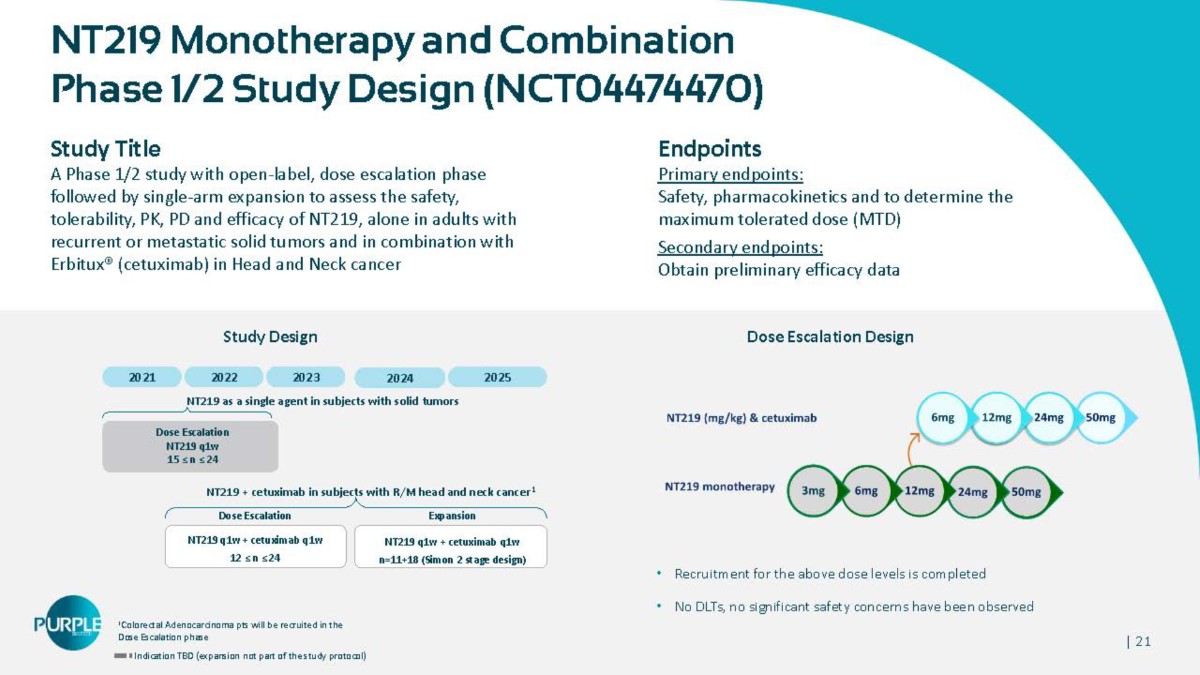
| 21 NT219 Monotherapy and Combination Phase 1/2 Study Design (NCT04474470) Study Title A Phase 1/2 study with open - label, dose escalation phase followed by single - arm expansion to assess the safety, tolerability, PK, PD and efficacy of NT219, alone in adults with recurrent or metastatic solid tumors and in combination with Erbitux® (cetuximab) in Head and Neck cancer 1 Colorectal Adenocarcinoma pts will be recruited in the Dose Escalation phase Indication TBD (expansion not part of the study protocol) 2021 NT219 q1w + cetuximab q1w 12 ≤ n ≤ 24 Dose Escalation NT219 q1w 15 ≤ n ≤ 24 NT219 + cetuximab in subjects with R/M head and neck cancer 1 Dose Escalation Expansion NT219 q1w + cetuximab q1w n=11+18 (Simon 2 stage design) Endpoints Primary endpoints: Safety, pharmacokinetics and to determine the maximum tolerated dose (MTD) Secondary endpoints: Obtain preliminary efficacy data Dose Escalation Design • Recruitment for the above dose levels is completed • No DLTs, no significant safety concerns have been observed Study Design 2022 2023 2024 NT219 as a single agent in subjects with solid tumors 2025

Advancing First - in - Class Oncology Therapies IM1240: CD3x5T4xNKG2A Conditionally - Activated Tri - Specific Antibody

| 23 A Novel Mechanism of Action Tri - Specific Antibody Cancer binder a - TAA Abs a - NK binder Abs T - cell - binder a - CD3 Abs aCD3 masking moiety Tumor cell T cell NK cell Cancer microenvironment Protease cleavage Removal of T cell engager’s masking by proteases of the tumor microenvironment T and NK Cell activation restricted to selected tumors • Multi - specific biologics is an expanding class of drugs getting a lot of interest in the industry • After initial success in hemato - oncology, new formats are being investigated in solid tumors • Technology displays several distinctive features: • Dual engagement of T cells and NK cells to mount an optimal anti - tumoral immune response • A tumor - restricted activation through a cleavable capping system designed to provide a wide therapeutic index • Carefully selected Tumor Associated Antigens allowing patient - centric development Masked pre - form Activated in TME Engagement
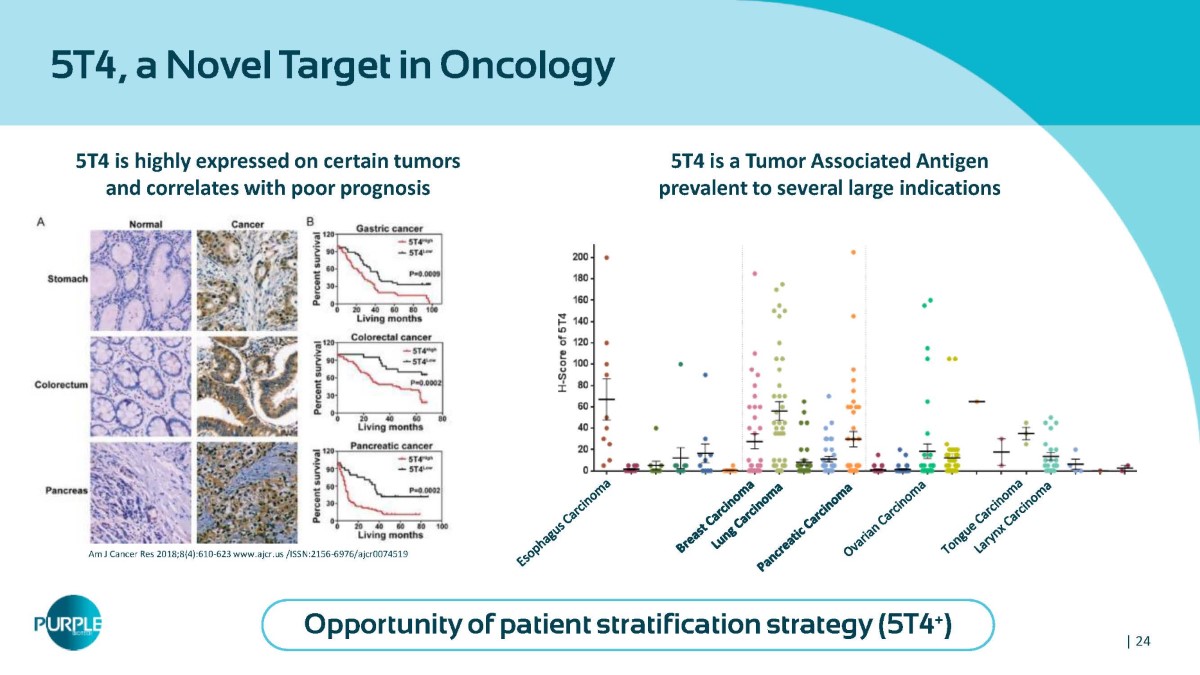
5T4, a Novel Target in Oncology 5T4 is highly expressed on certain tumors and correlates with poor prognosis Am J Cancer Res 2018;8(4):610 - 623 www.ajcr.us /ISSN:2156 - 6976/ajcr0074519 5T4 is a Tumor Associated Antigen prevalent to several large indications Opportunity of patient stratification strategy (5T4 + ) | 24
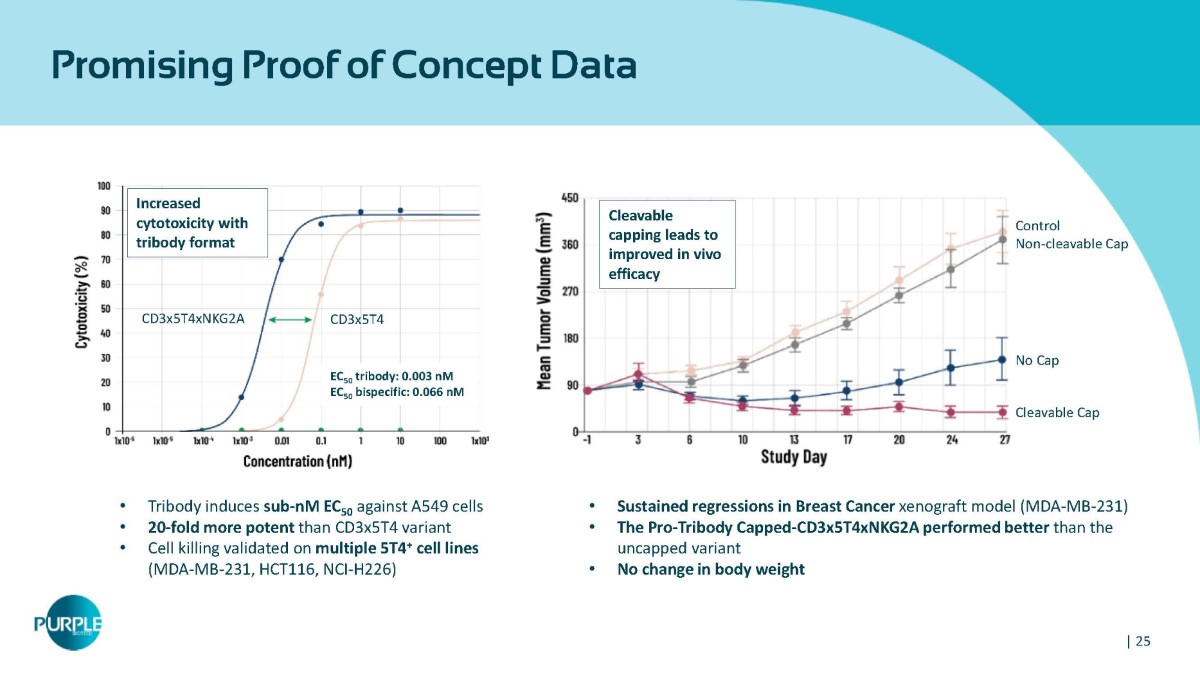
Promising Proof of Concept Data • Tribody induces sub - nM EC 50 against A549 cells • 20 - fold more potent than CD3x5T4 variant • Cell killing validated on multiple 5T4 + cell lines (MDA - MB - 231, HCT116, NCI - H226) • Sustained regressions in Breast Cancer xenograft model (MDA - MB - 231) • The Pro - Tribody Capped - CD3x5T4xNKG2A performed better than the uncapped variant • No change in body weight CD3x5T4xNKG2A CD3x5T4 Cleavable Cap No Cap Control Non - cleavable Cap Cleavable capping leads to improved in vivo efficacy Increased cytotoxicity with tribody format EC 50 tribody: 0.003 nM EC 50 bispecific: 0.066 nM | 25
Corporate Highlights Purple Biotech (NASDAQ/TASE: PPBT) As of June 30, 2023 • ADS Outstanding: 21.4 M • Cash Balance : $18 M Strong position to reach short and mid term value creating clinical data catalysts | 26 • Multiple data read - outs expected in 2023 & 2024 • Two First - in - Class clinical stage drugs • A preclinical tri - specific immuno - engagers platform • Lean & global operation • Cash runway into 1H25 Purple Biotech identifies promising first - in - class drug candidates to treat cancers with high unmet medical need

| 27 Contact Us: ir@purple - biotech.com THANK YOU

Appendix A | CM24
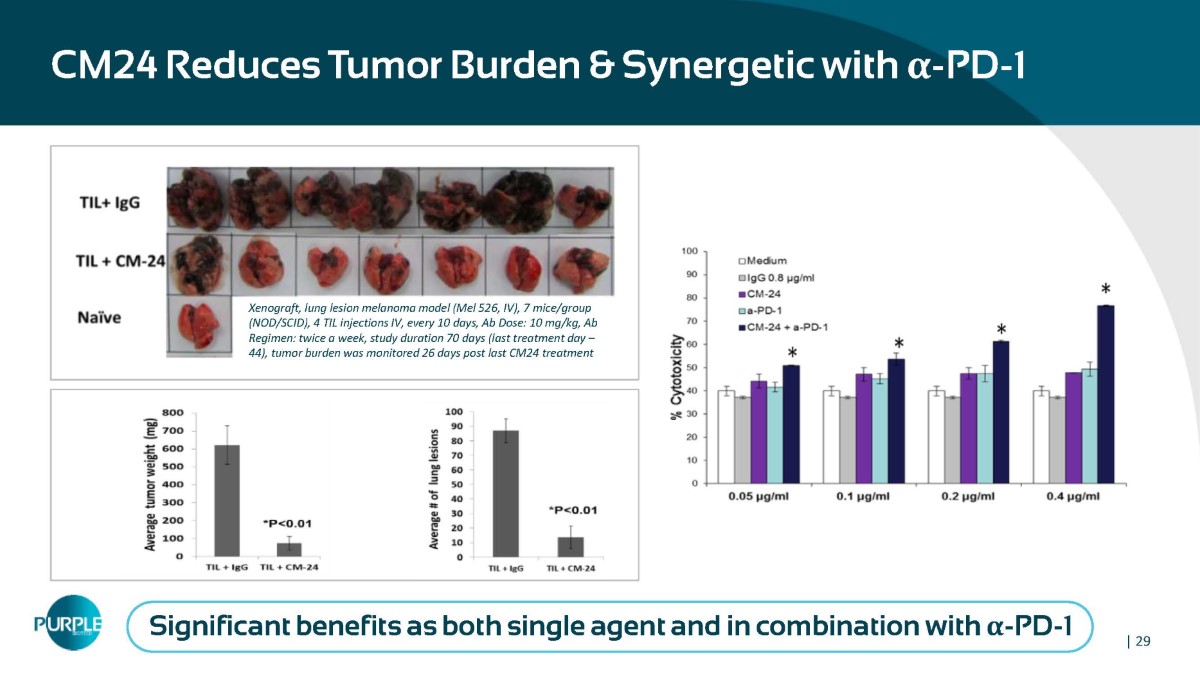
CM24 Reduces Tumor Burden & Synergetic with α - PD - 1 Xenograft, lung lesion melanoma model (Mel 526, IV), 7 mice/group (NOD/SCID), 4 TIL injections IV, every 10 days, Ab Dose: 10 mg/kg, Ab Regimen: twice a week, study duration 70 days (last treatment day – 44), tumor burden was monitored 26 days post last CM24 treatment Significant benefits as both single agent and in combination with α - PD - 1 | 29
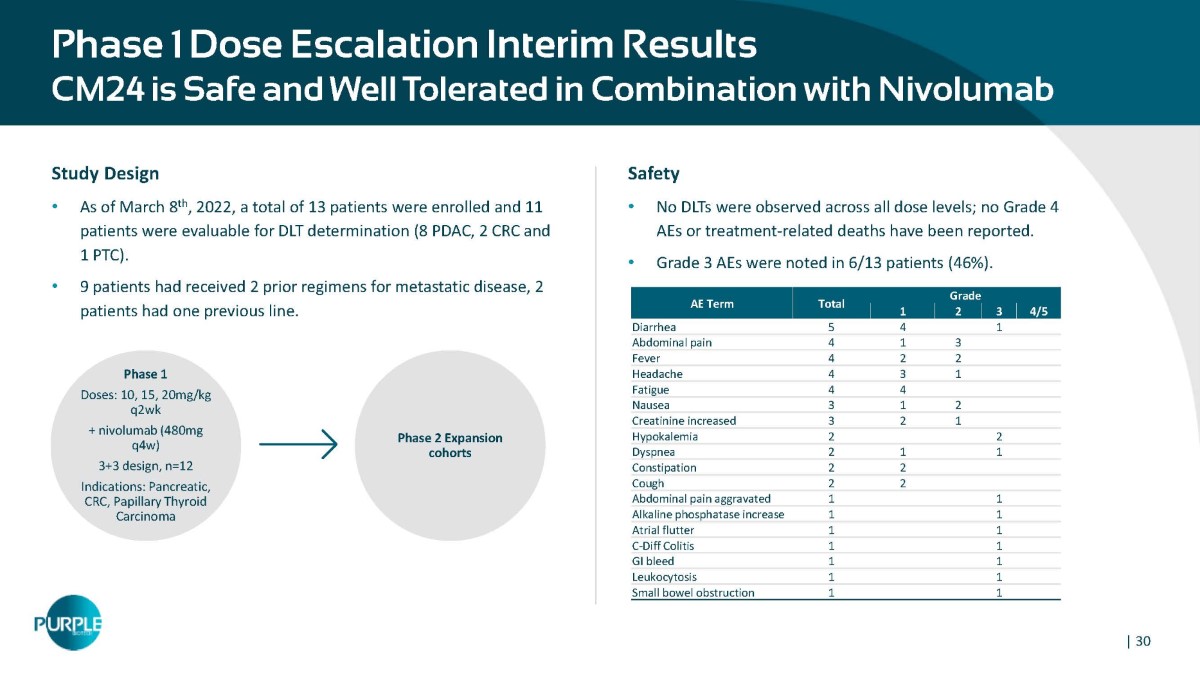
Phase 1 Dose Escalation Interim Results CM24 is Safe and Well Tolerated in Combination with Nivolumab Total Grade AE Term 4/5 3 2 1 1 4 5 Diarrhea 3 1 4 Abdominal pain 2 2 4 Fever 1 3 4 Headache 4 4 Fatigue 2 1 3 Nausea 1 2 3 Creatinine increased 2 2 Hypokalemia 1 1 2 Dyspnea 2 2 Constipation 2 2 Cough 1 1 Abdominal pain aggravated 1 1 Alkaline phosphatase increase 1 1 Atrial flutter 1 1 C - Diff Colitis 1 1 GI bleed 1 1 Leukocytosis 1 1 Small bowel obstruction Study Design • As of March 8 th , 2022, a total of 13 patients were enrolled and 11 patients were evaluable for DLT determination (8 PDAC, 2 CRC and 1 PTC). • 9 patients had received 2 prior regimens for metastatic disease, 2 patients had one previous line. Phase 1 Doses: 10, 15, 20mg/kg q2wk + nivolumab (480mg q4w) 3+3 design, n=12 Indications: Pancreatic, CRC, Papillary Thyroid Carcinoma Phase 2 Expansion cohorts Safety • No DLTs were observed across all dose levels; no Grade 4 AEs or treatment - related deaths have been reported. • Grade 3 AEs were noted in 6/13 patients (46%). | 30
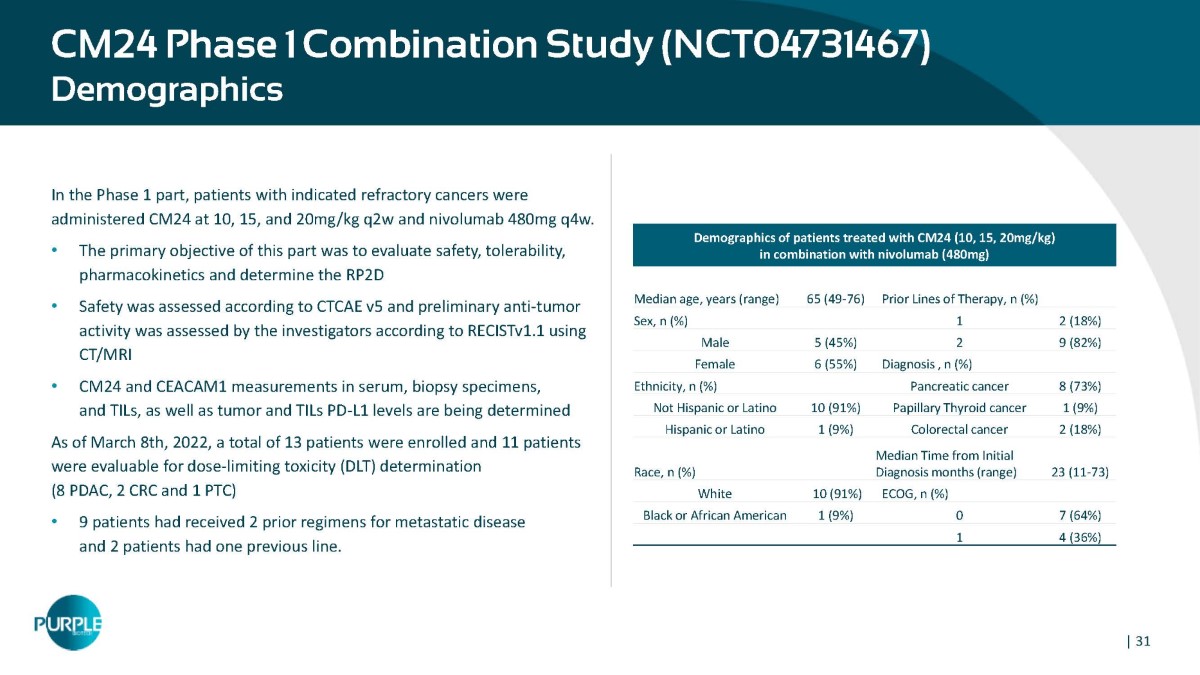
CM24 Phase 1 Combination Study (NCT04731467) Demographics In the Phase 1 part, patients with indicated refractory cancers were administered CM24 at 10, 15, and 20mg/kg q2w and nivolumab 480mg q4w. • The primary objective of this part was to evaluate safety, tolerability, pharmacokinetics and determine the RP2D • Safety was assessed according to CTCAE v5 and preliminary anti - tumor activity was assessed by the investigators according to RECISTv1.1 using CT/MRI • CM24 and CEACAM1 measurements in serum, biopsy specimens, and TILs, as well as tumor and TILs PD - L1 levels are being determined As of March 8th, 2022, a total of 13 patients were enrolled and 11 patients were evaluable for dose - limiting toxicity (DLT) determination (8 PDAC, 2 CRC and 1 PTC) • 9 patients had received 2 prior regimens for metastatic disease and 2 patients had one previous line. Demographics of patients treated with CM24 (10, 15, 20mg/kg) in combination with nivolumab (480mg) Prior Lines of Therapy, n (%) 65 (49 - 76) Median age, years (range) 2 (18%) 1 Sex, n (%) 9 (82%) 2 5 (45%) Male Diagnosis , n (%) 6 (55%) Female 8 (73%) Pancreatic cancer Ethnicity, n (%) 1 (9%) Papillary Thyroid cancer 10 (91%) Not Hispanic or Latino 2 (18%) Colorectal cancer 1 (9%) Hispanic or Latino 23 (11 - 73) Median Time from Initial Diagnosis months (range) Race, n (%) ECOG, n (%) 10 (91%) White 7 (64%) 0 1 (9%) Black or African American 4 (36%) 1 | 31
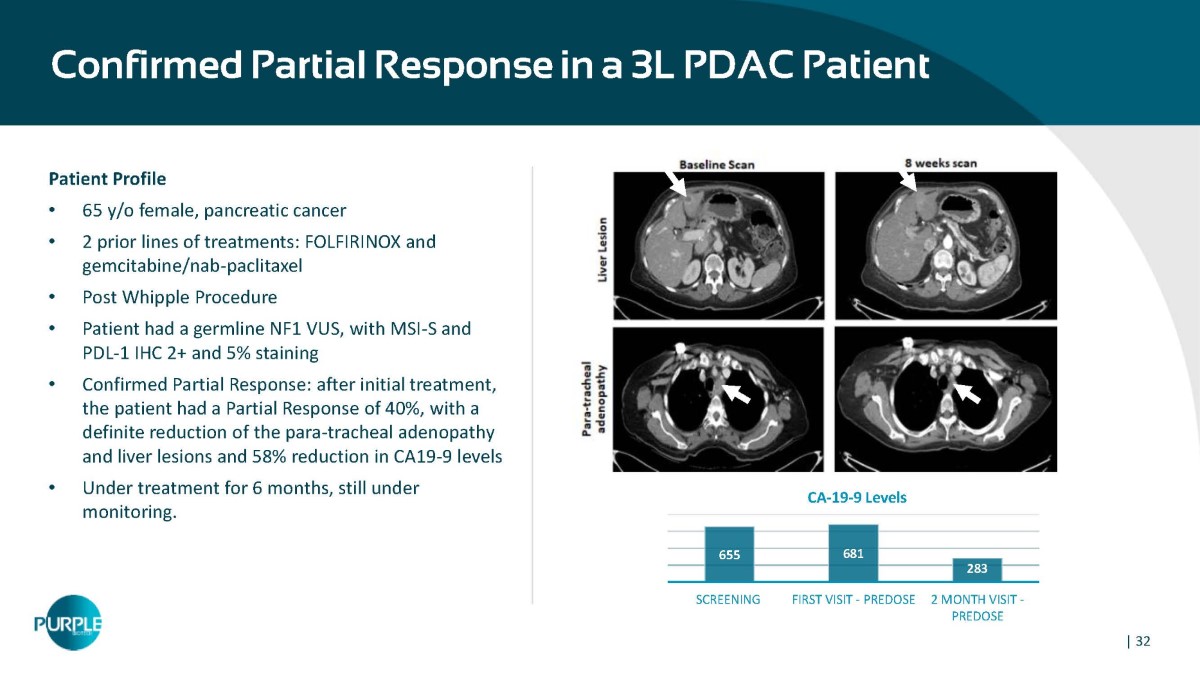
Confirmed Partial Response in a 3L PDAC Patient Patient Profile • 65 y/o female, pancreatic cancer • 2 prior lines of treatments: FOLFIRINOX and gemcitabine/nab - paclitaxel • Post Whipple Procedure • Patient had a germline NF1 VUS, with MSI - S and PDL - 1 IHC 2+ and 5% staining • Confirmed Partial Response: after initial treatment, the patient had a Partial Response of 40%, with a definite reduction of the para - tracheal adenopathy and liver lesions and 58% reduction in CA19 - 9 levels • Under treatment for 6 months, still under monitoring. 655 681 283 SCREENING FIRST VISIT - PREDOSE 2 MONTH VISIT - PREDOSE CA - 19 - 9 Levels | 32
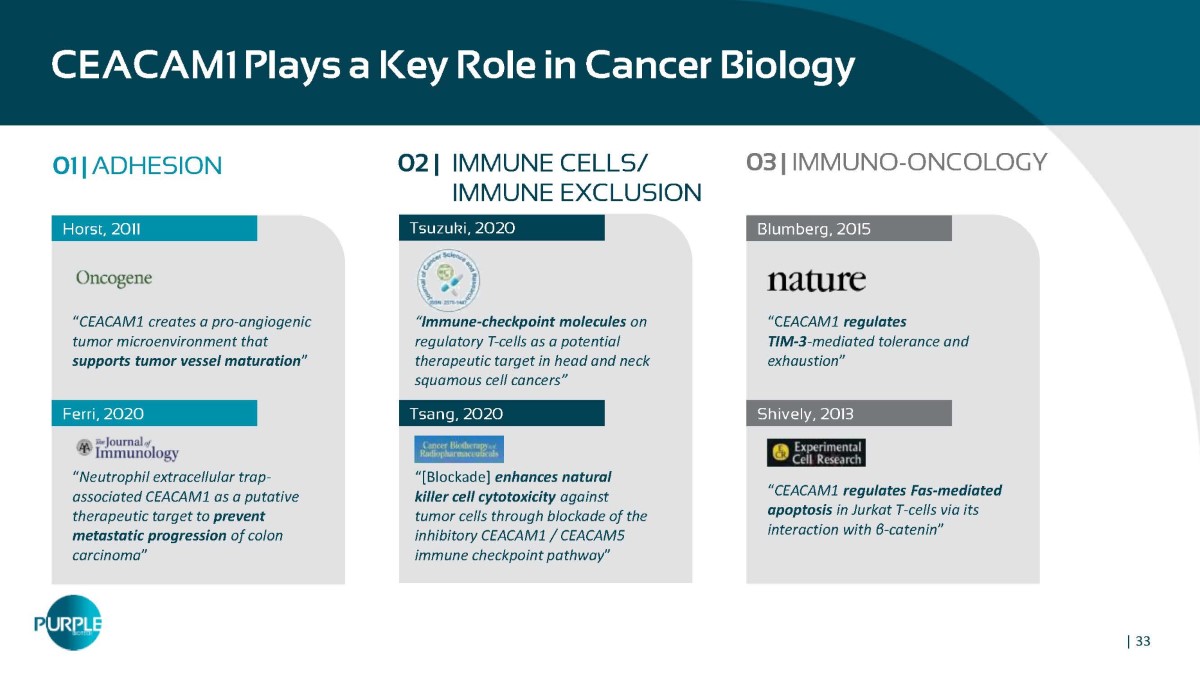
CEACAM1 Plays a Key Role in Cancer Biology 01 | ADHESION Horst, 2011 “ CEACAM1 creates a pro - angiogenic tumor microenvironment that supports tumor vessel maturation ” “ Neutrophil extracellular trap - associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma ” Ferri, 2020 Tsuzuki, 2020 Tsang, 2020 Blumberg, 2015 “C EACAM1 regulates TIM - 3 - mediated tolerance and exhaustion ” “ CEACAM1 regulates Fas - mediated apoptosis in Jurkat T - cells via its interaction with β - catenin ” Shively, 2013 02 | IMMUNE CELLS/ IMMUNE EXCLUSION 03 | IMMUNO - ONCOLOGY “ Immune-checkpoint molecules on regulatory T-cells as a potential therapeutic target in head and neck squamous cell cancers” “[Blockade] enhances natural killer cell cytotoxicity against tumor cells through blockade of the inhibitory CEACAM1 / CEACAM5 immune checkpoint pathway ” | 33

Appendix B | NT219
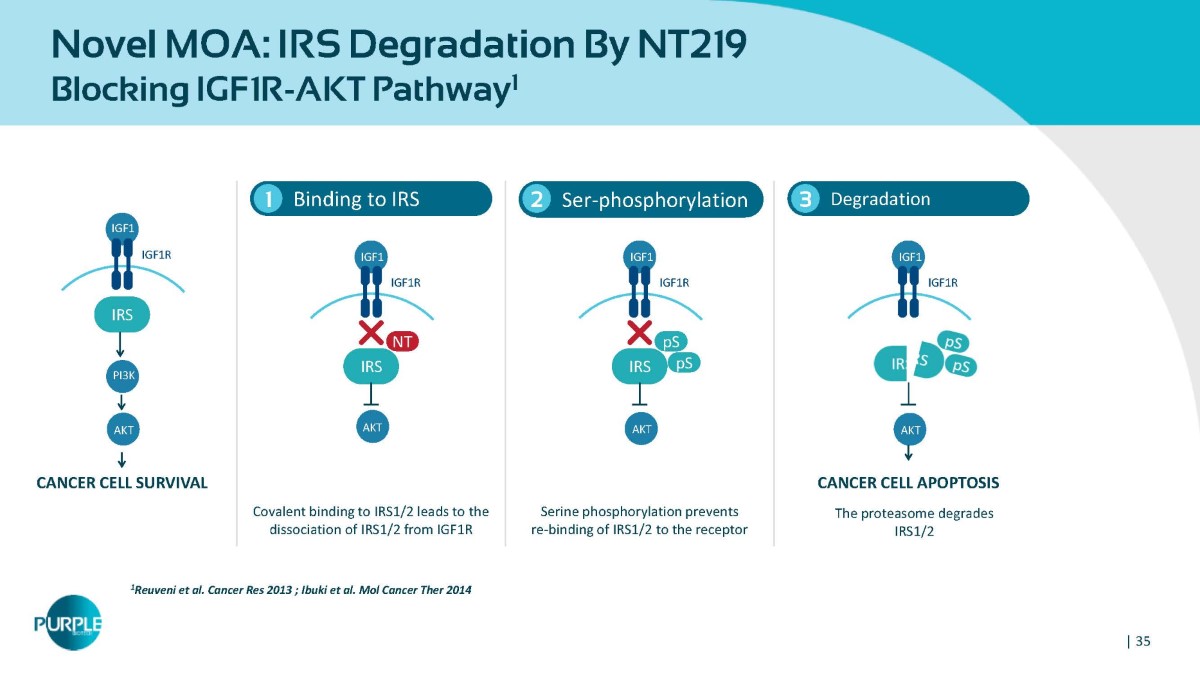
Novel MOA: IRS Degradation By NT219 Blocking IGF1R - AKT Pathway 1 Binding to IRS 1 1 Reuveni et al. Cancer Res 2013 ; Ibuki et al. Mol Cancer Ther 2014 2 Ser - phosphorylation Degradation 3 Covalent binding to IRS1/2 leads to the dissociation of IRS1/2 from IGF1R Serine phosphorylation prevents re - binding of IRS1/2 to the receptor CANCER CELL SURVIVAL CANCER CELL APOPTOSIS The proteasome degrades IRS1/2 IGF1 IGF1R IRS AKT PI3K IGF1 IGF1R IRS NT AKT IGF1 IGF1R IRS pS pS AKT IGF1 IGF1R AKT | 35
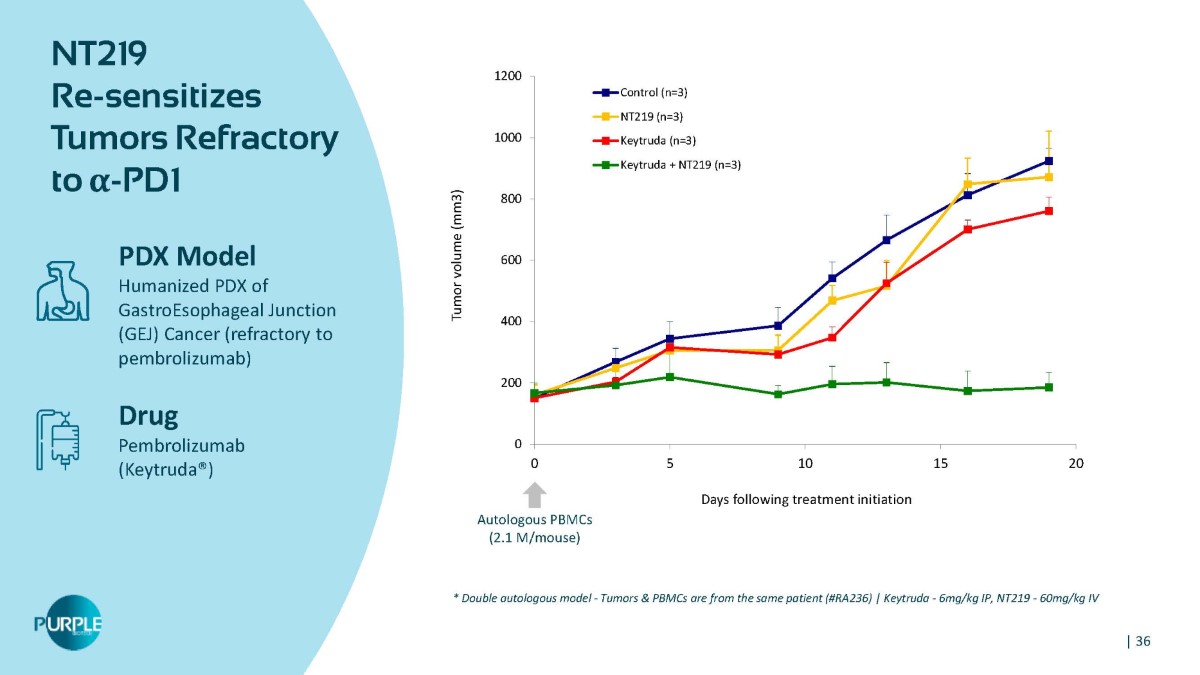
0 200 400 600 800 1000 1200 0 5 10 15 20 Tumor volume (mm3) Days following treatment initiation Control (n=3) NT219 (n=3) Keytruda (n=3) Keytruda + NT219 (n=3) Autologous PBMCs (2.1 M/mouse) NT219 Re - sensitizes Tumors Refractory to α - PD1 PDX Model Humanized PDX of GastroEsophageal Junction (GEJ) Cancer (refractory to pembrolizumab) Drug Pembrolizumab (Keytruda®) * Double autologous model - Tumors & PBMCs are from the same patient (#RA236) | Keytruda - 6mg/kg IP, NT219 - 60mg/kg IV | 36
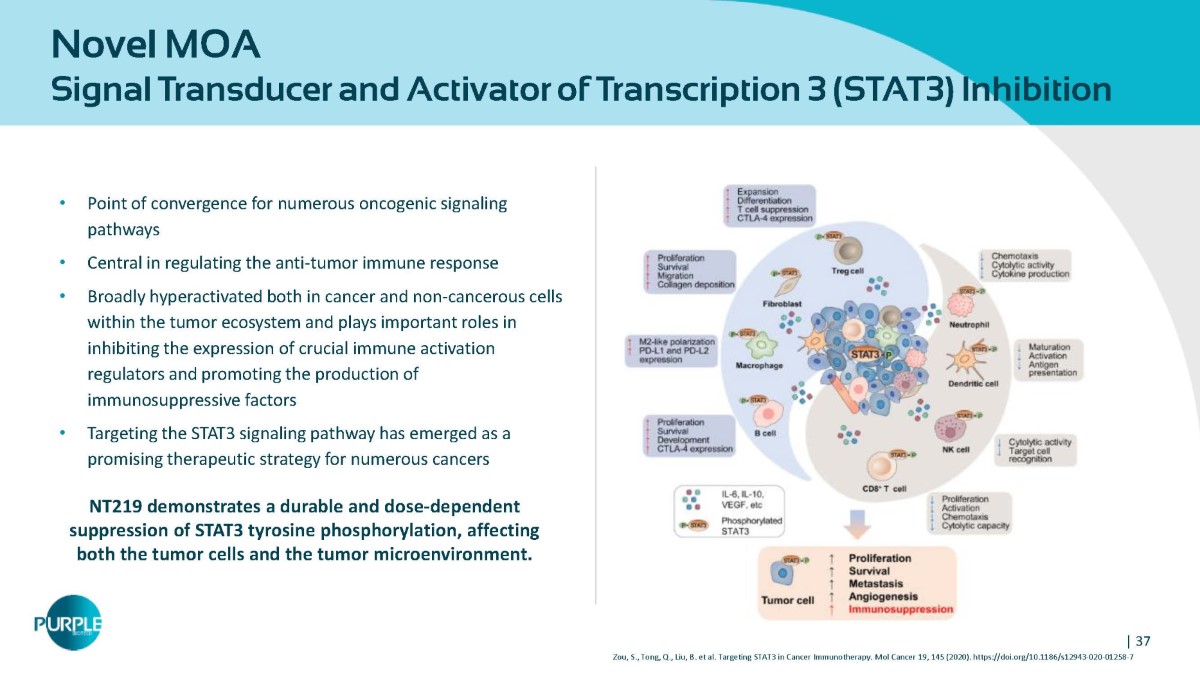
| 37 Zou, S., Tong, Q., Liu, B. et al. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer 19, 145 (2020). https://doi.org/10.1186/s12943 - 020 - 01258 - 7 Novel MOA Signal Transducer and Activator of Transcription 3 (STAT3) Inhibition • Point of convergence for numerous oncogenic signaling pathways • Central in regulating the anti - tumor immune response • Broadly hyperactivated both in cancer and non - cancerous cells within the tumor ecosystem and plays important roles in inhibiting the expression of crucial immune activation regulators and promoting the production of immunosuppressive factors • Targeting the STAT3 signaling pathway has emerged as a promising therapeutic strategy for numerous cancers NT219 demonstrates a durable and dose - dependent suppression of STAT3 tyrosine phosphorylation, affecting both the tumor cells and the tumor microenvironment.
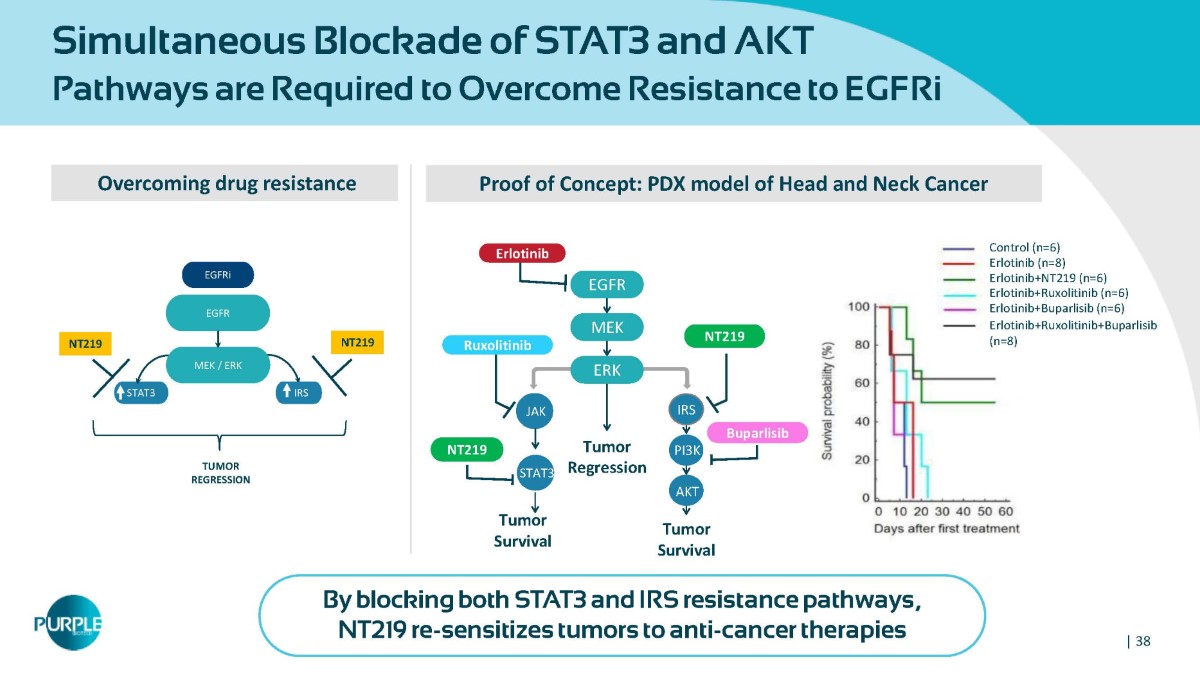
| 38 Simultaneous Blockade of STAT3 and AKT Pathways are Required to Overcome Resistance to EGFRi Overcoming drug resistance NT219 NT219 STAT3 IRS EGFR EGFRi MEK / ERK TUMOR REGRESSION Proof of Concept: PDX model of Head and Neck Cancer Control (n=6) Erlotinib (n=8) Erlotinib+NT219 (n=6) Erlotinib+Ruxolitinib (n=6) Erlotinib+Buparlisib (n=6) Erlotinib+Ruxolitinib+Buparlisib (n=8) STAT3 IRS EGFR MEK Tumor Regression ERK PI3K AKT Tumor Survival Tumor Survival Buparlisib Ruxolitinib Erlotinib NT219 NT219 JAK By blocking both STAT3 and IRS resistance pathways, NT219 re - sensitizes tumors to anti - cancer therapies
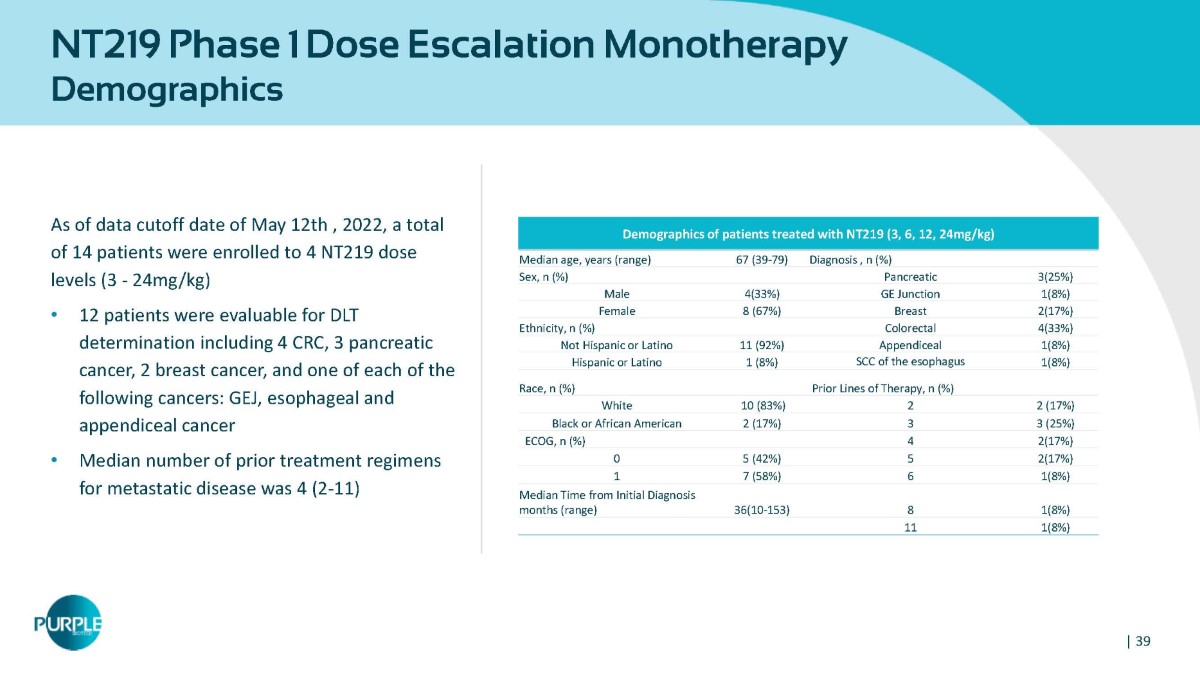
NT219 Phase 1 Dose Escalation Monotherapy Demographics As of data cutoff date of May 12th , 2022, a total of 14 patients were enrolled to 4 NT219 dose levels (3 - 24mg/kg) • 12 patients were evaluable for DLT determination including 4 CRC, 3 pancreatic cancer, 2 breast cancer, and one of each of the following cancers: GEJ, esophageal and appendiceal cancer • Median number of prior treatment regimens for metastatic disease was 4 (2 - 11) Demographics of patients treated with NT219 (3, 6, 12, 24mg/kg) Diagnosis , n (%) 67 (39 - 79) Median age, years (range) 3(25%) Pancreatic Sex, n (%) 1(8%) GE Junction 4(33%) Male 2(17%) Breast 8 (67%) Female 4(33%) Colorectal Ethnicity, n (%) 1(8%) Appendiceal 11 (92%) Not Hispanic or Latino 1(8%) SCC of the esophagus 1 (8%) Hispanic or Latino Prior Lines of Therapy, n (%) Race, n (%) 2 (17%) 2 10 (83%) White 3 (25%) 3 2 (17%) Black or African American 2(17%) 4 ECOG, n (%) 2(17%) 5 5 (42%) 0 1(8%) 6 7 (58%) 1 1(8%) 8 36(10 - 153) Median Time from Initial Diagnosis months (range) 1(8%) 11 | 39

Selected Publications Michael Karin Alexander Levitzki Menashe Bar - Eli Michael Cox | 40

Colon cancer LS - 513 cells overexpressing IRS2 demonstrate enhanced - catenin activity. Targeted inhibition of IRS2 by NT219 or IRS2 - SH RNA, suppresses the increased - catenin activity and inhibit LS - 513 cell viability. Combination of 5 - FU and NT219 significantly inhibited the growth of CRC tumors in brain, using intracranial model and extended mice survival. AACR Annual Meeting, April 2021, AACR Virtual Special Conference on Epigenetics and Metabolism, Oct 2020, Ido Wolf, MD, Head of Oncology Division, Tel Aviv Sourasky Medical Center IRS2 Active β - Catenin β - actin NT219 - + CRC Cell Viability (72hr) NT219 concentrations ( M) 0.5 1.0 1.5 *** ** DMEM iAKT Alpelisib NT - 219 IRS2 NT219 downregulates IRS2 and suppresses the - catenin activity Silencing IRS2 inhibits - catenin activity NT219 Suppresses β - Catenin activity in CRC Cells and Inhibited CRC Brain Metastasis 5 - FU NT219 + 5 - FU In Vitro | 41 In Vivo
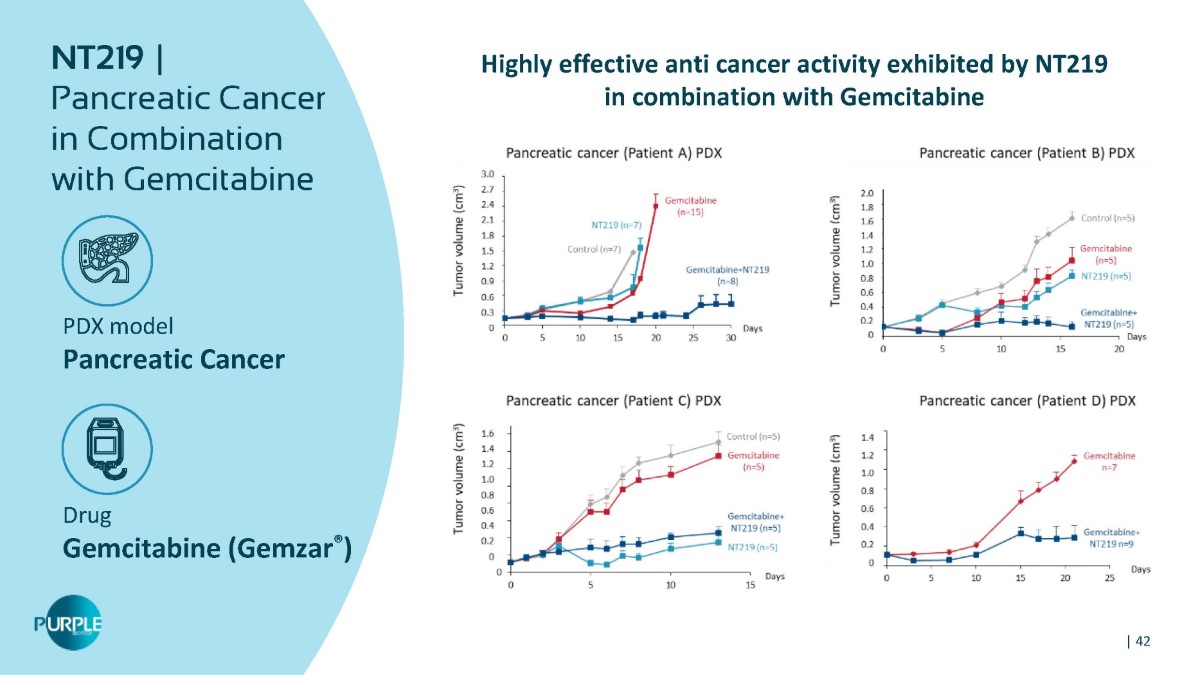
PDX model Pancreatic Cancer Drug Gemcitabine (Gemzar ® ) NT219 | Pancreatic Cancer in Combination with Gemcitabine | 42 Highly effective anti cancer activity exhibited by NT219 in combination with Gemcitabine
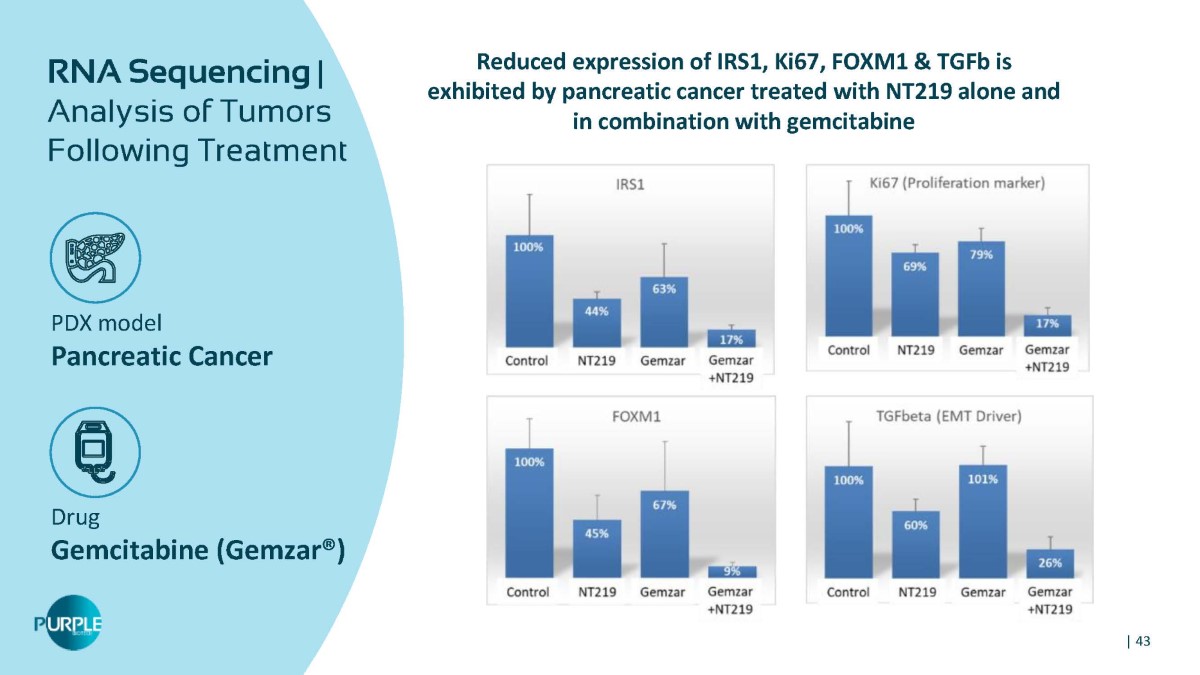
PDX model Pancreatic Cancer Drug Gemcitabine (Gemzar®) RNA Sequencing | Analysis of Tumors Following Treatment Reduced expression of IRS1, Ki67, FOXM1 & TGFb is exhibited by pancreatic cancer treated with NT219 alone and in combination with gemcitabine | 43
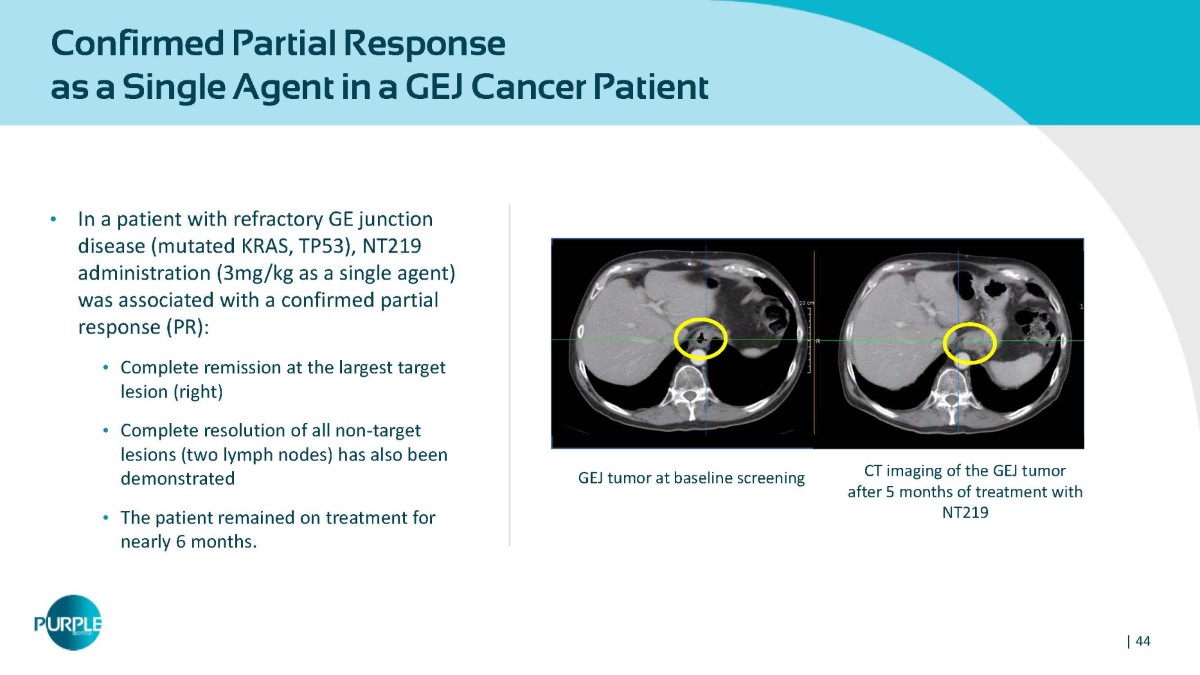
Confirmed Partial Response as a Single Agent in a GEJ Cancer Patient • In a patient with refractory GE junction disease (mutated KRAS, TP53), NT219 administration (3mg/kg as a single agent) was associated with a confirmed partial response (PR): • Complete remission at the largest target lesion (right) • Complete resolution of all non - target lesions (two lymph nodes) has also been demonstrated • The patient remained on treatment for nearly 6 months. Baseline scan 24 weeks scan CT imaging of the GEJ tumor after 5 months of treatment with NT219 GEJ tumor at baseline screening | 44
Purple Biotech (NASDAQ:PPBT)
過去 株価チャート
から 4 2024 まで 5 2024

Purple Biotech (NASDAQ:PPBT)
過去 株価チャート
から 5 2023 まで 5 2024
