UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the Month of October 2023
Commission
file number 001- 41291
Meihua
International Medical Technologies Co., Ltd.
(Translation of registrant’s name into English)
88 Tongda Road, Touqiao Town
Guangling District, Yangzhou, 225000
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F:
Form 20-F ☒
Form 40-F ☐
Explanatory Note
Meihua International
Medical Technologies Co., Ltd. (the “Company”) is furnishing its unaudited financial statements for the six months ended June
30, 2023 and incorporating such financial statements into the Company’s registration statement referenced below. The financial statements
and notes are attached as Exhibit 99.1 to this report. Management’s Discussion and Analysis of Financial Condition and Results of
Operations for the six months ended June 30, 2023 is attached as Exhibit 99.2 to this report.
This Form 6-K is hereby
incorporated by reference into the registration statement on Form F-3 of the Company (File Number 333-274194), as amended, and into the
prospectus contained within the foregoing registration statement, to the extent not superseded by documents or reports subsequently filed
or furnished by the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
Meihua International Medical Technologies Co., Ltd. |
| Dated: October 2, 2023 |
|
|
| |
By: |
/s/ Xin Wang |
| |
Name: |
Xin Wang |
| |
Title: |
Chief Executive Officer |
| |
|
(Principal Executive Officer) |
001-41291
Exhibit 99.1
INDEX TO THE UNAUDITED CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
TABLE OF CONTENTS
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
As of June 30, 2023 and December 31, 2022
(US$, except share data or otherwise noted)
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
| | |
| |
| Assets | |
| | |
| |
| Current Assets | |
| | |
| |
| Cash | |
$ | 17,861,214 | | |
$ | 26,736,700 | |
| Bank acceptance receivables | |
| 18,374,380 | | |
| 22,085,846 | |
| Accounts receivable | |
| 79,052,428 | | |
| 68,945,792 | |
| Inventories | |
| 1,647,146 | | |
| 1,122,038 | |
| Prepayment and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| Total current assets | |
| 132,264,679 | | |
| 135,319,155 | |
| | |
| | | |
| | |
| Property, plant and equipment | |
| 8,617,192 | | |
| 8,758,047 | |
| Intangible assets | |
| 3,876,027 | | |
| 497,600 | |
| Investment | |
| 5,997,634 | | |
| 6,669,655 | |
| Other noncurrent assets | |
| 11,856,920 | | |
| 12,333,122 | |
| Total assets | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
| | |
| | | |
| | |
| Liabilities and shareholders’ equity | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Short-term bank borrowings | |
$ | 7,171,128 | | |
$ | 6,089,428 | |
| Accounts payable | |
| 13,820,348 | | |
| 16,096,165 | |
| Taxes payable | |
| 1,451,855 | | |
| 1,131,276 | |
| Accrued expenses and other current liabilities | |
| 778,369 | | |
| 856,698 | |
| Total current liabilities | |
| 23,221,700 | | |
| 24,173,567 | |
| | |
| | | |
| | |
| Long term loan | |
| - | | |
| 724,932 | |
| Total liabilities | |
| 23,221,700 | | |
| 24,898,499 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| | | |
| | |
| Shareholders’ equity | |
| | | |
| | |
| Ordinary share, $0.0005 par value, 80,000,000 shares authorized, 23,940,000 and 23,940,000 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively | |
| 11,970 | | |
| 11,970 | |
| Preferred share, $0.0005 par value, 20,000,000 shares authorized, no shares issued and outstanding as of as of June 30, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Additional paid-in capital | |
| 42,967,006 | | |
| 42,967,006 | |
| Statutory surplus reserves | |
| 15,665,860 | | |
| 15,665,860 | |
| Retained earnings | |
| 90,392,246 | | |
| 83,330,239 | |
| Accumulated other comprehensive income (loss) | |
| (10,146,195 | ) | |
| (3,852,138 | ) |
| Total shareholders’ equity | |
| 138,890,887 | | |
| 138,122,937 | |
| Non-controlling interest | |
| 499,865 | | |
| 556,143 | |
| TOTAL EQUITY | |
| 139,390,752 | | |
| 138,679,080 | |
| | |
| | | |
| | |
| Total liabilities and shareholders’ equity | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
For the six months ended June 30, 2023 and 2022
(US$, except share data or otherwise noted)
| | |
For the Six months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| Third party sales | |
$ | 48,178,325 | | |
$ | 54,803,181 | |
| Related party sales | |
| 11,751 | | |
| 29,666 | |
| Total revenues | |
| 48,190,076 | | |
| 54,832,847 | |
| Cost of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| | |
| | | |
| | |
| Gross profit | |
| 17,170,729 | | |
| 20,891,732 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Selling | |
| 3,161,070 | | |
| 3,311,649 | |
| General and administrative | |
| 3,452,610 | | |
| 4,799,711 | |
| Research and development | |
| 1,460,376 | | |
| 1,642,204 | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Total operating expenses | |
| 8,074,056 | | |
| 12,223,030 | |
| | |
| | | |
| | |
| Income from operations | |
| 9,096,673 | | |
| 8,668,702 | |
| | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | |
| Interest expense | |
| 128,973 | | |
| 98,805 | |
| Interest income | |
| (361,532 | ) | |
| (19,725 | ) |
| Currency exchange gain | |
| 119,193 | | |
| (449,217 | ) |
| Other expense, net | |
| 114,298 | | |
| 50,180 | |
| Total other (income) expenses | |
| 932 | | |
| (319,957 | ) |
| | |
| | | |
| | |
| Income before income tax provision | |
| 9,095,741 | | |
| 8,988,659 | |
| Income taxes expense | |
| 2,064,212 | | |
| 2,433,772 | |
| Net income | |
| 7,031,529 | | |
$ | 6,554,887 | |
| Net loss attributable to non-controlling interests | |
| (30,478 | ) | |
| - | |
| Net income attributable to shareholders | |
| 7,062,007 | | |
| 6,554,887 | |
| | |
| | | |
| | |
| Foreign currency translation adjustment – gain / (loss) | |
| (6,319,857 | ) | |
| (6,133,093 | ) |
| Comprehensive (loss) income | |
$ | 711,672 | | |
$ | 421,794 | |
| Comprehensive loss attributable to non-controlling interests | |
| (56,278 | ) | |
| - | |
| Comprehensive (loss) income attributable to shareholders | |
| 767,950 | | |
| 421,794 | |
| | |
| | | |
| | |
Weighted average number of ordinary shares - basic and diluted | |
| 23,940,000 | | |
| 22,873,370 | |
| | |
| | | |
| | |
Basic & diluted net income per ordinary share | |
$ | 0.29 | | |
$ | 0.29 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
For the six months ended June 30, 2023 and 2022
(US$, except share data)
| | |
Ordinary
shares | | |
Ordinary
shares
amount | | |
Additional
paid-in
capital | | |
Ordinary
shares
subscribed | | |
Statutory
surplus
reserves | | |
Retained
earnings | | |
Accumulated
other
comprehensive
income (loss) | | |
Non-
controlling
interests | | |
Total
Equity | |
| Balance
as of December 31, 2021 | |
| 20,000,000 | | |
$ | 10,000 | | |
$ | 9,716,484 | | |
$ | - | | |
$ | 15,178,467 | | |
$ | 77,574,663 | | |
$ | 5,288,988 | | |
| - | | |
$ | 107,768,602 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Ordinary
shares subscribed | |
| 3,940,000 | | |
| 1,970 | | |
| 33,748,358 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 33,750,328 | |
| Net
income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6,554,887 | | |
| - | | |
| - | | |
| 6,554,887 | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,133,093 | ) | |
| - | | |
| (6,133,093 | ) |
| Balance
as of June 30, 2022 | |
| 23,940,000 | | |
$ | 11,970 | | |
$ | 43,464,842 | | |
| - | | |
$ | 15,178,467 | | |
$ | 84,129,550 | | |
$ | (844,105 | ) | |
| - | | |
$ | 141,940,724 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of December 31, 2022 | |
| 23,940,000 | | |
$ | 11,970 | | |
$ | 42,967,006 | | |
| - | | |
$ | 15,665,860 | | |
$ | 83,330,239 | | |
$ | (3,852,138 | ) | |
| 556,143 | | |
$ | 138,679,080 | |
| Net
income | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 7,062,007 | | |
| - | | |
| (30,478 | ) | |
| 7,031,529 | |
| Currency
translation adjustment | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (6,294,057 | ) | |
| (25,800 | ) | |
| (6,319,857 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance
as of June 30, 2023 | |
| 23,940,000 | | |
| 11,970 | | |
| 42,967,006 | | |
| - | | |
| 15,665,860 | | |
| 90,392,246 | | |
| (10,146,195 | ) | |
| 499,865 | | |
| 139,390,752 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS
OF CASH FLOWS
For the six months ended June 30, 2023 and 2022
(US$)
| | |
For The Six Months Ended June 30, | |
| | |
2023 | | |
2022 | |
| Cash Flows from operating activities: | |
| | |
| |
| Net income | |
$ | 7,031,529 | | |
$ | 6,554,887 | |
| Adjustments for items not affecting cash: | |
| | | |
| | |
| Depreciation | |
| 283,484 | | |
| 239,597 | |
| Amortization | |
| 13,733 | | |
| 13,417 | |
| Net loss from disposal of property, plant and equipment | |
| 104,572 | | |
| - | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Deferred tax expenses (benefit) | |
| - | | |
| (294,567 | ) |
| Currency exchange (gain) loss | |
| 119,193 | | |
| - | |
| Loss from equity method investments | |
| 1,632 | | |
| - | |
| Gain from cost method investments | |
| (202 | ) | |
| - | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Bank acceptance receivables | |
| 2,755,706 | | |
| (9,730,622 | ) |
| Accounts receivable | |
| (14,101,576 | ) | |
| 3,472,261 | |
| Inventories | |
| (606,934 | ) | |
| (7,197 | ) |
| Prepayments and other assets | |
| 178,920 | | |
| (5,268,377 | ) |
| Due from related parties | |
| - | | |
| (33,523 | ) |
| Accounts payable | |
| (1,559,255 | ) | |
| (4,851,578 | ) |
| Taxes payable | |
| 393,343 | | |
| (285,556 | ) |
| Accrued expenses and other current liabilities | |
| (38,714 | ) | |
| (64,985 | ) |
| Advance from customers | |
| - | | |
| (12,039 | ) |
| Net cash used in operating activities | |
| (5,424,569 | ) | |
| (7,798,816 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchases of property, plant and equipment | |
| (1,001,925 | ) | |
| (459,163 | ) |
| Additions to intangible assets | |
| (3,581,058 | ) | |
| - | |
| Proceeds from disposal of property, plant and equipment | |
| 355,993 | | |
| - | |
| Proceeds from disposal of long-term investment | |
| 360,839 | | |
| - | |
| Net cash used in investing activities | |
| (3,866,151 | ) | |
| (459,163 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from short-term bank borrowings | |
| 5,340,415 | | |
| 3,549,857 | |
| Repayments of short-term bank borrowings | |
| (4,618,738 | ) | |
| (2,932,491 | ) |
| Shareholder contribution | |
| - | | |
| 34,527,480 | |
| Net cash provided by financing activities | |
| 721,677 | | |
| 35,144,846 | |
| | |
| | | |
| | |
| Effect of foreign exchange rate changes | |
| (306,443 | ) | |
| (370,615 | ) |
| Net increase (decrease) in cash | |
| (8,875,486 | ) | |
| 26,516,252 | |
| Cash, beginning of year | |
| 26,736,700 | | |
| 8,149,276 | |
| Cash, end of year | |
$ | 17,861,214 | | |
$ | 34,665,528 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOWS INFORMATION: | |
| | | |
| | |
| Cash paid during the period for: | |
| | | |
| | |
| Cash paid for income tax | |
$ | 2,313,417 | | |
$ | 2,956,643 | |
| Cash paid for interest | |
$ | 128,973 | | |
$ | 98,804 | |
| | |
| | | |
| | |
| Non-cash transactions | |
| | | |
| | |
| Shareholder contribution through deferred cost | |
| - | | |
| 1,277,152 | |
The accompanying notes form an integral part
of these consolidated financial statements.
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS
1. Organization and principal activities
Principal Activities:
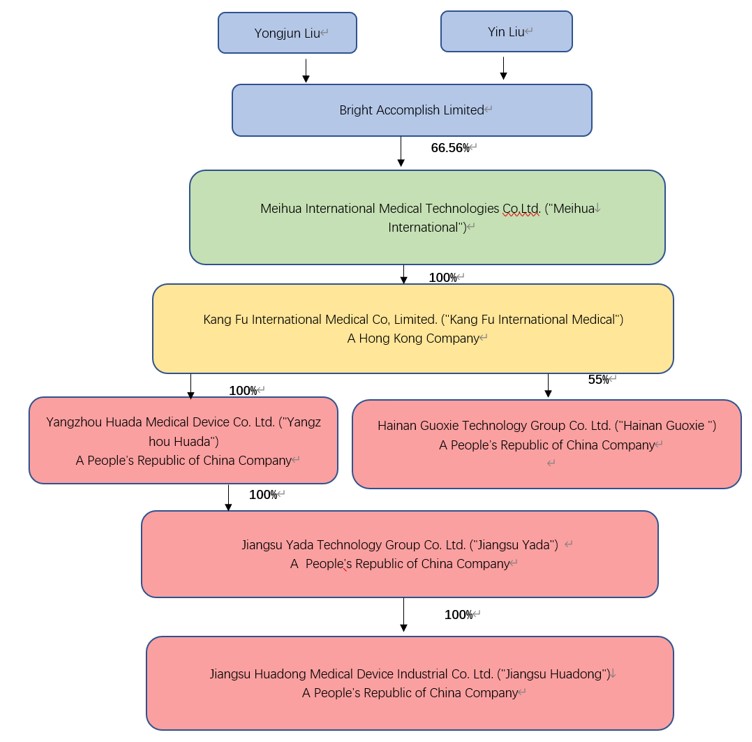
Meihua International Medical Technologies Co.,
Ltd. (“Meihua”) was incorporated on November 10, 2020 in the Cayman Islands. Meihua is a holding company with no operations.
Meihua produces and sells medical consumables through its wholly owned subsidiaries located in People’s Republic of China (“PRC”
or “China”). Below is Meihua’s organizational chart, as well as a description of the ownership structure.
Entity Name | |
Registered
Location | |
Percentage
of
ownership | |
Date of
incorporation | |
Principal
activities |
| Meihua International Medical Technologies Co., Ltd. (“Meihua”) | |
Cayman | |
Parent | |
November 10, 2020 | |
Investment holding |
| | |
| |
| |
| |
|
康复国际医疗有限公司
Kang Fu International Medical Co., Limited (“Kang Fu”) | |
Hong Kong | |
100% by Meihua | |
October 13, 2015 | |
Investment holding |
| | |
| |
| |
| |
|
扬州华达医疗器械有限公司
Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) | |
Yangzhou | |
100% by Kang Fu | |
December 24, 2001 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏亚达科技集团有限公司
Jiangsu Yada Technology Group Co., Ltd. (“Yada”) | |
Yangzhou | |
100% by Huada | |
December 5, 1991 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏华东医疗器械实业有限公司
Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) | |
Yangzhou | |
100% by Yada | |
November 18, 2000 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
扬州光辉医疗科技有限公司*
Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) | |
Yangzhou | |
100% by Huadong | |
December 22, 2020 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
海南国械医疗科技有限公司
Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) | |
Hainan | |
55% by Kang Fu | |
October 07, 2021 | |
Medical Equipment Sales |
Kang Fu was incorporated on October 13, 2015 with
a registered capital of HKD 53,911,815 ($6,911,771). Kang Fu is a holding company with no operations. The following operating entities
(Huada, Yada and Huadong) are all directly and indirectly 100% owned by Kang Fu for all the periods presented.
Huada is a subsidiary wholly owned by Kang Fu
and established in Yangzhou, China on December 24, 2001 with a registered capital of $17,193,021.
Yada is a subsidiary wholly owned by Huada and
was established in Yangzhou, China on December 5, 1991 with a registered capital of RMB51,390,000.
Huadong is a subsidiary wholly owned by Yada and
was established in Yangzhou, China on November 18, 2000 with a registered capital of RMB50,000,000.
Those three subsidiaries primarily manufacture
and sell Class I, II and III disposable medical devices under the Company’s own brands, and distribute Class I, II and III disposable
medical devices sourced from other manufacturers to our domestic and overseas customers.
Guanghui was a subsidiary wholly owned by Huadong
and was dissolved on June 1, 2023.
Hainan Guoxie is a subsidiary 55% owned by Kang
Fu and established in Hainan, China on October 07, 2021 with a registered capital of RMB100,000,000.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated
financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S.
GAAP”) pursuant to the rules and regulations of the Securities Exchange Commission (“SEC”). The interim results of operations
are not necessarily indicative of results to be expected for any other interim period or for a full year. In the opinion of management,
all adjustments, consisting only of normal recurring adjustments, considered necessary for a fair presentation of its financial position
and operating results have been included. These financial statements should be read in conjunction with the Company’s audited consolidated
financial statements and the related notes thereto for the fiscal years ended December 31, 2022 and 2021.
Use of Estimates
The preparation of unaudited condensed consolidated
financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the amounts
reported and disclosed in the consolidated financial statements and related notes.
The most significant estimates and judgments include
allowance for bad debts and the valuation of inventory. Actual amounts could differ from those estimates.
Non-controlling interests
Non-controlling interest represents the portion
of the net assets of subsidiaries attributable to interests that are not owned or controlled by the Company. The non-controlling interest
is presented in the consolidated balance sheets, separately from equity attributable to the shareholders of the Company. Non-controlling
interest’s operating results are presented on the face of the consolidated statements of income and comprehensive income as an allocation
of the total income for the year between non-controlling shareholders and the shareholders of the Company. As of June 30, 2023, non-controlling
interests represent one non-controlling shareholders’ proportionate share of equity interests in Hainan Guoxie.
Functional Currency and Foreign Currency
Translation
The Company’s reporting currency is the
United States dollar (“US$”). The Company’s operations are principally conducted through the PRC subsidiaries where
the local currency is the functional currency. Therefore, the functional currency of Kang Fu is the Hong Kong dollar and the functional
currency of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”).
Transactions denominated in currencies other than
the functional currencies are translated into the functional currency of the entity at the exchange rates prevailing on the transaction
dates. Monetary assets and liabilities denominated in currencies other than the applicable functional currency are translated into the
functional currency at the prevailing rates of exchange at the balance date. The resulting exchange differences are reported in the consolidated
statements of income and comprehensive income.
The assets and liabilities of the Company are
translated at the exchange spot rate at the balance sheet date, stockholders’ equity is translated at the historical rates and the
revenues and expenses are translated at the average exchange rates for the periods, except that the exchange rate used for translation
from Hong Kong dollar to US$ was 7.8000, a pegged rate determined by the linked exchange rate system in Hong Kong. This pegged rate was
used to translate Kang Fu’s balance sheets, income statement items and cash flow items for both the six months ended June 30, 2023
and 2022. The resulting translation adjustments are reported under other comprehensive income in the consolidated statements of income
and comprehensive income in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification
(“ASC”) 220, Comprehensive Income. The following are the exchange rates that were used in translating the Company’s
PRC subsidiaries’ financial statements into the consolidated financial statements:
| | |
June 30, 2023 | |
December 31, 2022 | |
June 30, 2022 |
| | |
| |
| |
|
| Period-end spot rate | |
US$1=RMB 7.2513 | |
US$1=RMB 6.8972 | |
US$1=RMB 6.6981 |
| | |
| |
| |
|
| Average rate | |
US$1=RMB 6.9283 | |
US$1=RMB 6.7290 | |
US$1=RMB 6.4791 |
Certain Risks and Concentration
The Company’s financial instruments that
potentially subject the Company to significant concentrations of credit risk consist primarily of cash and receivables. As of six months
ended June 30, 2023 and December 31, 2022 substantially all the Company’s cash were held in major financial institutions located
in Hong Kong and the PRC, which management considers to be of high credit quality.
For the six months ended June 30, 2023, two customers
accounted for approximately 18.18% and 10.01% of the Company’s total revenues. For the six months ended June 30, 2022, two customers
accounted for approximately 32.53% and 11.25% of the Company’s total revenues.
As of June 30, 2023, two customers accounted for
approximately 30.92% and 21.31% of the Company’s accounts receivable. As of December 31, 2022, two customers accounted for approximately
27.73% and 13.14% respectively, of the Company’s accounts receivable.
For the six months ended June 30, 2023, one supplier
accounted for approximately 14.73% of the Company’s total purchases. As of six months ended June 30, 2022, there was no supplier
that individually represented greater than 10% of the total purchase of the Company’s total purchases.
Fair Value Measurement
Fair value is the price that would be received
from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When
determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers
the principal or most advantageous market in which it would transact, and it considers assumptions that market participants would use
when pricing the asset or liability.
The Company adopted the guidance of Accounting
Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods
for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| |
Level 1: |
Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| |
|
|
| |
Level 3: |
Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The Company’s financial instruments include
cash, accounts receivable, bank acceptance receivables, due from related parties, accounts payable, other liabilities and accrued expenses
and short-term bank borrowings. The carrying amounts approximate their fair values due to their short maturities as of June 30, 2023 and
December 31, 2022.
The Company noted no transfers between levels
during any of the periods presented. The Company did not have any instruments that were measured at fair value on a recurring or non-recurring
basis as of June 30, 2023 and December 31, 2022.
Cash
Cash consists of petty cash on hand and cash held
in banks, which are highly liquid and are unrestricted as to withdrawal or use.
Bank Acceptance Receivables
Bank acceptance receivables are issued by bank
under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes until
maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party willing
to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year of the
original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers can
pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year, and are
non-interest bearing. As bank acceptance receivables are issued by the banks and payments are guaranteed. The Company has not discounted
any bank acceptances and there were no endorsed bank acceptances that are unmatured as of June 30, 2023. The Company collected approximately
$6.0 million as of August 31, 2023.
Accounts Receivable and Allowance for Doubtful
Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company follows the guidance under ASC 326,
Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Instruments. The standard uses a new forward-looking
“methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information
to inform credit loss estimates.” The Company has adopted the loss rate methodology to estimate historical losses on accounts receivables.
The Company has adopted the aging methodology to estimate the credit losses on accounts receivables. The historical data is adjusted to
account for forecasted changes in the macroeconomic environment in order to calculate the current expected credit loss. The provision
is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements of operations and
comprehensive income.
The Company historically has not had material
bad debts in accounts receivable. There were no bad debt expenses related to accounts receivable for six months ended June 30, 2023 and
2022, and there was no provision for doubtful accounts as of June 30, 2023 and December 31, 2022.
Inventories
Inventories are valued using the lower of cost
or net realizable value. Cost is principally determined using the weighted-average method. Manufactured inventories included cost of materials,
labor and overhead expenses. The Company records adjustments to inventory for excess quantities, obsolescence, or impairment, when appropriate,
to reflect inventory at net realizable value. These adjustments are based upon a combination of factors including current sales volume,
market conditions, lower of cost or market analysis and expected realizable value of the inventory.
There were no write-downs recognized of inventories
as of June 30, 2023 and December 31, 2022.
Prepayment and other current assets
As of June 30, 2023 and December 31, 2022, prepayment
and other current assets were $15,329,511 and $16,428,779.
Prepayment and other assets primarily consist
of prepayments for land use right and property, refundable tax credits and receivables, security deposits made to customers, advances
to employees, which are presented net of allowance for doubtful accounts. These balances are unsecured and are reviewed periodically to
determine whether their carrying value has become impaired. The Company considers the balances to be impaired if the utilization or refund
of the balances becomes doubtful. The Company uses the aging method to estimate the allowance for uncollectible balances. The allowance
is also based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical trends
of collections and utilizations. Actual amounts received or utilized may differ from management’s estimate of credit worthiness
and the economic environment. Delinquent account balances are written off against allowance for doubtful accounts after management has
determined that the likelihood of collection is not probable. The allowance for doubtful accounts amounted to nil as of June 30, 2023
and December 31, 2022.
Property, Plant and Equipment
Property, plant and equipment items are recorded
at their historic cost, less accumulated depreciation and impairment losses. The Company calculates depreciation using the straight-line
method, after consideration of the estimated residual values, over the following estimated useful lives:
| Category | |
Useful lives | |
Estimated
residual
value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
Major improvements are capitalized and expenditures
for maintenance and repairs are expensed as incurred. Construction in progress represents property, plant and equipment under construction
or being installed. Costs include original cost, installation, construction and other direct costs. Interest expenses directly related
to construction in progress would be capitalized. Construction in progress is transferred to the appropriate fixed asset account and depreciation
commences when the asset has been substantially completed and placed in service.
Intangible Assets
Intangible assets are non-monetary assets without
physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and
impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives.
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful lives, which is as
follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
Impairment of Long-Lived Assets
The Company accounts for impairment of long-lived
assets in accordance with Accounting Standards Codification (“ASC”) 360, Property, Plant and Equipment. (“ASC
360”). Long-lived assets consist primarily of property, plant and equipment, and intangible assets. In accordance with ASC 360,
the Company evaluates the carrying value of long-lived assets when it determines a triggering event has occurred, or whenever events or
changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When indicators exist, recoverability of
assets is measured by a comparison of the carrying value of the asset group to the estimated undiscounted future net cash flows expected
to be generated by the asset. Examples of such triggering events include a significant disposal of a portion of such assets, and adverse
change in the market involving the business employing the related assets. If such assets are determined not to be recoverable, the Company
performs an analysis of the fair value of the asset group and will recognize an impairment loss when the fair value is less than the carrying
amounts of such assets. The fair value, based on reasonable and supportable assumptions and projections, require subjective judgments.
Depending on the assumptions and estimates used, the appraised fair value projected in the evaluation of long-lived assets can vary within
a range of outcomes. The Company considers the likelihood of possible outcomes in determining the best estimate for the fair value of
the assets. The Company did not record any impairment charges for the six months ended June 30, 2023 and 2022. There can be no assurance
that future events will not have impact on company revenue or financial position which could result in impairment in the future.
Investment
In accordance with Financial Accounting Standards
Board (“FASB”) ASC 321, “Investment-Equity Securities,” the Company accounts for non-marketable securities on
a prospective basis. Equity investments that do not have readily determinable fair values and do not qualify for the net asset value practical
expedient are eligible for the measurement alternative.
On March 3, 2011, Yada invested in Yangzhou Juyuan
Guarantee Co., Ltd (“Juyuan”) and obtained 12% equity interest of Juyuan. For the Company’s passive and without significant
influence or control equity investment in private company which do not have readily determinable fair values, the Company has elected
the measurement alternative defined as cost, less impairment, plus or minus adjustments resulting from observable price changes in orderly
transactions for the identical or a similar investment of the Company. The investment is reviewed periodically to determine if its value
has been impaired and adjustments are recorded as necessary in profit or loss for the period. On January 5, 2023, majority shareholder
of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839 (RMB 2.5 million).
Investments in entities in which the Company can
exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting
in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method, the Company
initially records its investment at cost and the difference between the cost of the equity investee and the amount of the underlying equity
in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The share of earnings or
losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity method adjustments include the Company’s
proportionate share of investee income or loss, adjustments to recognize certain differences between the Company’s carrying value
and its equity in net assets of the investee at the date of investment, impairments, and other adjustments required by the equity method.
The Company assesses its equity investment for other-than-temporary impairment by considering factors as well as all relevant and available
information including, but not limited to, current economic and market conditions, the operating performance of the investees including
current earnings trends, the general market conditions in the investee’s industry or geographic area, factors related to the investee’s
ability to remain in business, such as the investee’s liquidity, debt ratios, and cash burn rate and other company-specific information.
Investments in equity securities without readily
determinable fair values are measured at cost minus impairment adjusted by observable price changes in orderly transactions for the identical
or a similar investment of the same issuer. These investments are measured at fair value on a nonrecurring basis when there are events
or changes in circumstances that may have a significant adverse effect. An impairment loss is recognized in the consolidated statements
of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment. Prior to the adoption
of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method of accounting, measured at cost less other-than-temporary
impairment.
On December 1, 2022, Huadong invested RMB 40 million
into Jiangsu Zhongxiangxin International Science and Technology Innovation Park Co., Ltd. (“Zhongxiangxin”), and obtained
25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the PRC. The Company accounted for
the investments using equity method, because the Company has significant influence but does not own a majority equity interest or otherwise
control over the equity investee. Under the equity method, the Company adjusts the carrying amount of the investment and recognizes investment
income or loss for its share of the earnings or loss of the investee after the date of investment. When the Company’s share of losses
in the equity investee equals or exceeds its interest in the equity investee, the Company does not recognize further losses, unless the
Company has incurred obligations or made payments or guarantees on behalf of the equity investee. For the six months ended June 30, 2023
and 2022, the investment loss from Zhongxiangxin was $1,632 and nil.
The Company continually reviews its investments
in equity investees to determine whether a decline in fair value below the carrying value is other-than-temporary. The primary factors
the Company considers in its determination include the financial condition, operating performance and the prospects of the equity investee;
other company specific information such as recent financing rounds; the geographic region, market and industry in which the equity investee
operates; and the length of time that the fair value of the investment is below its carrying value. If the decline in fair value is deemed
to be other-than-temporary, the carrying value of the equity investee is written down to fair value.
For the for the six months ended June 30, 2023
and 2022, no impairment indicators were identified of its investment in the private company was no impairment recorded.
Value-added Tax
Value-added taxes (“VAT”) collected
from customers relating to product sales and remitted to governmental authorities are presented on a net basis. VAT collected from customers
is excluded from revenue which is recorded in VAT payable. The Company is subject to a VAT rate of 13%. The VAT payable may be offset
by VAT paid by the Company on raw materials and other materials included in the cost of producing or acquiring its finished products.
Related Parties
Parties are considered to be related to the Company
if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with
the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal
owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly
influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully
pursuing its own separate interests. The Company discloses all significant related party transactions.
Revenue Recognition
Based on the requirements of ASC Topic 606, the
Company recognizes revenue when control of the promised goods or services is transferred to the customers in an amount that reflects the
consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells its products
to hospitals and medical equipment companies. Revenue is recognized when the following 5-step revenue recognition criteria are met:
| 1) | Identify
the contract with a customer |
| 2) | Identify
the performance obligations in the contract |
| 3) | Determine
the transaction price |
| 4) | Allocate
the transaction price |
| 5) | Recognize
revenue when or as the entity satisfies a performance obligation |
Revenue from product sales is recognized at the
point in time control of the products is transferred, generally upon customer receipt based upon the standard contract terms. Shipping
and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered to
be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard quality policy and
returns are generally not significant. Payment terms for product sales are generally set at 90 to 180 days after customer acceptance of
the product.
Revenue Disaggregation
The Company’s disaggregated revenues are
represented by two categories which are type of goods and type of customers.
Type of Goods
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 23,435,544 | | |
| 27,046,663 | |
| Resales of Sourced Disposable Medical Devices from Third Party Manufacturers | |
| 24,754,532 | | |
| 27,786,184 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Type of Customers
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Direct sales | |
| 4,305,506 | | |
| 4,582,321 | |
| Distributors | |
| 43,884,570 | | |
| 50,250,526 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Earnings per Ordinary Share
Earnings (loss) per ordinary share is calculated
in accordance with ASC 260, Earnings per Share. Basic earnings (loss) per ordinary share is computed by dividing the net income
(loss) attributable to shareholders of the Company by the weighted average number of ordinary shares outstanding during the year. Diluted
earnings per ordinary share is computed in accordance with the treasury stock method and based on the weighted average number of ordinary
shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents are excluded from the computation of diluted earnings
per ordinary share if their effects would be anti-dilutive. There is no ordinary share equivalent issued to date.
Comprehensive Income (Loss)
ASC 220, Comprehensive Income (“ASC 220”)
establishes rules for reporting and display of comprehensive income and its components. ASC 220 requires that unrealized gains and losses
on the Company’s foreign currency translation adjustments be included in comprehensive income (loss).
Advertising Costs
The Company’s advertising costs are expensed
as incurred. Advertising expenses are included in selling expenses in the accompanying consolidated statements of income and comprehensive
income. Advertising expenses were $8,275, and $7,603 for the six months ended June 30, 2023, and 2022, respectively.
Research and Development Costs
Research and development expenses are expensed
as incurred. Research and development expenses were $1,460,376 and $1,642,204 for the six months ended June 30, 2023, and 2022, respectively.
Income Tax
Current income taxes are provided on the basis
of net profit for financial reporting purposes, adjusted for income and expense items which are not assessable or deductible for income
tax purposes, in accordance with the regulations of the relevant tax jurisdictions.
Deferred income taxes are recognized for temporary
differences between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements, net operating
loss carry forwards and credits. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more
likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided in accordance
with the laws of the relevant taxing authorities. Deferred tax assets and liabilities are measured using enacted rates expected to apply
to taxable income in which temporary differences are expected to be reversed or settled. The effect on deferred tax assets and liabilities
of changes in tax rates is recognized in the statement of comprehensive income in the period of the enactment of the change.
The Company considers positive and negative evidence
when determining whether a portion or all of its deferred tax assets will more likely than not be realized. This assessment considers,
among other matters, the nature, frequency and severity of current and cumulative losses, forecasts of future profitability, the duration
of statutory carry-forward periods, its experience with tax attributes expiring unused, and its tax planning strategies. The ultimate
realization of deferred tax assets is dependent upon its ability to generate sufficient future taxable income within the carry-forward
periods provided for in the tax law and during the periods in which the temporary differences become deductible. When assessing the realization
of deferred tax assets, the Company has considered possible sources of taxable income including (i) future reversals of existing
taxable temporary differences, (ii) future taxable income exclusive of reversing temporary differences and carryforwards, (iii) future
taxable income arising from implementing tax planning strategies, and (iv) specific known trend of profits expected to be reflected
within the industry.
The Company recognizes a tax benefit associated
with an uncertain tax position when, in its judgment, it is more likely than not that the position will be sustained upon examination
by a taxing authority. For a tax position that meets the more-likely-than-not recognition threshold, the Company initially and subsequently
measures the tax benefit as the largest amount that the Company judges to have a greater than 50% likelihood of being realized upon ultimate
settlement with a taxing authority. The Company’s liability associated with unrecognized tax benefits is adjusted periodically due
to changing circumstances, such as the progress of tax audits, case law developments and new or emerging legislation. Such adjustments
are recognized entirely in the period in which they are identified. The Company’s effective tax rate includes the net impact of
changes in the liability for unrecognized tax benefits and subsequent adjustments as considered appropriate by management. The Company
classifies interest and penalties recognized on the liability for unrecognized tax benefits as income tax expense.
Segment Reporting
FASB ASC 280, “Segment Reporting,”
establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational
structure as well as information of the Company’s business segments, geographical areas, segments and major customers. The Company
uses the “management approach” in determining reportable operating segments. The management approach considers the internal
organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance
as the source for determining the Company’s reportable segments. The chief operating decision maker is the Company’s president
and Chief Executive Officer (“CEO”). Management, including the chief operating decision maker, reviews operating results of
different products at revenue level with no allocation of operating costs. Consequently, based on management’s assessment, the Company
has determined that it has only one operating segment as defined by FASB ASC 280.
The Company has disclosed the type of revenue by government category
as follows.
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 3,561,156 | | |
| 4,462,704 | | |
| 8,023,860 | | |
| 3,812,092 | | |
| 3,845,205 | | |
| 7,657,297 | |
| Class II | |
| 17,313,377 | | |
| 17,761,970 | | |
| 35,075,347 | | |
| 20,185,052 | | |
| 19,712,006 | | |
| 39,897,058 | |
| Class III | |
| 524,802 | | |
| 873,575 | | |
| 1,398,377 | | |
| 416,595 | | |
| 1,168,221 | | |
| 1,584,816 | |
| Others | |
| 2,036,209 | | |
| 1,656,283 | | |
| 3,692,492 | | |
| 2,632,924 | | |
| 3,060,752 | | |
| 5,693,676 | |
| Total | |
| 23,435,544 | | |
| 24,754,532 | | |
| 48,190,076 | | |
| 27,046,663 | | |
| 27,786,184 | | |
| 54,832,847 | |
Class I, II, and III medical devices are defined
by the National Medical Products Administration of China according to their risk levels under the Regulation on the Supervision and Administration
of Medical Devices (2021 Revision), Article 6 as follows:
| ● | “Class
I Medical Devices” means medical devices with low risks, whose safety and effectiveness can be ensured through routine administration. |
| ● | “Class
II Medical Devices” means medical devices with moderate risks, which shall be strictly controlled and administered to ensure their
safety and effectiveness. |
| ● | “Class
III Medical Devices” means medical devices with relatively high risks, which shall be strictly controlled and administered through
special measures to ensure their safety and effectiveness. |
Furthermore, the Company has disclosed revenue by major product type
included in each government category.
| | |
| |
June 30, 2023 | | |
June 30, 2022 | |
| Category | |
Products | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 1,073,853 | | |
| 1,346,164 | |
| | |
Oral medicine bottle | |
| 1,830,363 | | |
| 2,189,018 | |
| | |
Anal bag | |
| 849,099 | | |
| 395,292 | |
| | |
Other Class I | |
| 4,270,545 | | |
| 3,726,823 | |
| Subtotal-Class I | |
| |
| 8,023,860 | | |
| 7,657,297 | |
| Class II | |
Masks | |
| 47,946 | | |
| 211,468 | |
| | |
Identification tape | |
| 5,494,306 | | |
| 7,218,564 | |
| | |
Disposable medical brush | |
| 4,481,601 | | |
| 4,606,634 | |
| | |
Gynecological inspection kits | |
| 3,022,727 | | |
| 5,807,398 | |
| | |
Surgical kit | |
| 2,206,201 | | |
| 13,546,908 | |
| | |
Medical brush | |
| 2,809,448 | | |
| 2,586,945 | |
| | |
Medical kit | |
| 983,584 | | |
| 883,977 | |
| | |
Other Class II | |
| 16,029,534 | | |
| 5,035,164 | |
| Subtotal-Class II | |
| |
| 35,075,347 | | |
| 39,897,058 | |
| Class III | |
Electronic pump | |
| 138,751 | | |
| 67,866 | |
| | |
Anesthesia puncture kit | |
| 229,616 | | |
| 205,218 | |
| | |
Disposable infusion pump | |
| 113,335 | | |
| 78,453 | |
| | |
Infusion pump | |
| 178,461 | | |
| 90,036 | |
| | |
Electronic infusion pump | |
| 330 | | |
| 43,397 | |
| | |
Laparoscopic trocar | |
| 38 | | |
| 94,337 | |
| | |
Other Class III | |
| 737,846 | | |
| 1,005,509 | |
| Subtotal-Class III | |
| |
| 1,398,377 | | |
| 1,584,816 | |
| Others | |
| |
| 3,692,492 | | |
| 5,693,676 | |
| Total | |
| |
| 48,190,076 | | |
| 54,832,847 | |
For the six months ended June 30, 2023, and 2022,
revenues and assets within PRC contributed over 99.1% of the Company’s total revenues and assets.
The Outbreak of COVID-19
On January 30, 2020, the World Health Organization
declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency of International Concern,” and on March
11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” COVID-19 has had a severe and negative
impact on the Chinese and the global economy and such impact persists as of the date of this annual report.
In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply while the sales of products other than masks
declined due to an overall decrease in market demand. In fiscal year 2021, with the stable control of the domestic epidemic in China,
the market of masks was no longer in urgent shortage compared to the same period in 2020, and the production of epidemic prevention products
resumed more normal production levels. In general, with the precise control of the epidemic in China, our production and operations have
recovered smoothly, and the demand for other products has increased gradually. After the initial outbreak of COVID-19, from time to time,
some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the Omicron variants
in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series of restrictions and
quarantines were implemented to contain the spread.
Many of the restrictive measures previously adopted
by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more flexible
measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic could have
a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will be dependent
on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government actions
in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many other
factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the
expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the current
situation continues.
Recently Issued Accounting Standards
In October 2021, the FASB issued ASU No. 2021-08,
“‘Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers”
(“ASU 2021-08”). This ASU requires entities to apply Topic 606 to recognize and measure contract assets and contract liabilities
in a business combination. The amendments improve comparability after the business combination by providing consistent recognition and
measurement guidance for revenue contracts with customers acquired in a business combination and revenue contracts with customers not
acquired in a business combination. The amendments are effective for the Company beginning after December 15, 2023, and are applied prospectively
to business combinations that occur after the effective date. The Company does not expect the adoption of ASU 2021-04 will have a material
effect on the consolidated financial statements.
In June
2022, the FASB issued ASU 2022-03 Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual
Sale Restrictions. The update clarifies that a contractual restriction on the sale of an equity security is not considered part of the
unit of account of the equity security and, therefore, is not considered in measuring fair value. The update also clarifies that an entity
cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The update also requires certain additional
disclosures for equity securities subject to contractual sale restrictions. For public business entities, the amendments in this Update
are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities,
the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early
adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. As
an emerging growth company, the standard is effective for the Company for the year ended
December 31, 2025. The Company is in the process of evaluating the impact of the new guidance
on its consolidated financial statements.
3. Prepayments and other assets
Prepayments and other current assets consist of
the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Other receivable | |
| 159,817 | | |
| 239,148 | |
| Prepaid tax | |
| - | | |
| 250,410 | |
| Prepaid for land use right (1) | |
| 15,169,694 | | |
| 15,948,501 | |
| Prepaid for property (2) | |
| 11,856,920 | | |
| 12,323,842 | |
| Total | |
| 27,186,431 | | |
| 28,761,901 | |
| Less: non-current portion | |
| (11,856,920 | ) | |
| (12,333,122 | ) |
| Prepayments and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
4. Inventories
Inventories consist of the following:
| | |
June 30, 2023 | | |
December 31, 2021 | |
| | |
US$ | | |
US$ | |
| Raw material | |
| 493,143 | | |
| 177,474 | |
| Work-in-process | |
| 20,501 | | |
| 343,795 | |
| Finished goods | |
| 977,974 | | |
| 560,119 | |
| Goods in transit | |
| 95,814 | | |
| | |
| Low-value consumables | |
| 59,714 | | |
| 40,650 | |
| Total | |
| 1,647,146 | | |
| 1,122,038 | |
For the six months ended June 30, 2023 and 2022, there were no writes-down
of inventories.
5. Intangible Assets
Intangible assets consisted of the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Land use rights | |
| 4,134,521 | | |
| 752,887 | |
| Patents | |
| 27,582 | | |
| 28,997 | |
| Software | |
| 12,113 | | |
| 9,424 | |
| Trademarks | |
| 115,836 | | |
| 121,789 | |
| Total | |
| 4,290,052 | | |
| 913,097 | |
| Less: accumulated amortization | |
| 414,025 | | |
| 415,497 | |
| Intangible assets, net | |
| 3,876,027 | | |
| 497,600 | |
Amortization expense was $13,733 and $13,417 for
the six months ended June 30, 2023 and 2022, respectively. Hainan Guoxie spent $3.4 million in purchasing land use right for the six months
ended June 30, 2023 and the price was fully paid. The land will be used for manufacturing facility.
The following table sets forth the Company’s
amortization expenses for the twelve months ending December 31 of the following years:
| 2023 | |
$ | 41,870 | |
| 2024 | |
| 83,740 | |
| 2025 | |
| 83,740 | |
| 2026 | |
| 82,953 | |
| 2027 | |
| 82,690 | |
| Thereafter | |
| 3,501,034 | |
| | |
$ | 3,876,027 | |
As of June 30, 2023 and December 31, 2022, the
Company pledged land use rights to secure bank borrowings to the Company as disclosed in Note 7.
6. Investment
On March 3, 2011, Yada invested RMB 6 million
into Yangzhou Juyuan Guarantee Co., Ltd. (“Juyuan”) and obtained 12% equity interest of Juyuan. Juyuan mainly provides financing
guarantee services and relevant consulting services to customers. Juyuan has only one executive director and one supervisor. Neither the
executive director nor supervisor is related to Yada. Therefore, Yada has neither control nor significant influence over Juyuan. For the
Company’s passive and without significant influence or control equity investment in a private company which does not have readily
determinable fair values, the Company has elected the measurement alternative defined as cost, less impairment, plus or minus adjustments
resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. On January
5, 2023, majority shareholder of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839(RMB 2.5 million).
The carrying value of the investment amounted to approximately $0.5 million as of June 30, 2023.
On December 1, 2022, Huadong invested RMB40 million
into Zhongxiangxin, and obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the
PRC. The Company accounted for the investments using the equity method, because the Company has significant influence but does not own
a majority equity interest or otherwise control over the equity investee. Under the equity method, the Company adjusts the carrying amount
of the investment and recognizes investment income or loss for its share of the earnings or loss of the investee after the date of investment.
When the Company’s share of losses in the equity investee equals or exceeds its interest in the equity investee, the Company does
not recognize further losses, unless the Company has incurred obligations or made payments or guarantees on behalf of the equity investee.
For the six months ended June 30, 2023 and 2022, the investment loss from Zhongxiangxin was $1,632 and $nil.
For the six months ended June 30, 2023 and 2022,
no impairment indicators were identified related to revaluation of its investment in the private company was recorded.
7. Bank Borrowings
Bank borrowings are working capital loans from
banks in China. Short-term bank borrowings as of June 30, 2023 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
1/18/2023 | |
5/25/2024 | |
| 4,000,000 | | |
| 551,625 | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Agricultural Bank of China | |
Huadong | |
| 3.60 | % | |
8/12/2022 | |
7/12/2023 | |
| 9,000,000 | | |
| 1,241,157 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
1/30/2023 | |
2/15/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Bank of China | |
Huadong | |
| 3.80 | % | |
3/10/2023 | |
3/9/2024 | |
| 10,000,000 | | |
| 1,379,063 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023 | |
| 10,000,000 | | |
| 1,379,063 | |
| Industrial and Commercial Bank of China* | |
Yada | |
| 3.45 | % | |
2/17/2022 | |
2/16/2024 | |
| 9,000,000 | | |
| 1,241,156 | |
| Total | |
| |
| | | |
| |
| |
| 52,000,000 | | |
| 7,171,128 | |
Short-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 4,000,000 | | |
| 579,946 | |
| Agricultural Bank of China | |
Huadong | |
| 3.40 | % | |
12/8/2022 | |
12/7/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
2/17/2022 | |
3/2/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Bank of China | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023* | |
| 10,000,000 | | |
| 1,449,864 | |
| Industrial and Commercial Bank of China | |
Yada | |
| 3.70 | % | |
2/18/2022 | |
2/21/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| |
| | | |
| |
| |
| 42,000,000 | | |
| 6,089,428 | |
Interest expense was $128,973, and $98,805 for
the six months ended June 30, 2023 and 2022, respectively.
The Company’s short-term bank borrowings
are pledged by the Company’s assets and guaranteed by the Company’s major shareholders Yongjun Liu, Yin Liu and its subsidiary
Yada.
The carrying values of the Company’s pledged assets to secure
short-term borrowings by the Company are as follows:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| Buildings, net | |
| 3,432,150 | | |
| 2,777,379 | |
| Land use right, net | |
| 90,322 | | |
| 96,416 | |
| Total | |
| 3,522,472 | | |
| 2,873,795 | |
8. Long-term bank loan
There was no long-term bank loan as of June 30, 2023.
Long-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 724,932 | |
| Total | |
| |
| | | |
| |
| |
| 5,000,000 | | |
| 724,932 | |
On November 3, 2022, the Company signed a loan
agreement with Bank of Communications to obtain a two-year loan of RMB5 million ($724,932). The loan bears a floating interest rate of
a benchmark rate (3.50%). Huada mortgages the property and land for guaranteed repayment of the loan. The principal shall be repaid on
April 25, 2024. The balance was reclassified to short-term bank loan as of June 30, 2023.
9. Taxes Payable
Taxes payable consisted of the following:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| VAT payable | |
| 368,195 | | |
| 380,926 | |
| Income tax payable | |
| 1,012,775 | | |
| 690,824 | |
| Other tax payable | |
| 71,348 | | |
| 59,526 | |
| Total | |
| 1,452,318 | | |
| 1,131,276 | |
10. Income Taxes
Cayman Islands
Under the current laws of the Cayman Islands,
the Company is not subject to tax on income or capital gain. Additionally, upon payments of dividends to the shareholders, no Cayman Islands
withholding tax is imposed.
Hong Kong
Under the current Hong Kong Inland Revenue Ordinance,
the Company’s Hong Kong subsidiary, Kang Fu, is subject to 16.5% income tax on its taxable income generated from operations in Hong
Kong. On December 29, 2017, the Hong Kong government announced a two-tiered profit tax rate regime. Under the two-tiered tax rate regime,
the first HK$2.0 million earned in assessable profits will be subject to an 8.25% lower tax rate and the remaining taxable income will
continue to be taxed at the existing 16.5% tax rate. The two-tiered tax regime becomes effective from the assessment year of 2018 and
2019, which is on or after April 1, 2018. The application of the two-tiered rates is restricted to only one nominated enterprise among
connected entities. Kang Fu has been nominated by the Company as the entity to apply the two-tiered rates among the group for the assessment
years of 2023 and 2022.
PRC
Provisions for income tax are as follows:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Provisions for current income tax | |
| 2,064,697 | | |
| 2,433,772 | |
| Provisions for deferred income tax | |
| - | | |
| - | |
| Total | |
| 2,064,697 | | |
| 2,433,772 | |
The following is a reconciliation of the Company’s
total income tax expense to the income before income taxes for the six months ended June 30, 2023 and 2022, respectively:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Income before income tax provision | |
| 9,097,681 | | |
| 8,988,658 | |
| Tax at the PRC EIT tax rates | |
| 2,203,896 | | |
| 2,689,271 | |
| Change in valuation allowance | |
| 16,934 | | |
| - | |
| Tax effect of non-deductible expenses | |
| 208,961 | | |
| 157,007 | |
| Tax effect of R&D expenses additional deduction* | |
| (365,094 | ) | |
| (412,506 | ) |
| Income tax expense | |
| 2,064,697 | | |
| 2,433,772 | |
Under the Enterprise Income Tax Law (“EIT Law”), Foreign
Investment Enterprises (“FIEs”) and domestic companies are subject to Enterprise Income Tax (“EIT”) at a uniform
rate of 25%.
Huadong was granted a High and New Technology
Enterprise (“HNTE”) certificate and received a preferential tax rate of 15% for a three-year validity period from November
30, 2016 and the HNTE certificate was renewed on December 22, 2022 with a three-year validity period. Thus, Huadong will remain eligible
for a 15% preferential tax rate from January 1, 2016 through December 31, 2025.
The EIT Law also provides that an enterprise established
under the laws of a foreign country or region but whose “de facto management body” is located in the PRC be treated as a resident
enterprise for PRC tax purposes and consequently be subject to the PRC income tax at the rate of 25% for its global income. The Implementing
Rules of the EIT Law define the location of the “de facto management body” as “the place where the exercising, in substance,
of the overall management and control of the production and business operation, personnel, accounting, properties, etc., of a non-PRC
company is located.” Based on a review of surrounding facts and circumstances, the Company does not believe that it is likely that
its entities registered outside of the PRC should be considered as resident enterprises for the PRC tax purposes.
The EIT Law also imposes a withholding income
tax on dividends distributed by a FIE to its immediate holding company outside of the PRC. As a result, Kang Fu, which is the parent of
Huada, Yada and Huadong, is therefore subject to a maximum withholding tax of 10% on dividends distributed by Huada, Yada and Huadong.
In accordance with accounting guidance, all undistributed earnings are presumed to be transferred to the parent company and are subject
to the withholding taxes. The presumption may be overcome if the Company has sufficient evidence to demonstrate that the undistributed
dividends will be re-invested and the remittance of the dividends will be postponed indefinitely. As of June 30, 2023, the Company has
determined that the undistributed earnings in Huada, Yada and Huadong will be re-invested into the subsidiary for the expansion of the
Company’s business in mainland China and hence the remittance of dividends will be postponed indefinitely.
Uncertain tax positions
The Company evaluates each uncertain tax position
(including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits
associated with the tax positions. As of June 30, 2023, and 2022, the Company did not have any significant unrecognized uncertain tax
positions.
11. Commitments and Contingencies
Operating lease
The Company has an operating lease to rent an
office space in Shanghai. The lease term is 12 months, with the option to renew annually. Rent expense was $5,856 and $5,856 and is included
in general and administrative expenses for the six months ended June 30, 2023 and 2022, respectively. The Company has renewed the same
operating lease with a term from January 1, 2023 to December 31, 2023, with all other lease terms remaining the same.
Other commitments
The Company did not have other significant commitments,
long-term obligations or guarantees as of June 30, 2023 and 2022.
Contingencies
The Company is subject to legal proceedings and
regulatory actions in the ordinary course of business. The results of such proceedings cannot be predicted with certainty, but the Company
does not anticipate that the final outcome arising out of any such matter will have a material adverse effect on our consolidated business,
financial position, cash flows or results of operations taken as a whole.
On February 4, 2022, Macias Gini & O’Connell,
LLP (“Plaintiff”) initiated a lawsuit in San Francisco Superior Court. Plaintiff, a certified public accounting firm based
in the U.S., was hired by Kang Fu International Medical Co, and subsequently by Meihua International Medical Technologies Co, Ltd. (collectively,
“Meihua”), to audit Meihua’s consolidated financial statements for the 2018 and 2019 calendar years. Plaintiff seeks
damages from Meihua for its alleged failure to pay for services rendered in the amount of $210,000, plus interest and attorneys’
fees. The case was dismissed with prejudice in August of 2023.
On August 29, 2023, Zhu Cheng initiated a lawsuit
against Yada, Huada, Huadong and Kang Fu in Yangzhou Economic Development Zone Court. Zhu Cheng seeks damages from the above entities
for service fee of approximately $2.3 million (RMB 17.0 million). The Company is preparing to file a motion to dismiss the case. No contingent
liability has been accrued since the Company has deemed the possibility of loss to be remote.
12. Statutory Surplus Reserves and Restricted Net Assets
Pursuant to laws applicable to entities incorporated
in the PRC, the Company is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the
discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of
the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net
income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations
to the discretionary surplus reserve are made at the discretion of the Board of Directors. As of December 31, 2022 and June 30, 2023,
the Company did not have a discretionary surplus reserve. As of December 31, 2022, all of the Company’s PRC subsidiaries reserves
had reached 50% of their registered capital threshold and, as a result, the Company stopped being required to allocate after-tax profits
to this reserve.
As a result of these PRC laws and regulations
and the requirement that distributions by PRC entities can only be paid out of distributable profits computed in accordance with PRC GAAP,
the PRC entities are restricted from transferring a portion of their net assets to the Company. Amounts restricted include paid-in capital
and the statutory reserves of the Company’s PRC subsidiaries. The aggregate amounts of capital and statutory reserves restricted
which represented the amount of net assets of the relevant subsidiaries in the Company not available for distribution was $15,665,860
as of June 30, 2023 and December 31, 2022.
Under PRC laws and regulations, statutory surplus
reserves are restricted to set-off against losses, expansion of production and operation and increasing registered capital of the respective
company and are not distributable other than upon liquidation. The reserves are not allowed to be transferred to the Company in terms
of cash dividends, loans or advances, nor allowed for distribution except under liquidation.
13. Related Party Transactions and Balances
Related Parties:
| Name of related parties |
|
Relationship with the Company |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
| Yangzhou Meihua Import and Export Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
Related Party Sales
The Company sells products to its related parties
and the sales amount from related parties for the six months end 2023 and 2022 are as follows:
Sales:
| | |
For the Six Months ended
June 30, | |
| Name of related party | |
2023 | | |
2022 | |
| Yangzhou Meihua Import and Export Co., Ltd. | |
$ | 11,751 | | |
$ | 18,849 | |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. | |
| - | | |
| 10,818 | |
| Total | |
$ | 11,751 | | |
$ | 29,667 | |
14. Subsequent Events
The Company has evaluated the impact of events
that have occurred subsequent to June 30, 2023, through the issuance date of the consolidated financial statements, and concluded that
no subsequent events have occurred that would require recognition in the consolidated financial statements or disclosure in the notes
to the consolidated financial statements.
F-26
22873370
23940000
0.29
0.29
false
--12-31
Q2
2023-06-30
0001835615
0001835615
2023-01-01
2023-06-30
0001835615
2023-06-30
0001835615
2022-12-31
0001835615
2022-01-01
2022-06-30
0001835615
us-gaap:CommonStockMember
2021-12-31
0001835615
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0001835615
mhua:OrdinarySharesSubscribedMember
2021-12-31
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2021-12-31
0001835615
us-gaap:RetainedEarningsMember
2021-12-31
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-12-31
0001835615
mhua:NonControllingInterestsMember
2021-12-31
0001835615
2021-12-31
0001835615
us-gaap:CommonStockMember
2022-01-01
2022-06-30
0001835615
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-06-30
0001835615
mhua:OrdinarySharesSubscribedMember
2022-01-01
2022-06-30
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2022-01-01
2022-06-30
0001835615
us-gaap:RetainedEarningsMember
2022-01-01
2022-06-30
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-01-01
2022-06-30
0001835615
mhua:NonControllingInterestsMember
2022-01-01
2022-06-30
0001835615
us-gaap:CommonStockMember
2022-06-30
0001835615
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001835615
mhua:OrdinarySharesSubscribedMember
2022-06-30
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2022-06-30
0001835615
us-gaap:RetainedEarningsMember
2022-06-30
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001835615
mhua:NonControllingInterestsMember
2022-06-30
0001835615
2022-06-30
0001835615
us-gaap:CommonStockMember
2022-12-31
0001835615
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001835615
mhua:OrdinarySharesSubscribedMember
2022-12-31
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2022-12-31
0001835615
us-gaap:RetainedEarningsMember
2022-12-31
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-12-31
0001835615
mhua:NonControllingInterestsMember
2022-12-31
0001835615
us-gaap:CommonStockMember
2023-01-01
2023-06-30
0001835615
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-06-30
0001835615
mhua:OrdinarySharesSubscribedMember
2023-01-01
2023-06-30
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2023-01-01
2023-06-30
0001835615
us-gaap:RetainedEarningsMember
2023-01-01
2023-06-30
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-01-01
2023-06-30
0001835615
mhua:NonControllingInterestsMember
2023-01-01
2023-06-30
0001835615
us-gaap:CommonStockMember
2023-06-30
0001835615
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001835615
mhua:OrdinarySharesSubscribedMember
2023-06-30
0001835615
us-gaap:RetainedEarningsAppropriatedMember
2023-06-30
0001835615
us-gaap:RetainedEarningsMember
2023-06-30
0001835615
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001835615
mhua:NonControllingInterestsMember
2023-06-30
0001835615
2015-10-13
0001835615
mhua:KangFuMember
2023-06-30
0001835615
mhua:KangFuMember
2001-12-24
0001835615
mhua:HuadaMember
1991-12-05
0001835615
mhua:YadaMember
2000-11-18
0001835615
2021-10-07
2021-10-07
0001835615
mhua:HainanMember
2021-10-07
0001835615
mhua:MeihuaInternationalMedicalTechnologiesCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:KangFuInternationalMedicalCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:YangzhouHuadaMedicalEquipmentCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:JiangsuYadaTechnologyGroupCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:JiangsuHuadongMedicalDeviceIndustryCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:YangzhouGuanghuiMedicalTechnologyCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:HainanGuoxieTechnologyGroupCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:RevenueMember
2023-01-01
2023-06-30
0001835615
srt:MaximumMember
mhua:RevenueMember
2023-06-30
0001835615
srt:MinimumMember
mhua:RevenueMember
2023-06-30
0001835615
mhua:RevenueMember
2022-01-01
2022-06-30
0001835615
srt:MaximumMember
mhua:RevenueMember
2022-06-30
0001835615
srt:MinimumMember
mhua:RevenueMember
2022-06-30
0001835615
us-gaap:AccountsReceivableMember
2023-01-01
2023-06-30
0001835615
us-gaap:AccountsReceivableMember
2023-06-30
0001835615
srt:MaximumMember
us-gaap:AccountsReceivableMember
2023-06-30
0001835615
us-gaap:AccountsReceivableMember
2022-01-01
2022-12-31
0001835615
srt:MaximumMember
us-gaap:AccountsReceivableMember
2022-12-31
0001835615
srt:MinimumMember
us-gaap:AccountsReceivableMember
2022-12-31
0001835615
2023-08-31
0001835615
2022-01-01
2022-12-31
0001835615
mhua:InvestmentMember
2011-03-03
0001835615
mhua:InvestmentMember
2023-01-05
0001835615
2023-01-05
2023-01-05
0001835615
mhua:JiangsuHuadongMedicalDeviceIndustrialsCoLtdMember
2022-12-01
0001835615
mhua:ZhongxiangxinMember
2022-12-01
2022-12-01
0001835615
srt:MinimumMember
2023-01-01
2023-06-30
0001835615
srt:MaximumMember
2023-01-01
2023-06-30
0001835615
us-gaap:BuildingMember
2023-06-30
0001835615
us-gaap:BuildingMember
2023-01-01
2023-06-30
0001835615
us-gaap:MachineryAndEquipmentMember
2023-06-30
0001835615
us-gaap:MachineryAndEquipmentMember
2023-01-01
2023-06-30
0001835615
us-gaap:VehiclesMember
2023-06-30
0001835615
us-gaap:VehiclesMember
2023-01-01
2023-06-30
0001835615
us-gaap:ElectricGenerationEquipmentMember
2023-06-30
0001835615
us-gaap:ElectricGenerationEquipmentMember
2023-01-01
2023-06-30
0001835615
us-gaap:OfficeEquipmentMember
2023-06-30
0001835615
us-gaap:OfficeEquipmentMember
2023-01-01
2023-06-30
0001835615
mhua:InspectionEquipmentMember
2023-06-30
0001835615
mhua:InspectionEquipmentMember
2023-01-01
2023-06-30
0001835615
us-gaap:CopyrightsMember
2023-06-30
0001835615
us-gaap:PatentsMember
2023-06-30
0001835615
us-gaap:TrademarksMember
2023-06-30
0001835615
mhua:SelfManufacturedProductsMember
2023-01-01
2023-06-30
0001835615
mhua:SelfManufacturedProductsMember
2022-01-01
2022-06-30
0001835615
mhua:ResalesOfSourcedDisposableMedicalDevicesFromThirdPartyManufacturersMember
2023-01-01
2023-06-30
0001835615
mhua:ResalesOfSourcedDisposableMedicalDevicesFromThirdPartyManufacturersMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIIMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIIIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIIIMember
2022-01-01
2022-06-30
0001835615
mhua:OtherMember
2023-01-01
2023-06-30
0001835615
mhua:OtherMember
2022-01-01
2022-06-30
0001835615
mhua:EyeDropsBottleMember
2023-01-01
2023-06-30
0001835615
mhua:EyeDropsBottleMember
2022-01-01
2022-06-30
0001835615
mhua:OralMedicineBottleMember
2023-01-01
2023-06-30
0001835615
mhua:OralMedicineBottleMember
2022-01-01
2022-06-30
0001835615
mhua:AnalBagMember
2023-01-01
2023-06-30
0001835615
mhua:AnalBagMember
2022-01-01
2022-06-30
0001835615
mhua:OtherClassIMember
2023-01-01
2023-06-30
0001835615
mhua:OtherClassIMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIMember
2022-01-01
2022-06-30
0001835615
mhua:MasksMember
2023-01-01
2023-06-30
0001835615
mhua:MasksMember
2022-01-01
2022-06-30
0001835615
mhua:IdentificationTapeMember
2023-01-01
2023-06-30
0001835615
mhua:IdentificationTapeMember
2022-01-01
2022-06-30
0001835615
mhua:DisposableMedicalBrushMember
2023-01-01
2023-06-30
0001835615
mhua:DisposableMedicalBrushMember
2022-01-01
2022-06-30
0001835615
mhua:GynecologicalInspectionKitsMember
2023-01-01
2023-06-30
0001835615
mhua:GynecologicalInspectionKitsMember
2022-01-01
2022-06-30
0001835615
mhua:SurgicalKitMember
2023-01-01
2023-06-30
0001835615
mhua:SurgicalKitMember
2022-01-01
2022-06-30
0001835615
mhua:MedicalBrushMember
2023-01-01
2023-06-30
0001835615
mhua:MedicalBrushMember
2022-01-01
2022-06-30
0001835615
mhua:MedicalKitMember
2023-01-01
2023-06-30
0001835615
mhua:MedicalKitMember
2022-01-01
2022-06-30
0001835615
mhua:OtherClassIIMember
2023-01-01
2023-06-30
0001835615
mhua:OtherClassIIMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIIMember
2022-01-01
2022-06-30
0001835615
mhua:ElectronicPumpMember
2023-01-01
2023-06-30
0001835615
mhua:ElectronicPumpMember
2022-01-01
2022-06-30
0001835615
mhua:AnesthesiaPunctureKitMember
2023-01-01
2023-06-30
0001835615
mhua:AnesthesiaPunctureKitMember
2022-01-01
2022-06-30
0001835615
mhua:DisposableInfusionPumpMember
2023-01-01
2023-06-30
0001835615
mhua:DisposableInfusionPumpMember
2022-01-01
2022-06-30
0001835615
mhua:InfusionPumpMember
2023-01-01
2023-06-30
0001835615
mhua:InfusionPumpMember
2022-01-01
2022-06-30
0001835615
mhua:ElectronicInfusionPumpOneMember
2023-01-01
2023-06-30
0001835615
mhua:ElectronicInfusionPumpOneMember
2022-01-01
2022-06-30
0001835615
mhua:LaparoscopicTrocarMember
2023-01-01
2023-06-30
0001835615
mhua:LaparoscopicTrocarMember
2022-01-01
2022-06-30
0001835615
mhua:OtherClassIIIMember
2023-01-01
2023-06-30
0001835615
mhua:OtherClassIIIMember
2022-01-01
2022-06-30
0001835615
mhua:ClassIIIMember
2023-01-01
2023-06-30
0001835615
mhua:ClassIIIMember
2022-01-01
2022-06-30
0001835615
2018-10-22
2018-10-22
0001835615
2020-04-20
0001835615
mhua:LandUseRightsMember
2023-06-30
0001835615
mhua:LandUseRightsMember
2022-12-31
0001835615
us-gaap:PatentsMember
2022-12-31
0001835615
mhua:SoftwareMember
2023-06-30
0001835615
mhua:SoftwareMember
2022-12-31
0001835615
us-gaap:TrademarksMember
2022-12-31
0001835615
mhua:YangzhouJuyuanGuaranteeCoLtdMember
2011-03-03
0001835615
us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember
2011-03-03
0001835615
us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember
2023-01-05
0001835615
mhua:JiangsuHuadongMedicalDeviceIndustrialCoLtdMember
2022-12-01
0001835615
2022-12-01
0001835615
mhua:BankOfCommunicationsMember
2023-01-01
2023-06-30
0001835615
mhua:BankOfCommunicationsMember
2023-06-30
0001835615
mhua:BankOfCommunicationsOneMember
2023-01-01
2023-06-30
0001835615
mhua:BankOfCommunicationsOneMember
2023-06-30
0001835615
mhua:AgriculturalBankOfChinaMember
2023-01-01
2023-06-30
0001835615
mhua:AgriculturalBankOfChinaMember
2023-06-30
0001835615
mhua:JiangsuYangzhouRuralCommercialBankMember
2023-01-01
2023-06-30
0001835615
mhua:JiangsuYangzhouRuralCommercialBankMember
2023-06-30
0001835615
mhua:BankOfChinaMember
2023-01-01
2023-06-30
0001835615
mhua:BankOfChinaMember
2023-06-30
0001835615
mhua:AgriculturalBankOfChinaOneMember
2023-01-01
2023-06-30
0001835615
mhua:AgriculturalBankOfChinaOneMember
2023-06-30
0001835615
mhua:IndustrialAndCommercialBankOfChinaMember
2023-01-01
2023-06-30
0001835615
mhua:IndustrialAndCommercialBankOfChinaMember
2023-06-30
0001835615
mhua:BankOfCommunicationsMember
2022-01-01
2022-12-31
0001835615
mhua:BankOfCommunicationsMember
2022-12-31
0001835615
mhua:AgriculturalBankOfChinaMember
2022-01-01
2022-12-31
0001835615
mhua:AgriculturalBankOfChinaMember
2022-12-31
0001835615
mhua:JiangsuYangzhouRuralCommercialBankMember
2022-01-01
2022-12-31
0001835615
mhua:JiangsuYangzhouRuralCommercialBankMember
2022-12-31
0001835615
mhua:BankOfChinaMember
2022-01-01
2022-12-31
0001835615
mhua:BankOfChinaMember
2022-12-31
0001835615
mhua:AgriculturalBankOfChinaOneMember
2022-01-01
2022-12-31
0001835615
mhua:AgriculturalBankOfChinaOneMember
2022-12-31
0001835615
mhua:IndustrialAndCommercialBankOfChinaMember
2022-01-01
2022-12-31
0001835615
mhua:IndustrialAndCommercialBankOfChinaMember
2022-12-31
0001835615
2022-11-03
2022-11-03
0001835615
2022-11-03
0001835615
mhua:BankOfCommunicationsMember
2022-01-01
2022-12-31
0001835615
2017-12-29
2017-12-29
0001835615
2017-12-29
0001835615
2016-11-30
0001835615
2023-08-01
2023-08-29
0001835615
mhua:ShanghaiXinyaPharmaceuticalHanjiangCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:YangzhouMeihuaImportAndExportCoLtdMember
2023-01-01
2023-06-30
0001835615
mhua:YangzhouMeihuaImportAndExportCoLtdMember
2022-01-01
2022-06-30
0001835615
mhua:ShanghaiXinyaPharmaceuticalHanjiangCoLtdMember
2022-01-01
2022-06-30
iso4217:USD
iso4217:USD
xbrli:shares
xbrli:shares
iso4217:HKD
xbrli:pure
iso4217:CNY
Exhibit 99.2
Management’s discussion and analysis of
the financial condition and results of operations of Meihua International Medical Technologies Co., Ltd., a Cayman Islands company (“Meihua
International,” the “Company,” “we,” “our,” or “us”), for the semi-annual period
ended June 30, 2023 is set forth below:
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion
and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial
statements and the related notes included elsewhere in this filing. This discussion contains forward-looking statements that involve risks
and uncertainties. Actual results and the timing of selected events could differ materially from those anticipated in these forward-looking
statements as a result of various factors.
A. Operating Results
Business Overview
Meihua International Medical Technologies Co.,
Ltd., through its operating subsidiaries, is mainly engaged in the manufacture, research and development and sales of Class I, II and
III medical devices. It has a history of more than 30 years and has multiple product categories, with more than 800 domestic products
and more than 120 export products. The main product lines include disposable infusion pumps, anesthesia puncture kits, electronic pumps,
full anesthesia kits, urethral catheterization kits, gynecological examination kits, endotracheal intubation, dressing applications and
various tubes. It is the leading enterprise in China’s medical consumables industry. The Company has received qualification to manufacture
and produce China’s first, second and third type of medical device consumables and, at the same time, the Company has acquired FDA
registration and the European Union’s CE certification. Relevant permissions have been obtained in major sales markets to meet local
regulatory requirements.
The Company’s distribution network covers
major global markets. Internationally, the Company mainly exports medical devices through exporting distributors. To date, the Company
has 334 exporting distributors responsible for distributing its products to end users in Europe, North America, Asia, South America, Africa,
and Oceania. In the Chinese market, the Company sells products under its own brand to customers all over the country. The Company’s
product permeation for mainland China has reached major medical institutions and pharmacies through some 3,159 distributors. At the same
time, the Company has established a cooperative network with more than 531 hospitals through its own direct sales channels.
Revenues decreased by $6.6 million, or approximately
12.1%, to $48.2 million in the six months ended June 30, 2023 from $54.8 million in the six months ended June 30, 2022. The decrease was
mainly due to a decline in demand for customer orders.
Net income increased by $0.5 million, or approximately
7.3%, to $7.0 million in the six months ended June 30, 2023 from $6.6 million in the six months ended June 30, 2022. The increase was
mainly due to decrease in operating expenses in the six months ended June 30, 2023.
Recent Developments
On August 24, 2023, the
Company filed with the U.S. Securities and Exchange Commission (the “SEC”) a registration statement on Form F-3 under the
Securities Act of 1933, as amended (the “Securities Act”), utilizing a “shelf” registration process through which
the company may offer and selling, from time to time, up to $100,000,000 of the Company’s ordinary shares, par value $0.0005 per
share, debt securities, warrants, rights, and units, or any combination thereof, together or separately as described in the prospectus.
Coronavirus (COVID-19) Update
On January 30, 2020, the World Health Organization
declared the outbreak of the corona-virus disease (COVID-19) a “Public Health Emergency of International Concern,” and on
March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” Governments in affected countries
have imposed travel bans, quarantines and other emergency public health measures, which have caused material disruption to businesses
globally and result in an economic slowdown. These measures, though temporary in nature, may continue and increase depending on developments
in the COVID-19’s outbreak.
In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply, while at the same time sales of products other
than masks declined due to a decrease in market demand. In fiscal year 2021, as circumstances surrounding the COVID-19 epidemic stabilized
within the PRC, the market for masks was no longer in urgent shortage compared to the same period in 2020, and the production of other
epidemic prevention products resumed normal production levels. In general, with the precise control of the epidemic in the PRC, our production
and operations recovered smoothly, and the demand for other products has increased gradually.
After the initial outbreak of COVID-19, from time
to time, some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the Omicron
variants in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series of restrictions
and quarantines were implemented to contain the spread.
Many of the restrictive measures previously adopted
by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more flexible
measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic could have
a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will be dependent
on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government actions
in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many other
factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the
expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the current
situation continues.
In preparing these consolidated financial statements,
the Company has evaluated events and transactions for potential recognition or disclosure through six months ended June 30, 2023, the
date the consolidated financial statements were available to be issued. No events require additional adjustment to or disclosure in the
consolidated financial statements.
Results of Operations for the Six Months Ended June 30, 2023 and
2022
The following table sets forth a summary of our
consolidated results of operations for the periods presented. This information should be read together with our consolidated financial
statements and related notes included elsewhere in this Annual Report. The results of operations in any period are not necessarily indicative
of our future trends.
(Amounts expressed in U.S. dollars, except share
data and per share data, or otherwise noted)
For the Six months Ended June 30, 2023
and 2022
| | |
For the Six months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| Third party sales | |
$ | 48,178,325 | | |
$ | 54,803,181 | |
| Related party sales | |
| 11,751 | | |
| 29,666 | |
| Total revenues | |
| 48,190,076 | | |
| 54,832,847 | |
| Cost of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| | |
| | | |
| | |
| Gross profit | |
| 17,170,729 | | |
| 20,891,732 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Selling | |
| 3,161,070 | | |
| 3,311,649 | |
| General and administrative | |
| 3,452,610 | | |
| 4,799,711 | |
| Research and development | |
| 1,460,376 | | |
| 1,642,204 | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Total operating expenses | |
| 8,074,056 | | |
| 12,223,030 | |
| | |
| | | |
| | |
| Income from operations | |
| 9,096,673 | | |
| 8,668,702 | |
| | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | |
| Interest expense | |
| 128,973 | | |
| 98,805 | |
| Interest income | |
| (361,532 | ) | |
| (19,725 | ) |
| Currency exchange (gain) loss | |
| 119,193 | | |
| (449,217 | ) |
| Other expense, net | |
| 114,298 | | |
| 50,180 | |
| Total other (income) expenses | |
| 932 | | |
| (319,957 | ) |
| | |
| | | |
| | |
| Income before income tax provision | |
| 9,095,741 | | |
| 8,988,659 | |
| Income taxes expense | |
| 2,064,212 | | |
| 2,433,772 | |
| Net income | |
$ | 7,031,529 | | |
$ | 6,554,887 | |
For the Six months Ended June 30, 2023 and
2022
Revenues
Revenues decreased by $6.6 million, or approximately
12.1%, to $48.2 million for the six months ended June 30, 2023 from $54.8 million in the six months ended June 30, 2022. The decrease
was mainly due to a decline in demand for customer orders.
Cost of revenues
Cost of revenues primarily include cost of materials,
direct labor costs, overhead, and other related incidental expenses that are directly attributable to the Company’s principal operations.
Cost of revenues decreased by $2.9 million, or approximately 8.6%, to $31.0 million in the six months ended June 30, 2023 from $33.9 million
in the six months ended June 30, 2022. The decrease was generally in line with decrease in revenue except some fixed cost such as lease
expense and salary of administrative employees in production department.
Gross profit margin
The following table sets forth the overall gross
profit margin of the Company:
| | |
For the Six months Ended
June 30 | |
| | |
2023 | | |
2022 | |
| Revenues | |
$ | 48,190,076 | | |
$ | 54,832,847 | |
| Costs of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| Gross profit | |
$ | 17,170,729 | | |
$ | 20,891,732 | |
| Gross profit margin % | |
| 35.6 | % | |
| 38.1 | % |
Gross profit decreased by $3.7 million, or approximately
17.8%, to $17.2 million in the six months ended June 30, 2023 from $20.9 million in the six months ended June 30, 2022. Gross profit margin
decreased from 38.1% in the six months ended June 30, 2022 to 35.6% in the six months ended June 30, 2023 due to some fixed cost did not
decrease proportionately with revenue.
Operating costs and expenses
Our operating costs and expenses consist of selling
expenses, general and administrative expenses and research and development expenses.
Selling
The following table sets forth a breakdown of
the selling expenses of the Company:
For the Six months Ended June 30,2023
and 2022
| | |
2023 | | |
2022 | |
| Transportation expenses | |
$ | 1,137,936 | | |
$ | 1,404,106 | |
| Salaries and benefits | |
| 698,086 | | |
| 787,279 | |
| Entertainment expenses | |
| 500,485 | | |
| 427,447 | |
| Conference expenses | |
| 493,850 | | |
| 415,750 | |
| Travel allowance | |
| 177,807 | | |
| 159,228 | |
| Auto expenses | |
| 118,629 | | |
| 101,031 | |
| Advertising expenses | |
| 8,275 | | |
| 7,603 | |
| Other expenses | |
| 26,002 | | |
| 9,205 | |
| Total | |
$ | 3,161,070 | | |
$ | 3,311,649 | |
Selling expenses decreased by $0.1 million, or
approximately 4.5%, to $3.2 million in the six months ended June 30, 2023 from $3.3 million in the six months ended June 30, 2022. The
decrease was mainly attributable to the combined effects of the followings:
| |
(a) |
Our conference expenses increased by $78,100, or approximately 18.8% to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the six months ended June 30, 2022. Conference expenses are mainly related to the company’s market expansion, business development, business negotiation, medical expo, and exhibition affairs. These expenditures helped the Company promote its products, develop markets and channels, strengthen customer communication, and establish long-term and stable cooperative relations; |
| |
(b) |
Our transportation expenses decreased by $0.3 million, or approximately 19.0%, to $1.1 million in the six months ended June 30, 2023 from $1.4 million in the six months ended June 30, 2022. The reduction in business travel was due to a decline in demand for customer orders. |
| |
(c) |
Our salaries and benefits expenses decreased by $89,193 or approximately 11.3%, to $0.7 million in the six months ended June 30, 2023 from $0.8 million in the six months ended June 30, 2022. The decrease was due to a decrease in the salary and benefits of the sales team, which was in line with revenue decrease. |
| |
(d) |
Our entertainment expenses increased by $73,038, or approximately 17.1%, to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the six months ended June 30, 2022. The increase was mainly attributable to new customer acquisition efforts. |
General and administrative
General and administrative expenses primarily
consist of the following expenses:
For the Six months Ended June 30, 2023
and 2022
| | |
2023 | | |
2022 | |
| Salaries and benefits | |
$ | 738,077 | | |
$ | 600,682 | |
| Entertainment expenses | |
| 606,820 | | |
| 481,334 | |
| Conference fee | |
| 374,022 | | |
| 311,786 | |
| Auto expenses | |
| 113,810 | | |
| 83,363 | |
| Maintenance expenses | |
| 50,199 | | |
| 65,278 | |
| Depreciation expenses | |
| 45,951 | | |
| 16,793 | |
| Travel allowance | |
| 72,283 | | |
| 63,044 | |
| Office expenses | |
| 45,664 | | |
| 50,288 | |
| Surtax expenses | |
| 302,376 | | |
| 338,190 | |
| Amortization expenses | |
| 19,696 | | |
| 13,416 | |
| Rental expenses | |
| 7,299 | | |
| 7,805 | |
| Insurance expenses | |
| 118,000 | | |
| 50,336 | |
| Service expenses | |
| 726,302 | | |
| 2,496,058 | |
| Other expenses | |
| 232,111 | | |
| 221,338 | |
| Total | |
$ | 3,452,610 | | |
$ | 4,799,711 | |
General and administrative expenses decreased
by $1.3 million, or approximately 28.1%, to $3.5 million in the six months ended June 30, 2023 from $4.8 million in the six months ended
June 30, 2022. The decrease was primarily due to (a) service expenses decreasing by approximately $1.8 million from approximately $2.5
million in the six months ended June 30, 2022 to approximately $0.7 million in the six months ended June 30, 2023, offset by (b) salaries
and benefits increasing by approximately $137,000 from approximately $601,000 in the six months ended June 30, 2022 to approximately $738,000
in the six months ended June 30, 2023, and (c) entertainment expenses increasing by $125,000 or 26.1% from approximately $481,000 in the
six months ended June 30, 2022 to approximately $607,000 in the six months ended June 30, 2023
Research and development
The following table sets forth a breakdown of
the research and development expenses of the Company:
For the Six months Ended June 30,2023
and 2022
| | |
2023 | | |
2022 | |
| Sample manufacturing expenses | |
$ | 676,283 | | |
$ | 850,532 | |
| Salaries and benefits | |
| 564,114 | | |
| 586,062 | |
| Travel allowance | |
| 58,886 | | |
| 64,201 | |
| Depreciation expenses | |
| 5,113 | | |
| 3,993 | |
| Design expenses | |
| 38,985 | | |
| 52,337 | |
| Material expenses | |
| 13,495 | | |
| 11,943 | |
| Other expenses | |
| 103,500 | | |
| 73,136 | |
| Total | |
$ | 1,460,376 | | |
$ | 1,642,204 | |
The research and development expenses decreased
by $0.2 million, or approximately 11.1%, to $1.5 million in the six months ended June 30, 2023, from $1.6 million in the six months ended
June 30, 2022. The decrease was mainly due to decrease in sample manufacturing expenses.
Income from operations
As a result of the factors described above, our
income from operations increased by $0.4 million, or approximately 4.9%, to $9.1 million in the six months ended June 30, 2023 from $8.7
million in the six months ended June 30, 2022.
Income tax expense
The provision for income taxes decreased by $0.4
million, or approximately 15.2%, to $2.1 million in the six months ended June 30, 2023 from $2.4 million in the six months ended June
30, 2022. The decrease was mainly due to the decrease of taxable income for the six months ended June 30, 2023.
Net income
As a result of the factors described above, our
net income increased by $0.5 million, or approximately 7.3%, to $7.0 million in the six months ended June 30, 2023 from $6.6 million in
the six months ended June 30, 2022.
Unrealized foreign currency translation adjustment
The Company’s reporting currency is the
United States dollar (“US$”). The Company’s operations are primarily conducted through the PRC subsidiaries where the
local currency is the functional currency. The functional currency of Kang Fu HK is the Hong Kong dollar and the functional currency of
Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”). Results of operations and cash flows are translated at average
exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period and equity
is translated at historical exchange rates. Translation adjustments resulting from the process of translating the financial statements
denominated in RMB into U.S. dollars are included in other comprehensive income. Our foreign currency translation loss in the six months
ended June 30, 2023 was $6.3 million, compared to the foreign currency translation loss of $6.1 million in the six months ended June 30,
2022. The change was primarily due to the exchange rate fluctuation of RMB against the U.S. dollars.
B. Liquidity and Capital Resources
Cash Flows and Working Capital
As of June 30, 2023 and 2022, we had cash of $17.9
million and $34.7 million, respectively. We believe that our current cash, cash to be generated from our operations and access to capital
market will be sufficient to meet our working capital needs for at least the next twelve months. We do not have any amounts committed
to be provided by our related party. We are also not dependent upon future financing to meet our liquidity needs for the next twelve months.
In order to implement our growth strategies, we plan to expand our business. With additional capacity, and varied product offerings, the
Company will provide tailored “one-stop” services from wound care, to surgical auxiliary supplies, to disease prevention.
To do so, we may need more capital through equity financing to expand our production and meet market demands.
Substantially all of our operations are conducted
in China and all of our revenues, expense and cash are denominated in RMB. RMB is subject to the exchange control regulation in China,
and, as a result, we may have difficulty in distributing any dividends outside of China due to PRC exchange control regulations which
restrict the ability to convert RMB into U.S. Dollars.
Under applicable PRC regulations, foreign-invested
enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards
and regulations. In addition, a foreign-invested enterprise in China is required to set aside at least 10% of its after-tax profit every
year as its general reserves based on PRC accounting standards until the accumulative amount of such reserves reaches 50% of its registered
capital. These reserves can’t be distributed as cash dividends. The board of directors of a foreign-invested enterprise has the
discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds, which can’t be distributed to equity
owners except liquidation. Under PRC law, RMB can be converted into U.S. Dollars under the company’s “current account”
(including dividends, trade and service-related foreign exchange transactions) rather than the “capital account” (including
foreign direct investments and loans, without the prior approval of the SAFE).
For retained earnings accrued after such date,
the board of directors will declare dividends after taking into account our operations, earnings, financial condition, the demand for
cash and availability and other relevant factors. Any declaration, payment and amount of dividends should be subject to our By-laws, charter
and applicable Chinese and U.S. state and federal laws and regulations, including the approval from the shareholders of each subsidiary
which intends to declare such dividends, if applicable.
We have limited financial obligations dominated
in U.S. dollars, thus the foreign currency restrictions and regulations in PRC on the dividends distribution will not have a material
impact on the liquidity, financial condition and results of operations of our Company.
Cash Flow Summary
For the Six months Ended June 30, 2023 and 2022
| | |
2023 | | |
2022 | |
| Net Cash Used in Operating Activities | |
$ | (5,424,569 | ) | |
$ | (7,798,816 | ) |
| | |
| | | |
| | |
| Net Cash Used in Investing Activities | |
| (3,866,151 | ) | |
| (459,163 | ) |
| | |
| | | |
| | |
| Net Cash Provided by Financing Activities | |
| 721,677 | | |
| 35,144,846 | |
| | |
| | | |
| | |
| Effect of Exchange Rate Changes on Cash | |
| (306,443 | ) | |
| (370,615 | ) |
| | |
| | | |
| | |
| Cash at Beginning of Year | |
| 26,736,700 | | |
| 8,149,276 | |
| | |
| | | |
| | |
| Cash at End of Year | |
$ | 17,861,214 | | |
$ | 34,665,528 | |
For the Six months Ended June 30, 2023 and 2022
Cash Flow in Operating Activities
Net cash used in operating activities was $5.4
million in the six months ended June 30, 2023, primarily comprised of net income of approximately $7.0 million and adjusted for non-cash
items approximately $0.5 million, increase in accounts receivable of approximately $14.1 million, decrease of bank acceptance receivable
of approximately $2.8 million, decrease of accounts payable of approximately $1.6 million, increase in inventory of approximately $0.6
million.
Net cash used in operating activities was $7.8
million in the six months ended June 30, 2022, primarily comprised of net income of approximately $6.6 million and adjusted for non-cash
items approximately $2.4 million, decrease in accounts receivable of approximately $3.5 million, increase of bank acceptance receivable
of approximately $9.7 million, decrease of accounts payable of approximately $4.9 million, increase in prepayments and other assets of
approximately $5.3 million.
Cash Flow in Investing Activities
Net cash used in investing activities was $3.9
million in the six months ended June 30, 2023. It consisted of purchases of property and equipment of $1.0 million, intangible assets
of $3.6 million, proceeds from disposal of fixed assets of $0.4 million, and proceeds from disposal of long-term investment of $0.4 million.
Net cash used in investing activities was $0.5
million in the six months ended June 30, 2022. It consisted of purchases of property and equipment.
Cash Flow in Financing Activities
For the six months ended June 30, 2023, the Company
had net cash provided by financing activities of $0.7 million, which consisted of the proceeds from short-term bank loans of $5.3 million,
and repayments of short-term bank loans of $4.6 million.
For the six months ended June 30, 2022, the Company
had net cash provided by financing activities of $35.1 million, which consisted of the proceeds from short-term bank loans of $3.5 million,
and repayments of short-term bank loans of $2.9 million.
Off-Balance Sheet Arrangements
As of June 30, 2023 and December 31, 2022, there
were no off-balance sheet arrangements.
C. Research and Development, Patents and Licenses, etc.
Please see Item 4.A. “Information on the
Company—Business Overview—Intellectual Property,” in 20-F/A filed on August 29, 2023.
D. Trend Information
Other than as disclosed elsewhere in this Annual
Report, we are not aware of any trends, uncertainties, demands, commitments or events for the six months ended June 30, 2023 that are
reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that
would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Critical Accounting Estimates
Our discussion and analysis of our financial condition
and results of operations are based upon our unaudited condensed consolidated financial statements. These financial statements are prepared
in accordance with U.S. GAAP, which requires us to make estimates and assumptions that affect the reported amounts of our assets and liabilities
and revenue and expenses, to disclose contingent assets and liabilities on the date of the unaudited condensed consolidated financial
statements, and to disclose the reported amounts of revenue and expenses incurred during the financial reporting period. The most significant
estimates and assumptions include allowance for bad debts and the valuation of inventory. We continue to evaluate these estimates and
assumptions that we believe to be reasonable under the circumstances. We rely on these evaluations as the basis for making judgments about
the carrying values of assets and liabilities that are not readily apparent from other sources. Since the use of estimates is an integral
component of the financial reporting process, actual results could differ from those estimates. Some of our accounting policies require
higher degrees of judgment than others in their application. We believe critical accounting policies as disclosed in this report reflect
the more significant judgments and estimates used in preparation of our consolidated financial statements. We believe there have been
no material changes to our critical accounting policies and estimates.
Accounts
Receivable and Allowance for Doubtful Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company records impairment losses for accounts
receivable based on assessments of the recoverability of the trade and other receivables and individual account analysis, including the
current creditworthiness and the past collection history of each debtor. Impairments arise when there is objective evidence indicating
that the balances may not be collectible. The identification of bad and doubtful debts, in particular of a loss event, requires the use
of judgment and estimates, which involve the estimates of specific losses on individual exposures, as well as a provision on historical
trends of collections. Based on management of customers’ credit and ongoing relationship, management makes conclusions whether any
balances outstanding at the end of the period will be deemed non-collectible on an individual basis and on aging analysis basis. The provision
is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements of operations and
comprehensive income. Delinquent account balances are written-off against the allowance for doubtful accounts after management has determined
that the likelihood of collection is not probable.
Inventories
Inventories are valued using the lower of cost
or net realizable value. Cost is principally determined using the weighted-average method. The Company records adjustments to inventory
for excess quantities, obsolescence, or impairment, when appropriate, to reflect inventory at net realizable value. These adjustments
are based upon a combination of factors including current sales volume, market conditions, lower of cost or market analysis and expected
realizable value of the inventory.
Exhibit 99.3
Meihua International Medical Technologies Co.,
Ltd. Reports Unaudited 2023 First Half Financial Results
YANGZHOU, China, October 2, 2023 /PRNewswire/
-- Meihua International Medical Technologies Co., Ltd. (“MHUA” or the “Company”) (NASDAQ: MHUA), a reputable manufacturer
and provider of Class I, II, and III disposable medical devices with operating subsidiaries in China, today reported its unaudited financial
results for the six months ended June 30, 2023. All amounts below are in U.S. dollars.
First Half 2023 Financial Metrics:
| ● | Revenues
decreased by 12.1% to approximately $48.2 million for the six months ended June 30, 2023, from approximately $54.8 million in the
same period of fiscal year 2022. |
| ● | Gross
profit decreased by 17.8% to approximately $17.2 million for the six months ended June 30, 2023, from approximately $20.9 million
in the same period of fiscal year 2022. |
| ● | Gross
margin decreased from 38.1% in the six months ended June 30, 2022 to 35.6% in the six months ended June 30, 2023. |
| ● | Income
from operations increased by 4.9% to $9.1 million for the six months ended June 30, 2023, from $8.7 million for the six months ended
June 30, 2022. |
| ● | Net
income increased by 7.3% to approximately $7.0 million for the six months ended June 30, 2023 from $6.6 million in the same period
of fiscal year 2022. |
| | |
For the six months ended June 30, | |
| (in $ millions, except earnings per share; difference due to rounding) | |
2023 | | |
2022 | | |
% Change | |
| Revenues | |
$ | 48.2 | | |
$ | 54.8 | | |
| (12.1 | )% |
| Gross profit | |
$ | 17.2 | | |
$ | 20.9 | | |
| (17.8 | )% |
| Gross margin | |
| 35.6 | % | |
| 38.1 | % | |
| (2.5 percentage point | ) |
| Income from operation | |
$ | 9.1 | | |
$ | 8.7 | | |
| 4.9 | % |
| Net income | |
$ | 7.0 | | |
$ | 6.6 | | |
| 7.3 | % |
| Net income per share - Basic and Diluted | |
$ | 0.29 | | |
$ | 0.29 | | |
| 0.0 | % |
Mr. Yongjun Liu, Chairman of the Company, commented:
“With a rich legacy spanning over three decades, we have forged enduring partnerships with 334 distributors across Europe, North
America, Asia, South America, Africa, and Oceania. We believe that this extensive network empowers us to cater to the discerning clientele
on a global scale. Domestically, we have established a formidable presence, leveraging a robust network of 3,159 distributors nationwide
to propel our brand. Moreover, we believe that our strategic collaborations with over 531 hospitals and direct sales channels have further
propelled our market penetration, solidifying our influence.”
“While we experienced a temporary downturn
in revenue for the period ended June 30, 2023, our overall financial performance remains resilient. In the first half of 2023, we achieved
revenues of $48.2 million, exhibiting a slight decrease from the corresponding period in 2022, which stood at $54.8 million. Notwithstanding
this decrease in revenues, which we believe is temporary, we believe our unwavering focus on optimizing operational costs has yielded
substantial growth in net income. During the same timeframe, our net income increased to $7.0 million, reflecting a commendable 7.3% increase
from $6.6 million in 2022.”
“Despite the impact of the global COVID-19
pandemic on the sales and market dynamics of certain essential medical products, leading to a partial decline in profits, our proactive
strategic planning has helped to ensure the smooth progress in the development of our state-of-the-art medical facility in China’s
Hainan Province. As production and operations gradually regain momentum, we believe market demand for our future products will be poised
to surge. We firmly believe that Meihua International’s strategic transformation is on the cusp of yielding tangible results, gradually
evolving into a cohesive entity. Through planning and strategic execution, we believe our future endeavors will enable us to transcend
the confines of the traditional medical consumables market, and allow us to channel our efforts towards high-value products imbued with
technological prowess. Consequently, we anticipate Meihua International will experience a stable rebound across our overall business,
generating confidence in the future market landscape.”
“Looking ahead, we remain steadfast in our
commitment to continued growth and expansion. Through continuous innovation, expansion of our product portfolio, and an unwavering dedication
to excellence, we are poised to reinforce our position as a global leader in the medical equipment industry. Harnessing our expertise
and expanding our market influence, we are confident that we will continue to offer groundbreaking solutions that revolutionize healthcare
outcomes on a global scale.”
First Half 2023 Financial Results
Revenues
Revenue decreased by approximately $6.6 million,
or 12.1%, to approximately $48.2 million for the six months ended June 30, 2023 from $54.8 million for the six months ended June 30, 2022.
The decrease in revenues was primarily driven by a decline in demand for customer orders.
Cost of revenues
Cost of revenues primarily include cost of materials,
direct labor costs, overhead, and other related incidental expenses that are directly attributable to the Company’s principal operations.
Cost of revenues decreased by $2.9 million, or approximately 8.6%, to $31.0 million in the six months ended June 30, 2023 from $33.9 million
in the six months ended June 30, 2022. The decrease was driven by an increase related to some ongoing fixed costs such as lease expense
and salary of administrative employees, which was generally in line with our decrease in revenue experienced during the period.
Gross profit and margin
Gross profit decreased by $3.7 million, or approximately
17.8%, to $17.2 million in the six months ended June 30, 2023 from $20.9 million in the six months ended June 30, 2022. Gross profit margin
decreased from 38.1% in the six months ended June 30, 2022 to 35.6% in the six months ended June 30, 2023. This decrease was due to some
fixed cost that did not decrease proportionately with revenue.
Operating costs and expenses
Operating costs and expenses consist of selling
expenses, general and administrative expenses and research and development expenses.
Selling
Selling expenses decreased by $0.1 million, or
approximately 4.5%, to $3.2 million in the six months ended June 30, 2023 from $3.3 million in the six months ended June 30, 2022. The
decrease was mainly attributable to the combined effects of the following:
| (a) | Conference
expenses increased by $78,100, or approximately 18.8% to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the
six months ended June 30, 2022. Conference expenses are mainly related to the company’s market expansion, business development,
business negotiation, medical expo and exhibition affairs. These expenditures helped the Company promote its products, develop markets
and channels, strengthen customer communication, and establish long-term and stable cooperative relations; |
| (b) | Transportation
expenses decreased by $0.3 million, or approximately 19.0%, to $1.1 million in the six months ended June 30, 2023 from $1.4 million in
the six months ended June 30, 2022. The reduction in business travel was due to a decline in demand for customer orders; |
| (c) | Salaries
and benefits expenses decreased by $89,193, or approximately 11.3%, to $0.7 million in the six months ended June 30, 2023 from $0.8 million
in the six months ended June 30, 2022. The decrease was due to a decrease in salaries and benefits paid to the sales team, which was
in line with the decrease in revenue; and |
| (d) | Entertainment
expenses increased by $73,038, or approximately 17.1%, to $0.5 million in the six months ended June 30, 2023 from $0.4 million in the
six months ended June 30, 2022. The increase was mainly attributable to new customer acquisition efforts. |
General and administrative expenses
General and administrative expenses decreased
by $1.3 million, or approximately 28.1%, to $3.5 million in the six months ended June 30, 2023 from $4.8 million in the six months ended
June 30, 2022. The decrease was primarily due to (a) service expenses decreasing by approximately $1.8 million from approximately $2.5
million in the six months ended June 30, 2022 to approximately $0.7 million in the six months ended June 30, 2023, offset by (b) salaries
and benefits increasing by approximately $137,000 from approximately $601,000 in the six months ended June 30, 2022 to approximately $738,000
in the six months ended June 30, 2023, and (c) entertainment expenses increasing by $125,000 or 26.1% from approximately $481,000 in the
six months ended June 30, 2022 to approximately $607,000 in the six months ended June 30, 2023.
Research and development expenses
Research and development expenses decreased by
$0.2 million, or approximately 11.1%, to $1.5 million in the six months ended June 30, 2023 from $1.6 million in the six months ended
June 30, 2022. The decrease was mainly due to decrease in sample manufacturing expenses.
Income from operations
Income from operations, as a result of the factors
described above, increased by $0.4 million, or approximately 4.9%, to $9.1 million in the six months ended June 30, 2023 from $8.7 million
in the six months ended June 30, 2022.
Net income
Net income increased by $0.5 million, or approximately
7.3%, to $7.0 million in the six months ended June 30, 2023 from $6.6 million in the six months ended June 30, 2022.
Recent development
On May 15, 2023, the Company announced that its
wholly-owned subsidiary, Jiangsu Huadong Medical Device Industrial Co., Ltd., has been invited to participate for the 12th consecutive
year in the 87th China International Medical Equipment Fair, the largest and most prestigious medical industry fair in China.
The event, which runs from May 14th to 17th, 2023, will provide an excellent platform for Jiangsu Huadong to showcase
its cutting-edge medical devices and technologies.
On May 1, 2023, the Company announced that via
its subsidiary Hainan Guoxie Technology Group Co., Ltd., has completed the purchase of land use rights in the South of Hainan Free Trade
Port Boao Hope City in Qionghai city for investment and construction of an integrated medical industrial park. The Company plans to develop
a comprehensive industrial park on the land, including a product research and development, production and sales training center, and import/export
product assembly and technology license-in projects. The site will also include a hospital and a retirement and rehabilitation-integrated
service center.
About Meihua International Medical Technologies
Co., Ltd.
Meihua International
Medical Technologies, Ltd. (“Meihua International” of the “Company”) is a reputable manufacturer and provider
of Class I, II, and III disposable medical devices with operating subsidiaries in China. The Company manufactures and sells Class
I disposable medical devices, such as HDPE bottles for tablets and LDPE bottles for eye drops, throat strip and anal bags, and Class II
and III disposable medical devices, such as disposable identification bracelets, gynecological examination kits, inspection kits, surgical
kits, medical brushes, medical dressing, medical catheters, uterine tissue suction tables, virus sampling tubes, disposable infusion pumps,
electronic pumps and anesthesia puncture kits, among other products which are sold under Meihua’s brands and are also sourced and
distributed from other manufacturers. The Company has received international “CE” certification and ISO 13485 system certification
and has also registered with the FDA (registration number: 3006554788) for over 20 Class I products. The Company has served hospitals,
pharmacies, medical institutions and medical equipment companies for more than 30 years, providing over 1,000 types of products for domestic
sales, as well as over 120 products which are exported to more than 30 countries internationally across Europe, North America, South
America, Asia, Africa and Oceania. For more information, please visit www.meihuamed.com.
Forward-Looking Statement
This press
release contains forward-looking statements as defined by the Private Securities Litigation Reform Act of 1995. Forward-looking statements
include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other
statements that are other than statements of historical facts. When the Company uses words such as “may,” “will,”
“intend,” “should,” “believe,” “expect,” “anticipate,” “project,”
“estimate” or similar expressions that do not relate solely to historical matters, it is making forward-looking statements.
Forward-looking statements are not guarantees of future performance and involve risks and uncertainties that may cause the actual results
to differ materially from the Company’s expectations discussed in the forward-looking statements. These statements are subject to
uncertainties and risks including, but not limited to, the following: the Company’s ability to achieve its goals and strategies,
and its ability to fully execute on the planned agreement, the Company’s future business development and plans of future business
development, including its ability to successfully develop robotic assisted surgery systems and obtain licensure and certification for
such systems, financial conditions and results of operations, product and service demand and acceptance, reputation and brand, the impact
of competition and pricing, changes in technology, government regulations, fluctuations in general economic and business conditions in China,
and assumptions underlying or related to any of the foregoing and other risks contained in reports filed by the Company with the U.S.
Securities and Exchange Commission (“SEC”). For these reasons, among others, investors are cautioned not to place undue reliance
upon any forward-looking statements in this press release. Additional factors are discussed in the Company’s filings with the SEC,
including under the section entitled “Risk Factors” in its annual report on Form 20-F, as filed with the SEC on April
14, 2023, as amended and most recently filed on August 29, 2023, as well as its current reports on Form 6-K and other filings, all
of which are available for review at www.sec.gov. The Company undertakes no obligation to publicly revise these forward-looking statements
to reflect events or circumstances that arise after the date hereof.
For more information, please contact:
Janice Wang
Wealth Financial Services LLC
Phone:
+86 13811768599
+1 (628) 283 9214
Email: services@wealthfsllc.com
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
As of June 30, 2023 and December 31, 2022
(US$, except share data or otherwise noted)
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
| | |
| |
| Assets | |
| | |
| |
| Current Assets | |
| | |
| |
| Cash | |
$ | 17,861,214 | | |
$ | 26,736,700 | |
| Bank acceptance receivables | |
| 18,374,380 | | |
| 22,085,846 | |
| Accounts receivable | |
| 79,052,428 | | |
| 68,945,792 | |
| Inventories | |
| 1,647,146 | | |
| 1,122,038 | |
| Prepayment and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| Total current assets | |
| 132,264,679 | | |
| 135,319,155 | |
| | |
| | | |
| | |
| Property, plant and equipment | |
| 8,617,192 | | |
| 8,758,047 | |
| Intangible assets | |
| 3,876,027 | | |
| 497,600 | |
| Investment | |
| 5,997,634 | | |
| 6,669,655 | |
| Other noncurrent assets | |
| 11,856,920 | | |
| 12,333,122 | |
| Total assets | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
| | |
| | | |
| | |
| Liabilities and shareholders’ equity | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Short-term bank borrowings | |
$ | 7,171,128 | | |
$ | 6,089,428 | |
| Accounts payable | |
| 13,820,348 | | |
| 16,096,165 | |
| Taxes payable | |
| 1,451,855 | | |
| 1,131,276 | |
| Accrued expenses and other current liabilities | |
| 778,369 | | |
| 856,698 | |
| Total current liabilities | |
| 23,221,700 | | |
| 24,173,567 | |
| | |
| | | |
| | |
| Long term loan | |
| - | | |
| 724,932 | |
| Total liabilities | |
| 23,221,700 | | |
| 24,898,499 | |
| | |
| | | |
| | |
| Commitments and contingencies | |
| | | |
| | |
| Shareholders’ equity | |
| | | |
| | |
| Ordinary share, $0.0005 par value, 80,000,000 shares authorized, 23,940,000 and 23,940,000 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively | |
| 11,970 | | |
| 11,970 | |
| Preferred share, $0.0005 par value, 20,000,000 shares authorized, no shares issued and outstanding as of as of June 30, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Additional paid-in capital | |
| 42,967,006 | | |
| 42,967,006 | |
| Statutory surplus reserves | |
| 15,665,860 | | |
| 15,665,860 | |
| Retained earnings | |
| 90,392,246 | | |
| 83,330,239 | |
| Accumulated other comprehensive income (loss) | |
| (10,146,195 | ) | |
| (3,852,138 | ) |
| Total shareholders’ equity | |
| 138,890,887 | | |
| 138,122,937 | |
| Non-controlling interest | |
| 499,865 | | |
| 556,143 | |
| TOTAL EQUITY | |
| 139,390,752 | | |
| 138,679,080 | |
| | |
| | | |
| | |
| Total liabilities and shareholders’ equity | |
$ | 162,612,452 | | |
$ | 163,577,579 | |
MEIHUA INTERNATIONAL MEDICAL TECHNOLOGIES CO.,
LTD.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS
OF INCOME AND COMPREHENSIVE INCOME
For the six months ended June 30, 2023 and 2022
(US$, except share data or otherwise noted)
| | |
For the Six months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| Revenues | |
| | |
| |
| Third party sales | |
$ | 48,178,325 | | |
$ | 54,803,181 | |
| Related party sales | |
| 11,751 | | |
| 29,666 | |
| Total revenues | |
| 48,190,076 | | |
| 54,832,847 | |
| Cost of revenues | |
| 31,019,347 | | |
| 33,941,115 | |
| | |
| | | |
| | |
| Gross profit | |
| 17,170,729 | | |
| 20,891,732 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Selling | |
| 3,161,070 | | |
| 3,311,649 | |
| General and administrative | |
| 3,452,610 | | |
| 4,799,711 | |
| Research and development | |
| 1,460,376 | | |
| 1,642,204 | |
| Written-off Tai He deposit | |
| - | | |
| 2,469,466 | |
| Total operating expenses | |
| 8,074,056 | | |
| 12,223,030 | |
| | |
| | | |
| | |
| Income from operations | |
| 9,096,673 | | |
| 8,668,702 | |
| | |
| | | |
| | |
| Other (income) expense: | |
| | | |
| | |
| Interest expense | |
| 128,973 | | |
| 98,805 | |
| Interest income | |
| (361,532 | ) | |
| (19,725 | ) |
| Currency exchange gain | |
| 119,193 | | |
| (449,217 | ) |
| Other expense, net | |
| 114,298 | | |
| 50,180 | |
| Total other (income) expenses | |
| 932 | | |
| (319,957 | ) |
| | |
| | | |
| | |
| Income before income tax provision | |
| 9,095,741 | | |
| 8,988,659 | |
| Income taxes expense | |
| 2,064,212 | | |
| 2,433,772 | |
| Net income | |
| 7,031,529 | | |
$ | 6,554,887 | |
| Net loss attributable to non-controlling interests | |
| (30,478 | ) | |
| - | |
| Net income attributable to shareholders | |
| 7,062,007 | | |
| 6,554,887 | |
| | |
| | | |
| | |
| Foreign currency translation adjustment – gain / (loss) | |
| (6,319,857 | ) | |
| (6,133,093 | ) |
| Comprehensive (loss) income | |
$ | 711,672 | | |
$ | 421,794 | |
| Comprehensive loss attributable to non-controlling interests | |
| (56,278 | ) | |
| - | |
| Comprehensive (loss) income attributable to shareholders | |
| 767,950 | | |
| 421,794 | |
| | |
| | | |
| | |
| Weighted average number of ordinary shares - basic and diluted | |
| 23,940,000 | | |
| 22,873,370 | |
| | |
| | | |
| | |
| Basic & diluted net income per ordinary share | |
$ | 0.29 | | |
$ | 0.29 | |
6
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Unaudited Condensed Consolidated Balance Sheets - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Current Assets |
|
|
| Cash |
$ 17,861,214
|
$ 26,736,700
|
| Bank acceptance receivables |
18,374,380
|
22,085,846
|
| Accounts receivable |
79,052,428
|
68,945,792
|
| Inventories |
1,647,146
|
1,122,038
|
| Prepayment and other current assets |
15,329,511
|
16,428,779
|
| Total current assets |
132,264,679
|
135,319,155
|
| Property, plant and equipment |
8,617,192
|
8,758,047
|
| Intangible assets |
3,876,027
|
497,600
|
| Investment |
5,997,634
|
6,669,655
|
| Other noncurrent assets |
11,856,920
|
12,333,122
|
| Total assets |
162,612,452
|
163,577,579
|
| Current liabilities |
|
|
| Short-term bank borrowings |
7,171,128
|
6,089,428
|
| Accounts payable |
13,820,348
|
16,096,165
|
| Taxes payable |
1,451,855
|
1,131,276
|
| Accrued expenses and other current liabilities |
778,369
|
856,698
|
| Total current liabilities |
23,221,700
|
24,173,567
|
| Long term loan |
|
724,932
|
| Total liabilities |
23,221,700
|
24,898,499
|
| Commitments and contingencies |
|
|
| Shareholders’ equity |
|
|
| Ordinary share, $0.0005 par value, 80,000,000 shares authorized, 23,940,000 and 23,940,000 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively |
11,970
|
11,970
|
| Preferred share, $0.0005 par value, 20,000,000 shares authorized, no shares issued and outstanding as of as of June 30, 2023 and December 31, 2022 |
|
|
| Additional paid-in capital |
42,967,006
|
42,967,006
|
| Statutory surplus reserves |
15,665,860
|
15,665,860
|
| Retained earnings |
90,392,246
|
83,330,239
|
| Accumulated other comprehensive income (loss) |
(10,146,195)
|
(3,852,138)
|
| Total shareholders’ equity |
138,890,887
|
138,122,937
|
| Non-controlling interest |
499,865
|
556,143
|
| TOTAL EQUITY |
139,390,752
|
138,679,080
|
| Total liabilities and shareholders’ equity |
$ 162,612,452
|
$ 163,577,579
|
| X |
- DefinitionAmount of liabilities incurred to vendors for goods and services received, and accrued liabilities classified as other, payable within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_AccountsPayableAndOtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph ((a)(1),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe total amount of investments that are intended to be held for an extended period of time (longer than one operating cycle). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of all notes and loans payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesAndLoans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_MinorityInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost, after allowance for credit loss, of financing receivable. Excludes financing receivable covered under loss sharing agreement and net investment in lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-5
| Name: |
us-gaap_NotesReceivableNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionReflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ShortTermBorrowings |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of statutory capital required to be maintained as of the balance sheet date under prescribed or permitted statutory accounting practices. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 944
-SubTopic 505
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479989/944-505-50-1
| Name: |
us-gaap_StatutoryAccountingPracticesStatutoryCapitalAndSurplusRequired |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for statutory income, sales, use, payroll, excise, real, property and other taxes. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TaxesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Unaudited Condensed Consolidated Balance Sheets (Parentheticals) - $ / shares
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Statement of Financial Position [Abstract] |
|
|
| Ordinary share, par value (in Dollars per share) |
$ 0.0005
|
$ 0.0005
|
| Ordinary share, shares authorized |
80,000,000
|
80,000,000
|
| Ordinary share, shares issued |
23,940,000
|
23,940,000
|
| Ordinary share, shares outstanding |
23,940,000
|
23,940,000
|
| Preferred share, par value (in Dollars per share) |
$ 0.0005
|
$ 0.0005
|
| Preferred share, shares authorized |
20,000,000
|
20,000,000
|
| Preferred share, shares issued |
|
|
| Preferred share, shares outstanding |
|
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Unaudited Condensed Consolidated Statements of Income and Comprehensive Income - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Revenues |
|
|
| Third party sales |
$ 48,178,325
|
$ 54,803,181
|
| Related party sales |
11,751
|
29,666
|
| Total revenues |
48,190,076
|
54,832,847
|
| Cost of revenues |
31,019,347
|
33,941,115
|
| Gross profit |
17,170,729
|
20,891,732
|
| Operating expenses |
|
|
| Selling |
3,161,070
|
3,311,649
|
| General and administrative |
3,452,610
|
4,799,711
|
| Research and development |
1,460,376
|
1,642,204
|
| Written-off Tai He deposit |
|
2,469,466
|
| Total operating expenses |
8,074,056
|
12,223,030
|
| Income from operations |
9,096,673
|
8,668,702
|
| Other (income) expense: |
|
|
| Interest expense |
128,973
|
98,805
|
| Interest income |
(361,532)
|
(19,725)
|
| Currency exchange gain |
119,193
|
(449,217)
|
| Other expense, net |
114,298
|
50,180
|
| Total other (income) expenses |
932
|
(319,957)
|
| Income before income tax provision |
9,095,741
|
8,988,659
|
| Income taxes expense |
2,064,212
|
2,433,772
|
| Net income |
7,031,529
|
6,554,887
|
| Net loss attributable to non-controlling interests |
(30,478)
|
|
| Net income attributable to shareholders |
7,062,007
|
6,554,887
|
| Foreign currency translation adjustment – gain / (loss) |
(6,319,857)
|
(6,133,093)
|
| Comprehensive (loss) income |
711,672
|
421,794
|
| Comprehensive loss attributable to non-controlling interests |
(56,278)
|
|
| Comprehensive (loss) income attributable to shareholders |
$ 767,950
|
$ 421,794
|
| Weighted average number of ordinary shares - basic (in Shares) |
23,940,000
|
22,873,370
|
| Basic net income per ordinary share (in Dollars per share) |
$ 0.29
|
$ 0.29
|
| X |
- References
+ Details
| Name: |
mhua_OtherIncomeExpenseAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue generated from sales of medical devices to third parties.
| Name: |
mhua_RevenueFromThirdPartySales |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income (loss) and other comprehensive income (loss), attributable to noncontrolling interests. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.1,2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of operating interest income (expense). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.10)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_InterestIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses related to the generation of investment income. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeInvestmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of Net Income (Loss) attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
| Name: |
us-gaap_NetIncomeLossAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationAdjustmentNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of other operating income and expenses, the components of which are not separately disclosed on the income statement, from items that are associated with the entity's normal revenue producing operations.
| Name: |
us-gaap_OtherOperatingIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, including tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value-added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479941/924-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerIncludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenuesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpenses recognized in the period that are directly related to the selling and distribution of products or services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Unaudited Condensed Consolidated Statements of Changes in Shareholders’ Equity - USD ($)
|
Ordinary shares |
Additional paid-in capital |
Ordinary shares subscribed |
Statutory surplus reserves |
Retained earnings |
Accumulated other comprehensive income (loss) |
Non- controlling interests |
Total |
| Balance at Dec. 31, 2021 |
$ 10,000
|
$ 9,716,484
|
|
$ 15,178,467
|
$ 77,574,663
|
$ 5,288,988
|
|
$ 107,768,602
|
| Balance (in Shares) at Dec. 31, 2021 |
20,000,000
|
|
|
|
|
|
|
|
| Ordinary shares subscribed |
$ 1,970
|
33,748,358
|
|
|
|
|
|
33,750,328
|
| Ordinary shares subscribed (in Shares) |
3,940,000
|
|
|
|
|
|
|
|
| Net income |
|
|
|
|
6,554,887
|
|
|
6,554,887
|
| Currency translation adjustment |
|
|
|
|
|
(6,133,093)
|
|
(6,133,093)
|
| Balance at Jun. 30, 2022 |
$ 11,970
|
43,464,842
|
|
15,178,467
|
84,129,550
|
(844,105)
|
|
141,940,724
|
| Balance (in Shares) at Jun. 30, 2022 |
23,940,000
|
|
|
|
|
|
|
|
| Balance at Dec. 31, 2022 |
$ 11,970
|
42,967,006
|
|
15,665,860
|
83,330,239
|
(3,852,138)
|
556,143
|
138,679,080
|
| Balance (in Shares) at Dec. 31, 2022 |
23,940,000
|
|
|
|
|
|
|
|
| Net income |
|
|
|
|
7,062,007
|
|
(30,478)
|
7,031,529
|
| Currency translation adjustment |
|
|
|
|
|
(6,294,057)
|
(25,800)
|
(6,319,857)
|
| Balance at Jun. 30, 2023 |
$ 11,970
|
$ 42,967,006
|
|
$ 15,665,860
|
$ 90,392,246
|
$ (10,146,195)
|
$ 499,865
|
$ 139,390,752
|
| Balance (in Shares) at Jun. 30, 2023 |
23,940,000
|
|
|
|
|
|
|
|
| X |
- DefinitionAmount after tax, before reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a-c)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-12
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationGainLossArisingDuringPeriodNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent and noncontrolling interest. Excludes temporary equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 23
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483421/250-10-45-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-5
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (h)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (a)(3)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 15
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480336/718-10-65-15
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 34: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 38: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 39: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 40: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 41: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-15
Reference 42: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-16
Reference 43: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 55
-Paragraph 4I
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4I
| Name: |
us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Unaudited Condensed Consolidated Statements of Cash Flows - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Cash Flows from operating activities: |
|
|
| Net income |
$ 7,031,529
|
$ 6,554,887
|
| Adjustments for items not affecting cash: |
|
|
| Depreciation |
283,484
|
239,597
|
| Amortization |
13,733
|
13,417
|
| Net loss from disposal of property, plant and equipment |
104,572
|
|
| Written-off Tai He deposit |
|
2,469,466
|
| Deferred tax expenses (benefit) |
|
(294,567)
|
| Currency exchange (gain) loss |
119,193
|
|
| Loss from equity method investments |
1,632
|
|
| Gain from cost method investments |
(202)
|
|
| Changes in operating assets and liabilities: |
|
|
| Bank acceptance receivables |
2,755,706
|
(9,730,622)
|
| Accounts receivable |
(14,101,576)
|
3,472,261
|
| Inventories |
(606,934)
|
(7,197)
|
| Prepayments and other assets |
178,920
|
(5,268,377)
|
| Due from related parties |
|
(33,523)
|
| Accounts payable |
(1,559,255)
|
(4,851,578)
|
| Taxes payable |
393,343
|
(285,556)
|
| Accrued expenses and other current liabilities |
(38,714)
|
(64,985)
|
| Advance from customers |
|
(12,039)
|
| Net cash used in operating activities |
(5,424,569)
|
(7,798,816)
|
| Cash flows from investing activities: |
|
|
| Purchases of property, plant and equipment |
(1,001,925)
|
(459,163)
|
| Additions to intangible assets |
(3,581,058)
|
|
| Proceeds from disposal of property, plant and equipment |
355,993
|
|
| Proceeds from disposal of long-term investment |
360,839
|
|
| Net cash used in investing activities |
(3,866,151)
|
(459,163)
|
| Cash flows from financing activities: |
|
|
| Proceeds from short-term bank borrowings |
5,340,415
|
3,549,857
|
| Repayments of short-term bank borrowings |
(4,618,738)
|
(2,932,491)
|
| Shareholder contribution |
|
34,527,480
|
| Net cash provided by financing activities |
721,677
|
35,144,846
|
| Effect of foreign exchange rate changes |
(306,443)
|
(370,615)
|
| Net increase (decrease) in cash |
(8,875,486)
|
26,516,252
|
| Cash, beginning of year |
26,736,700
|
8,149,276
|
| Cash, end of year |
17,861,214
|
34,665,528
|
| Cash paid during the period for: |
|
|
| Cash paid for income tax |
2,313,417
|
2,956,643
|
| Cash paid for interest |
128,973
|
98,804
|
| Non-cash transactions |
|
|
| Shareholder contribution through deferred cost |
|
$ 1,277,152
|
| X |
- DefinitionAmount of bank acceptance receivables.
| Name: |
mhua_BankAcceptanceReceivables |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of proceeds from issuance of shares.
| Name: |
mhua_ProceedsFromIssuanceOfShares |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionShareholder contribution through deferred cost amount.
| Name: |
mhua_ShareholderContributionThroughDeferredCost |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of an equity method investment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(9)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_EquityMethodInvestmentRealizedGainLossOnDisposal |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossUnrealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on sale or disposal of property, plant and equipment assets, excluding oil and gas property and timber property. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-3
| Name: |
us-gaap_GainLossOnDispositionOfAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in accrued expenses, and obligations classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilitiesAndOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in right to consideration in exchange for good or service transferred to customer when right is conditioned on something other than passage of time. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInContractWithCustomerAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NoncashInvestingAndFinancingItemsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to acquire asset without physical form usually arising from contractual or other legal rights, excluding goodwill. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from an entity that is affiliated with the entity by means of direct or indirect ownership. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromContributionsFromAffiliates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the sale of equity method investments, which are investments in joint ventures and entities in which the entity has an equity ownership interest normally of 20 to 50 percent and exercises significant influence. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfEquityMethodInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the sale of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for the payment of debt classified as other, maturing within one year or the operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_RepaymentsOfOtherShortTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrealized gain (loss) on investment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_UnrealizedGainLossOnInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Organization and Principal Activities
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Organization and Principal Activities [Abstract] |
|
| Organization and principal activities |
1. Organization and principal activities
Principal Activities:
Meihua International Medical Technologies Co.,
Ltd. (“Meihua”) was incorporated on November 10, 2020 in the Cayman Islands. Meihua is a holding company with no operations.
Meihua produces and sells medical consumables through its wholly owned subsidiaries located in People’s Republic of China (“PRC”
or “China”). Below is Meihua’s organizational chart, as well as a description of the ownership structure.
Entity Name | |
Registered
Location | |
Percentage
of
ownership | |
Date of
incorporation | |
Principal
activities |
| Meihua International Medical Technologies Co., Ltd. (“Meihua”) | |
Cayman | |
Parent | |
November 10, 2020 | |
Investment holding |
| | |
| |
| |
| |
|
康复国际医疗有限公司
Kang Fu International Medical Co., Limited (“Kang Fu”) | |
Hong Kong | |
100% by Meihua | |
October 13, 2015 | |
Investment holding |
| | |
| |
| |
| |
|
扬州华达医疗器械有限公司
Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) | |
Yangzhou | |
100% by Kang Fu | |
December 24, 2001 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏亚达科技集团有限公司
Jiangsu Yada Technology Group Co., Ltd. (“Yada”) | |
Yangzhou | |
100% by Huada | |
December 5, 1991 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏华东医疗器械实业有限公司
Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) | |
Yangzhou | |
100% by Yada | |
November 18, 2000 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
扬州光辉医疗科技有限公司*
Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) | |
Yangzhou | |
100% by Huadong | |
December 22, 2020 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
海南国械医疗科技有限公司
Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) | |
Hainan | |
55% by Kang Fu | |
October 07, 2021 | |
Medical Equipment Sales |
Kang Fu was incorporated on October 13, 2015 with
a registered capital of HKD 53,911,815 ($6,911,771). Kang Fu is a holding company with no operations. The following operating entities
(Huada, Yada and Huadong) are all directly and indirectly 100% owned by Kang Fu for all the periods presented.
Huada is a subsidiary wholly owned by Kang Fu
and established in Yangzhou, China on December 24, 2001 with a registered capital of $17,193,021.
Yada is a subsidiary wholly owned by Huada and
was established in Yangzhou, China on December 5, 1991 with a registered capital of RMB51,390,000.
Huadong is a subsidiary wholly owned by Yada and
was established in Yangzhou, China on November 18, 2000 with a registered capital of RMB50,000,000.
Those three subsidiaries primarily manufacture
and sell Class I, II and III disposable medical devices under the Company’s own brands, and distribute Class I, II and III disposable
medical devices sourced from other manufacturers to our domestic and overseas customers.
Guanghui was a subsidiary wholly owned by Huadong
and was dissolved on June 1, 2023.
Hainan Guoxie is a subsidiary 55% owned by Kang
Fu and established in Hainan, China on October 07, 2021 with a registered capital of RMB100,000,000.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Summary of Significant Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated
financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S.
GAAP”) pursuant to the rules and regulations of the Securities Exchange Commission (“SEC”). The interim results of operations
are not necessarily indicative of results to be expected for any other interim period or for a full year. In the opinion of management,
all adjustments, consisting only of normal recurring adjustments, considered necessary for a fair presentation of its financial position
and operating results have been included. These financial statements should be read in conjunction with the Company’s audited consolidated
financial statements and the related notes thereto for the fiscal years ended December 31, 2022 and 2021.
Use of Estimates
The preparation of unaudited condensed consolidated
financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the amounts
reported and disclosed in the consolidated financial statements and related notes.
The most significant estimates and judgments include
allowance for bad debts and the valuation of inventory. Actual amounts could differ from those estimates.
Non-controlling interests
Non-controlling interest represents the portion
of the net assets of subsidiaries attributable to interests that are not owned or controlled by the Company. The non-controlling interest
is presented in the consolidated balance sheets, separately from equity attributable to the shareholders of the Company. Non-controlling
interest’s operating results are presented on the face of the consolidated statements of income and comprehensive income as an allocation
of the total income for the year between non-controlling shareholders and the shareholders of the Company. As of June 30, 2023, non-controlling
interests represent one non-controlling shareholders’ proportionate share of equity interests in Hainan Guoxie.
Functional Currency and Foreign Currency
Translation
The Company’s reporting currency is the
United States dollar (“US$”). The Company’s operations are principally conducted through the PRC subsidiaries where
the local currency is the functional currency. Therefore, the functional currency of Kang Fu is the Hong Kong dollar and the functional
currency of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”).
Transactions denominated in currencies other than
the functional currencies are translated into the functional currency of the entity at the exchange rates prevailing on the transaction
dates. Monetary assets and liabilities denominated in currencies other than the applicable functional currency are translated into the
functional currency at the prevailing rates of exchange at the balance date. The resulting exchange differences are reported in the consolidated
statements of income and comprehensive income. The assets and liabilities of the Company are
translated at the exchange spot rate at the balance sheet date, stockholders’ equity is translated at the historical rates and the
revenues and expenses are translated at the average exchange rates for the periods, except that the exchange rate used for translation
from Hong Kong dollar to US$ was 7.8000, a pegged rate determined by the linked exchange rate system in Hong Kong. This pegged rate was
used to translate Kang Fu’s balance sheets, income statement items and cash flow items for both the six months ended June 30, 2023
and 2022. The resulting translation adjustments are reported under other comprehensive income in the consolidated statements of income
and comprehensive income in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification
(“ASC”) 220, Comprehensive Income. The following are the exchange rates that were used in translating the Company’s
PRC subsidiaries’ financial statements into the consolidated financial statements:
| | |
June 30, 2023 | |
December 31, 2022 | |
June 30, 2022 |
| | |
| |
| |
|
| Period-end spot rate | |
US$1=RMB 7.2513 | |
US$1=RMB 6.8972 | |
US$1=RMB 6.6981 |
| | |
| |
| |
|
| Average rate | |
US$1=RMB 6.9283 | |
US$1=RMB 6.7290 | |
US$1=RMB 6.4791 |
Certain Risks and Concentration
The Company’s financial instruments that
potentially subject the Company to significant concentrations of credit risk consist primarily of cash and receivables. As of six months
ended June 30, 2023 and December 31, 2022 substantially all the Company’s cash were held in major financial institutions located
in Hong Kong and the PRC, which management considers to be of high credit quality.
For the six months ended June 30, 2023, two customers
accounted for approximately 18.18% and 10.01% of the Company’s total revenues. For the six months ended June 30, 2022, two customers
accounted for approximately 32.53% and 11.25% of the Company’s total revenues.
As of June 30, 2023, two customers accounted for
approximately 30.92% and 21.31% of the Company’s accounts receivable. As of December 31, 2022, two customers accounted for approximately
27.73% and 13.14% respectively, of the Company’s accounts receivable.
For the six months ended June 30, 2023, one supplier
accounted for approximately 14.73% of the Company’s total purchases. As of six months ended June 30, 2022, there was no supplier
that individually represented greater than 10% of the total purchase of the Company’s total purchases. Fair Value Measurement
Fair value is the price that would be received
from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When
determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers
the principal or most advantageous market in which it would transact, and it considers assumptions that market participants would use
when pricing the asset or liability.
The Company adopted the guidance of Accounting
Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods
for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| |
Level 1: |
Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| |
|
|
| |
Level 3: |
Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The Company’s financial instruments include
cash, accounts receivable, bank acceptance receivables, due from related parties, accounts payable, other liabilities and accrued expenses
and short-term bank borrowings. The carrying amounts approximate their fair values due to their short maturities as of June 30, 2023 and
December 31, 2022.
The Company noted no transfers between levels
during any of the periods presented. The Company did not have any instruments that were measured at fair value on a recurring or non-recurring
basis as of June 30, 2023 and December 31, 2022.
Cash
Cash consists of petty cash on hand and cash held
in banks, which are highly liquid and are unrestricted as to withdrawal or use.
Bank Acceptance Receivables
Bank acceptance receivables are issued by bank
under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes until
maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party willing
to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year of the
original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers can
pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year, and are
non-interest bearing. As bank acceptance receivables are issued by the banks and payments are guaranteed. The Company has not discounted
any bank acceptances and there were no endorsed bank acceptances that are unmatured as of June 30, 2023. The Company collected approximately
$6.0 million as of August 31, 2023. Accounts Receivable and Allowance for Doubtful
Accounts
Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment.
The Company follows the guidance under ASC 326,
Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Instruments. The standard uses a new forward-looking
“methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information
to inform credit loss estimates.” The Company has adopted the loss rate methodology to estimate historical losses on accounts receivables.
The Company has adopted the aging methodology to estimate the credit losses on accounts receivables. The historical data is adjusted to
account for forecasted changes in the macroeconomic environment in order to calculate the current expected credit loss. The provision
is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements of operations and
comprehensive income.
The Company historically has not had material
bad debts in accounts receivable. There were no bad debt expenses related to accounts receivable for six months ended June 30, 2023 and
2022, and there was no provision for doubtful accounts as of June 30, 2023 and December 31, 2022.
Inventories
Inventories are valued using the lower of cost
or net realizable value. Cost is principally determined using the weighted-average method. Manufactured inventories included cost of materials,
labor and overhead expenses. The Company records adjustments to inventory for excess quantities, obsolescence, or impairment, when appropriate,
to reflect inventory at net realizable value. These adjustments are based upon a combination of factors including current sales volume,
market conditions, lower of cost or market analysis and expected realizable value of the inventory.
There were no write-downs recognized of inventories
as of June 30, 2023 and December 31, 2022.
Prepayment and other current assets
As of June 30, 2023 and December 31, 2022, prepayment
and other current assets were $15,329,511 and $16,428,779.
Prepayment and other assets primarily consist
of prepayments for land use right and property, refundable tax credits and receivables, security deposits made to customers, advances
to employees, which are presented net of allowance for doubtful accounts. These balances are unsecured and are reviewed periodically to
determine whether their carrying value has become impaired. The Company considers the balances to be impaired if the utilization or refund
of the balances becomes doubtful. The Company uses the aging method to estimate the allowance for uncollectible balances. The allowance
is also based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical trends
of collections and utilizations. Actual amounts received or utilized may differ from management’s estimate of credit worthiness
and the economic environment. Delinquent account balances are written off against allowance for doubtful accounts after management has
determined that the likelihood of collection is not probable. The allowance for doubtful accounts amounted to nil as of June 30, 2023
and December 31, 2022. Property, Plant and Equipment
Property, plant and equipment items are recorded
at their historic cost, less accumulated depreciation and impairment losses. The Company calculates depreciation using the straight-line
method, after consideration of the estimated residual values, over the following estimated useful lives:
| Category | |
Useful lives | |
Estimated
residual
value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
Major improvements are capitalized and expenditures
for maintenance and repairs are expensed as incurred. Construction in progress represents property, plant and equipment under construction
or being installed. Costs include original cost, installation, construction and other direct costs. Interest expenses directly related
to construction in progress would be capitalized. Construction in progress is transferred to the appropriate fixed asset account and depreciation
commences when the asset has been substantially completed and placed in service.
Intangible Assets
Intangible assets are non-monetary assets without
physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and
impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives.
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful lives, which is as
follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
Impairment of Long-Lived Assets
The Company accounts for impairment of long-lived
assets in accordance with Accounting Standards Codification (“ASC”) 360, Property, Plant and Equipment. (“ASC
360”). Long-lived assets consist primarily of property, plant and equipment, and intangible assets. In accordance with ASC 360,
the Company evaluates the carrying value of long-lived assets when it determines a triggering event has occurred, or whenever events or
changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When indicators exist, recoverability of
assets is measured by a comparison of the carrying value of the asset group to the estimated undiscounted future net cash flows expected
to be generated by the asset. Examples of such triggering events include a significant disposal of a portion of such assets, and adverse
change in the market involving the business employing the related assets. If such assets are determined not to be recoverable, the Company
performs an analysis of the fair value of the asset group and will recognize an impairment loss when the fair value is less than the carrying
amounts of such assets. The fair value, based on reasonable and supportable assumptions and projections, require subjective judgments.
Depending on the assumptions and estimates used, the appraised fair value projected in the evaluation of long-lived assets can vary within
a range of outcomes. The Company considers the likelihood of possible outcomes in determining the best estimate for the fair value of
the assets. The Company did not record any impairment charges for the six months ended June 30, 2023 and 2022. There can be no assurance
that future events will not have impact on company revenue or financial position which could result in impairment in the future. Investment
In accordance with Financial Accounting Standards
Board (“FASB”) ASC 321, “Investment-Equity Securities,” the Company accounts for non-marketable securities on
a prospective basis. Equity investments that do not have readily determinable fair values and do not qualify for the net asset value practical
expedient are eligible for the measurement alternative.
On March 3, 2011, Yada invested in Yangzhou Juyuan
Guarantee Co., Ltd (“Juyuan”) and obtained 12% equity interest of Juyuan. For the Company’s passive and without significant
influence or control equity investment in private company which do not have readily determinable fair values, the Company has elected
the measurement alternative defined as cost, less impairment, plus or minus adjustments resulting from observable price changes in orderly
transactions for the identical or a similar investment of the Company. The investment is reviewed periodically to determine if its value
has been impaired and adjustments are recorded as necessary in profit or loss for the period. On January 5, 2023, majority shareholder
of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839 (RMB 2.5 million).
Investments in entities in which the Company can
exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting
in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method, the Company
initially records its investment at cost and the difference between the cost of the equity investee and the amount of the underlying equity
in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The share of earnings or
losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity method adjustments include the Company’s
proportionate share of investee income or loss, adjustments to recognize certain differences between the Company’s carrying value
and its equity in net assets of the investee at the date of investment, impairments, and other adjustments required by the equity method.
The Company assesses its equity investment for other-than-temporary impairment by considering factors as well as all relevant and available
information including, but not limited to, current economic and market conditions, the operating performance of the investees including
current earnings trends, the general market conditions in the investee’s industry or geographic area, factors related to the investee’s
ability to remain in business, such as the investee’s liquidity, debt ratios, and cash burn rate and other company-specific information.
Investments in equity securities without readily
determinable fair values are measured at cost minus impairment adjusted by observable price changes in orderly transactions for the identical
or a similar investment of the same issuer. These investments are measured at fair value on a nonrecurring basis when there are events
or changes in circumstances that may have a significant adverse effect. An impairment loss is recognized in the consolidated statements
of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment. Prior to the adoption
of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method of accounting, measured at cost less other-than-temporary
impairment.
On December 1, 2022, Huadong invested RMB 40 million
into Jiangsu Zhongxiangxin International Science and Technology Innovation Park Co., Ltd. (“Zhongxiangxin”), and obtained
25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the PRC. The Company accounted for
the investments using equity method, because the Company has significant influence but does not own a majority equity interest or otherwise
control over the equity investee. Under the equity method, the Company adjusts the carrying amount of the investment and recognizes investment
income or loss for its share of the earnings or loss of the investee after the date of investment. When the Company’s share of losses
in the equity investee equals or exceeds its interest in the equity investee, the Company does not recognize further losses, unless the
Company has incurred obligations or made payments or guarantees on behalf of the equity investee. For the six months ended June 30, 2023
and 2022, the investment loss from Zhongxiangxin was $1,632 and nil. The Company continually reviews its investments
in equity investees to determine whether a decline in fair value below the carrying value is other-than-temporary. The primary factors
the Company considers in its determination include the financial condition, operating performance and the prospects of the equity investee;
other company specific information such as recent financing rounds; the geographic region, market and industry in which the equity investee
operates; and the length of time that the fair value of the investment is below its carrying value. If the decline in fair value is deemed
to be other-than-temporary, the carrying value of the equity investee is written down to fair value.
For the for the six months ended June 30, 2023
and 2022, no impairment indicators were identified of its investment in the private company was no impairment recorded.
Value-added Tax
Value-added taxes (“VAT”) collected
from customers relating to product sales and remitted to governmental authorities are presented on a net basis. VAT collected from customers
is excluded from revenue which is recorded in VAT payable. The Company is subject to a VAT rate of 13%. The VAT payable may be offset
by VAT paid by the Company on raw materials and other materials included in the cost of producing or acquiring its finished products.
Related Parties
Parties are considered to be related to the Company
if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with
the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal
owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly
influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully
pursuing its own separate interests. The Company discloses all significant related party transactions.
Revenue Recognition
Based on the requirements of ASC Topic 606, the
Company recognizes revenue when control of the promised goods or services is transferred to the customers in an amount that reflects the
consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells its products
to hospitals and medical equipment companies. Revenue is recognized when the following 5-step revenue recognition criteria are met:
| 1) | Identify
the contract with a customer |
| 2) | Identify
the performance obligations in the contract |
| 3) | Determine
the transaction price |
| 4) | Allocate
the transaction price |
| 5) | Recognize
revenue when or as the entity satisfies a performance obligation |
Revenue from product sales is recognized at the
point in time control of the products is transferred, generally upon customer receipt based upon the standard contract terms. Shipping
and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered to
be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard quality policy and
returns are generally not significant. Payment terms for product sales are generally set at 90 to 180 days after customer acceptance of
the product. Revenue Disaggregation
The Company’s disaggregated revenues are
represented by two categories which are type of goods and type of customers.
Type of Goods
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 23,435,544 | | |
| 27,046,663 | |
| Resales of Sourced Disposable Medical Devices from Third Party Manufacturers | |
| 24,754,532 | | |
| 27,786,184 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Type of Customers
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Direct sales | |
| 4,305,506 | | |
| 4,582,321 | |
| Distributors | |
| 43,884,570 | | |
| 50,250,526 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Earnings per Ordinary Share
Earnings (loss) per ordinary share is calculated
in accordance with ASC 260, Earnings per Share. Basic earnings (loss) per ordinary share is computed by dividing the net income
(loss) attributable to shareholders of the Company by the weighted average number of ordinary shares outstanding during the year. Diluted
earnings per ordinary share is computed in accordance with the treasury stock method and based on the weighted average number of ordinary
shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents are excluded from the computation of diluted earnings
per ordinary share if their effects would be anti-dilutive. There is no ordinary share equivalent issued to date.
Comprehensive Income (Loss)
ASC 220, Comprehensive Income (“ASC 220”)
establishes rules for reporting and display of comprehensive income and its components. ASC 220 requires that unrealized gains and losses
on the Company’s foreign currency translation adjustments be included in comprehensive income (loss).
Advertising Costs
The Company’s advertising costs are expensed
as incurred. Advertising expenses are included in selling expenses in the accompanying consolidated statements of income and comprehensive
income. Advertising expenses were $8,275, and $7,603 for the six months ended June 30, 2023, and 2022, respectively.
Research and Development Costs
Research and development expenses are expensed
as incurred. Research and development expenses were $1,460,376 and $1,642,204 for the six months ended June 30, 2023, and 2022, respectively. Income Tax
Current income taxes are provided on the basis
of net profit for financial reporting purposes, adjusted for income and expense items which are not assessable or deductible for income
tax purposes, in accordance with the regulations of the relevant tax jurisdictions.
Deferred income taxes are recognized for temporary
differences between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements, net operating
loss carry forwards and credits. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more
likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided in accordance
with the laws of the relevant taxing authorities. Deferred tax assets and liabilities are measured using enacted rates expected to apply
to taxable income in which temporary differences are expected to be reversed or settled. The effect on deferred tax assets and liabilities
of changes in tax rates is recognized in the statement of comprehensive income in the period of the enactment of the change.
The Company considers positive and negative evidence
when determining whether a portion or all of its deferred tax assets will more likely than not be realized. This assessment considers,
among other matters, the nature, frequency and severity of current and cumulative losses, forecasts of future profitability, the duration
of statutory carry-forward periods, its experience with tax attributes expiring unused, and its tax planning strategies. The ultimate
realization of deferred tax assets is dependent upon its ability to generate sufficient future taxable income within the carry-forward
periods provided for in the tax law and during the periods in which the temporary differences become deductible. When assessing the realization
of deferred tax assets, the Company has considered possible sources of taxable income including (i) future reversals of existing
taxable temporary differences, (ii) future taxable income exclusive of reversing temporary differences and carryforwards, (iii) future
taxable income arising from implementing tax planning strategies, and (iv) specific known trend of profits expected to be reflected
within the industry.
The Company recognizes a tax benefit associated
with an uncertain tax position when, in its judgment, it is more likely than not that the position will be sustained upon examination
by a taxing authority. For a tax position that meets the more-likely-than-not recognition threshold, the Company initially and subsequently
measures the tax benefit as the largest amount that the Company judges to have a greater than 50% likelihood of being realized upon ultimate
settlement with a taxing authority. The Company’s liability associated with unrecognized tax benefits is adjusted periodically due
to changing circumstances, such as the progress of tax audits, case law developments and new or emerging legislation. Such adjustments
are recognized entirely in the period in which they are identified. The Company’s effective tax rate includes the net impact of
changes in the liability for unrecognized tax benefits and subsequent adjustments as considered appropriate by management. The Company
classifies interest and penalties recognized on the liability for unrecognized tax benefits as income tax expense.
Segment Reporting
FASB ASC 280, “Segment Reporting,”
establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational
structure as well as information of the Company’s business segments, geographical areas, segments and major customers. The Company
uses the “management approach” in determining reportable operating segments. The management approach considers the internal
organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance
as the source for determining the Company’s reportable segments. The chief operating decision maker is the Company’s president
and Chief Executive Officer (“CEO”). Management, including the chief operating decision maker, reviews operating results of
different products at revenue level with no allocation of operating costs. Consequently, based on management’s assessment, the Company
has determined that it has only one operating segment as defined by FASB ASC 280. The Company has disclosed the type of revenue by government category
as follows.
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 3,561,156 | | |
| 4,462,704 | | |
| 8,023,860 | | |
| 3,812,092 | | |
| 3,845,205 | | |
| 7,657,297 | |
| Class II | |
| 17,313,377 | | |
| 17,761,970 | | |
| 35,075,347 | | |
| 20,185,052 | | |
| 19,712,006 | | |
| 39,897,058 | |
| Class III | |
| 524,802 | | |
| 873,575 | | |
| 1,398,377 | | |
| 416,595 | | |
| 1,168,221 | | |
| 1,584,816 | |
| Others | |
| 2,036,209 | | |
| 1,656,283 | | |
| 3,692,492 | | |
| 2,632,924 | | |
| 3,060,752 | | |
| 5,693,676 | |
| Total | |
| 23,435,544 | | |
| 24,754,532 | | |
| 48,190,076 | | |
| 27,046,663 | | |
| 27,786,184 | | |
| 54,832,847 | |
Class I, II, and III medical devices are defined
by the National Medical Products Administration of China according to their risk levels under the Regulation on the Supervision and Administration
of Medical Devices (2021 Revision), Article 6 as follows:
| ● | “Class
I Medical Devices” means medical devices with low risks, whose safety and effectiveness can be ensured through routine administration. |
| ● | “Class
II Medical Devices” means medical devices with moderate risks, which shall be strictly controlled and administered to ensure their
safety and effectiveness. |
| ● | “Class
III Medical Devices” means medical devices with relatively high risks, which shall be strictly controlled and administered through
special measures to ensure their safety and effectiveness. |
Furthermore, the Company has disclosed revenue by major product type
included in each government category.
| | |
| |
June 30, 2023 | | |
June 30, 2022 | |
| Category | |
Products | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 1,073,853 | | |
| 1,346,164 | |
| | |
Oral medicine bottle | |
| 1,830,363 | | |
| 2,189,018 | |
| | |
Anal bag | |
| 849,099 | | |
| 395,292 | |
| | |
Other Class I | |
| 4,270,545 | | |
| 3,726,823 | |
| Subtotal-Class I | |
| |
| 8,023,860 | | |
| 7,657,297 | |
| Class II | |
Masks | |
| 47,946 | | |
| 211,468 | |
| | |
Identification tape | |
| 5,494,306 | | |
| 7,218,564 | |
| | |
Disposable medical brush | |
| 4,481,601 | | |
| 4,606,634 | |
| | |
Gynecological inspection kits | |
| 3,022,727 | | |
| 5,807,398 | |
| | |
Surgical kit | |
| 2,206,201 | | |
| 13,546,908 | |
| | |
Medical brush | |
| 2,809,448 | | |
| 2,586,945 | |
| | |
Medical kit | |
| 983,584 | | |
| 883,977 | |
| | |
Other Class II | |
| 16,029,534 | | |
| 5,035,164 | |
| Subtotal-Class II | |
| |
| 35,075,347 | | |
| 39,897,058 | |
| Class III | |
Electronic pump | |
| 138,751 | | |
| 67,866 | |
| | |
Anesthesia puncture kit | |
| 229,616 | | |
| 205,218 | |
| | |
Disposable infusion pump | |
| 113,335 | | |
| 78,453 | |
| | |
Infusion pump | |
| 178,461 | | |
| 90,036 | |
| | |
Electronic infusion pump | |
| 330 | | |
| 43,397 | |
| | |
Laparoscopic trocar | |
| 38 | | |
| 94,337 | |
| | |
Other Class III | |
| 737,846 | | |
| 1,005,509 | |
| Subtotal-Class III | |
| |
| 1,398,377 | | |
| 1,584,816 | |
| Others | |
| |
| 3,692,492 | | |
| 5,693,676 | |
| Total | |
| |
| 48,190,076 | | |
| 54,832,847 | |
For the six months ended June 30, 2023, and 2022,
revenues and assets within PRC contributed over 99.1% of the Company’s total revenues and assets. The Outbreak of COVID-19
On January 30, 2020, the World Health Organization
declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency of International Concern,” and on March
11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” COVID-19 has had a severe and negative
impact on the Chinese and the global economy and such impact persists as of the date of this annual report.
In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply while the sales of products other than masks
declined due to an overall decrease in market demand. In fiscal year 2021, with the stable control of the domestic epidemic in China,
the market of masks was no longer in urgent shortage compared to the same period in 2020, and the production of epidemic prevention products
resumed more normal production levels. In general, with the precise control of the epidemic in China, our production and operations have
recovered smoothly, and the demand for other products has increased gradually. After the initial outbreak of COVID-19, from time to time,
some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the Omicron variants
in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series of restrictions and
quarantines were implemented to contain the spread.
Many of the restrictive measures previously adopted
by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more flexible
measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic could have
a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will be dependent
on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government actions
in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many other
factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the
expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the current
situation continues. Recently Issued Accounting Standards
In October 2021, the FASB issued ASU No. 2021-08,
“‘Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers”
(“ASU 2021-08”). This ASU requires entities to apply Topic 606 to recognize and measure contract assets and contract liabilities
in a business combination. The amendments improve comparability after the business combination by providing consistent recognition and
measurement guidance for revenue contracts with customers acquired in a business combination and revenue contracts with customers not
acquired in a business combination. The amendments are effective for the Company beginning after December 15, 2023, and are applied prospectively
to business combinations that occur after the effective date. The Company does not expect the adoption of ASU 2021-04 will have a material
effect on the consolidated financial statements.
In June
2022, the FASB issued ASU 2022-03 Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual
Sale Restrictions. The update clarifies that a contractual restriction on the sale of an equity security is not considered part of the
unit of account of the equity security and, therefore, is not considered in measuring fair value. The update also clarifies that an entity
cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The update also requires certain additional
disclosures for equity securities subject to contractual sale restrictions. For public business entities, the amendments in this Update
are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities,
the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early
adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. As
an emerging growth company, the standard is effective for the Company for the year ended
December 31, 2025. The Company is in the process of evaluating the impact of the new guidance
on its consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Prepayments and Other Assets
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Prepayments and Other Assets [Abstract] |
|
| Prepayments and other assets |
3. Prepayments and other assets
Prepayments and other current assets consist of
the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Other receivable | |
| 159,817 | | |
| 239,148 | |
| Prepaid tax | |
| - | | |
| 250,410 | |
| Prepaid for land use right (1) | |
| 15,169,694 | | |
| 15,948,501 | |
| Prepaid for property (2) | |
| 11,856,920 | | |
| 12,323,842 | |
| Total | |
| 27,186,431 | | |
| 28,761,901 | |
| Less: non-current portion | |
| (11,856,920 | ) | |
| (12,333,122 | ) |
| Prepayments and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| (1) | On
October 22, 2018, the Company signed a land use right agreement with the government of Touqiao Town, Yangzhou City and paid RMB 50 million
($6.9 million) and RMB 60 million ($8.27 million), respectively, in 2018 and 2019 according to the agreement. As a result of COVID-19,
the land use right had not been transferred to the Company as scheduled. Both parties agreed to cancel the transaction and the funds
prepaid for land use right will be returned to the Company before December 31, 2023. |
| (2) | On
April 20, 2020, the Company signed a factory building purchase agreement with Jiangsu Qionghua Group Co., Ltd. and paid deposit of RMB
85 million ($11.72 million). As a result of COVID-19, the factory building had not been completed as scheduled. Both parties agreed to
cancel the transaction and that the deposit for the building would be returned to the Company on or before December 31, 2025, with such
deposit accumulating interest at an annual interest rate of 3.5%. |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherAssetsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for other current assets.
| Name: |
us-gaap_OtherCurrentAssetsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Inventories
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Inventories [Abstract] |
|
| Inventories |
4. Inventories
Inventories consist of the following:
| | |
June 30, 2023 | | |
December 31, 2021 | |
| | |
US$ | | |
US$ | |
| Raw material | |
| 493,143 | | |
| 177,474 | |
| Work-in-process | |
| 20,501 | | |
| 343,795 | |
| Finished goods | |
| 977,974 | | |
| 560,119 | |
| Goods in transit | |
| 95,814 | | |
| | |
| Low-value consumables | |
| 59,714 | | |
| 40,650 | |
| Total | |
| 1,647,146 | | |
| 1,122,038 | |
For the six months ended June 30, 2023 and 2022, there were no writes-down
of inventories.
|
| X |
- DefinitionThe entire disclosure for inventory. Includes, but is not limited to, the basis of stating inventory, the method of determining inventory cost, the classes of inventory, and the nature of the cost elements included in inventory. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
| Name: |
us-gaap_InventoryDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible Assets
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Intangible Assets [Abstract] |
|
| Intangible Assets |
5. Intangible Assets
Intangible assets consisted of the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Land use rights | |
| 4,134,521 | | |
| 752,887 | |
| Patents | |
| 27,582 | | |
| 28,997 | |
| Software | |
| 12,113 | | |
| 9,424 | |
| Trademarks | |
| 115,836 | | |
| 121,789 | |
| Total | |
| 4,290,052 | | |
| 913,097 | |
| Less: accumulated amortization | |
| 414,025 | | |
| 415,497 | |
| Intangible assets, net | |
| 3,876,027 | | |
| 497,600 | |
Amortization expense was $13,733 and $13,417 for
the six months ended June 30, 2023 and 2022, respectively. Hainan Guoxie spent $3.4 million in purchasing land use right for the six months
ended June 30, 2023 and the price was fully paid. The land will be used for manufacturing facility.
The following table sets forth the Company’s
amortization expenses for the twelve months ending December 31 of the following years:
| 2023 | |
$ | 41,870 | |
| 2024 | |
| 83,740 | |
| 2025 | |
| 83,740 | |
| 2026 | |
| 82,953 | |
| 2027 | |
| 82,690 | |
| Thereafter | |
| 3,501,034 | |
| | |
$ | 3,876,027 | |
As of June 30, 2023 and December 31, 2022, the
Company pledged land use rights to secure bank borrowings to the Company as disclosed in Note 7.
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all or part of the information related to intangible assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-30/tableOfContent
| Name: |
us-gaap_IntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Investment
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Investment [Abstract] |
|
| Investment |
6. Investment
On March 3, 2011, Yada invested RMB 6 million
into Yangzhou Juyuan Guarantee Co., Ltd. (“Juyuan”) and obtained 12% equity interest of Juyuan. Juyuan mainly provides financing
guarantee services and relevant consulting services to customers. Juyuan has only one executive director and one supervisor. Neither the
executive director nor supervisor is related to Yada. Therefore, Yada has neither control nor significant influence over Juyuan. For the
Company’s passive and without significant influence or control equity investment in a private company which does not have readily
determinable fair values, the Company has elected the measurement alternative defined as cost, less impairment, plus or minus adjustments
resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. On January
5, 2023, majority shareholder of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839(RMB 2.5 million).
The carrying value of the investment amounted to approximately $0.5 million as of June 30, 2023.
On December 1, 2022, Huadong invested RMB40 million
into Zhongxiangxin, and obtained 25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the
PRC. The Company accounted for the investments using the equity method, because the Company has significant influence but does not own
a majority equity interest or otherwise control over the equity investee. Under the equity method, the Company adjusts the carrying amount
of the investment and recognizes investment income or loss for its share of the earnings or loss of the investee after the date of investment.
When the Company’s share of losses in the equity investee equals or exceeds its interest in the equity investee, the Company does
not recognize further losses, unless the Company has incurred obligations or made payments or guarantees on behalf of the equity investee.
For the six months ended June 30, 2023 and 2022, the investment loss from Zhongxiangxin was $1,632 and $nil.
For the six months ended June 30, 2023 and 2022,
no impairment indicators were identified related to revaluation of its investment in the private company was recorded.
|
| X |
- DefinitionThe entire disclosure for investment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//320/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Topic 321
-Publisher FASB
-URI https://asc.fasb.org//321/tableOfContent
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Topic 325
-Publisher FASB
-URI https://asc.fasb.org//325/tableOfContent
| Name: |
us-gaap_InvestmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Bank Borrowings
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Bank Borrowings [Abstract] |
|
| Bank Borrowings |
7. Bank Borrowings
Bank borrowings are working capital loans from
banks in China. Short-term bank borrowings as of June 30, 2023 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
1/18/2023 | |
5/25/2024 | |
| 4,000,000 | | |
| 551,625 | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Agricultural Bank of China | |
Huadong | |
| 3.60 | % | |
8/12/2022 | |
7/12/2023 | |
| 9,000,000 | | |
| 1,241,157 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
1/30/2023 | |
2/15/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Bank of China | |
Huadong | |
| 3.80 | % | |
3/10/2023 | |
3/9/2024 | |
| 10,000,000 | | |
| 1,379,063 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023 | |
| 10,000,000 | | |
| 1,379,063 | |
| Industrial and Commercial Bank of China* | |
Yada | |
| 3.45 | % | |
2/17/2022 | |
2/16/2024 | |
| 9,000,000 | | |
| 1,241,156 | |
| Total | |
| |
| | | |
| |
| |
| 52,000,000 | | |
| 7,171,128 | |
Short-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 4,000,000 | | |
| 579,946 | |
| Agricultural Bank of China | |
Huadong | |
| 3.40 | % | |
12/8/2022 | |
12/7/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
2/17/2022 | |
3/2/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Bank of China | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023* | |
| 10,000,000 | | |
| 1,449,864 | |
| Industrial and Commercial Bank of China | |
Yada | |
| 3.70 | % | |
2/18/2022 | |
2/21/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| |
| | | |
| |
| |
| 42,000,000 | | |
| 6,089,428 | |
| * | These
loans were renewed upon maturity. |
Interest expense was $128,973, and $98,805 for
the six months ended June 30, 2023 and 2022, respectively.
The Company’s short-term bank borrowings
are pledged by the Company’s assets and guaranteed by the Company’s major shareholders Yongjun Liu, Yin Liu and its subsidiary
Yada.
The carrying values of the Company’s pledged assets to secure
short-term borrowings by the Company are as follows:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| Buildings, net | |
| 3,432,150 | | |
| 2,777,379 | |
| Land use right, net | |
| 90,322 | | |
| 96,416 | |
| Total | |
| 3,522,472 | | |
| 2,873,795 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShortTermBorrowingsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for short-term debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//470/tableOfContent
| Name: |
us-gaap_ShortTermDebtTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Long-Term Bank Loan
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Long-Term Bank Loan [Abstract] |
|
| Long-term bank loan |
8. Long-term bank loan
There was no long-term bank loan as of June 30, 2023.
Long-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 724,932 | |
| Total | |
| |
| | | |
| |
| |
| 5,000,000 | | |
| 724,932 | |
On November 3, 2022, the Company signed a loan
agreement with Bank of Communications to obtain a two-year loan of RMB5 million ($724,932). The loan bears a floating interest rate of
a benchmark rate (3.50%). Huada mortgages the property and land for guaranteed repayment of the loan. The principal shall be repaid on
April 25, 2024. The balance was reclassified to short-term bank loan as of June 30, 2023.
|
| X |
- References
+ Details
| Name: |
us-gaap_LongTermDebtAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-term debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//470/tableOfContent
| Name: |
us-gaap_LongTermDebtTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Taxes Payable
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Taxes Payable [Abstract] |
|
| Taxes Payable |
9. Taxes Payable
Taxes payable consisted of the following:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| VAT payable | |
| 368,195 | | |
| 380,926 | |
| Income tax payable | |
| 1,012,775 | | |
| 690,824 | |
| Other tax payable | |
| 71,348 | | |
| 59,526 | |
| Total | |
| 1,452,318 | | |
| 1,131,276 | |
|
| X |
- References
+ Details
| Name: |
mhua_TaxesPayableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TaxesPayableCurrentAndNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Income Taxes [Abstract] |
|
| Income Taxes |
10. Income Taxes
Cayman Islands
Under the current laws of the Cayman Islands,
the Company is not subject to tax on income or capital gain. Additionally, upon payments of dividends to the shareholders, no Cayman Islands
withholding tax is imposed.
Hong Kong
Under the current Hong Kong Inland Revenue Ordinance,
the Company’s Hong Kong subsidiary, Kang Fu, is subject to 16.5% income tax on its taxable income generated from operations in Hong
Kong. On December 29, 2017, the Hong Kong government announced a two-tiered profit tax rate regime. Under the two-tiered tax rate regime,
the first HK$2.0 million earned in assessable profits will be subject to an 8.25% lower tax rate and the remaining taxable income will
continue to be taxed at the existing 16.5% tax rate. The two-tiered tax regime becomes effective from the assessment year of 2018 and
2019, which is on or after April 1, 2018. The application of the two-tiered rates is restricted to only one nominated enterprise among
connected entities. Kang Fu has been nominated by the Company as the entity to apply the two-tiered rates among the group for the assessment
years of 2023 and 2022. PRC
Provisions for income tax are as follows:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Provisions for current income tax | |
| 2,064,697 | | |
| 2,433,772 | |
| Provisions for deferred income tax | |
| - | | |
| - | |
| Total | |
| 2,064,697 | | |
| 2,433,772 | |
The following is a reconciliation of the Company’s
total income tax expense to the income before income taxes for the six months ended June 30, 2023 and 2022, respectively:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Income before income tax provision | |
| 9,097,681 | | |
| 8,988,658 | |
| Tax at the PRC EIT tax rates | |
| 2,203,896 | | |
| 2,689,271 | |
| Change in valuation allowance | |
| 16,934 | | |
| - | |
| Tax effect of non-deductible expenses | |
| 208,961 | | |
| 157,007 | |
| Tax effect of R&D expenses additional deduction* | |
| (365,094 | ) | |
| (412,506 | ) |
| Income tax expense | |
| 2,064,697 | | |
| 2,433,772 | |
| * | According
to PRC tax regulations, an additional of 100% of current year R&D expenses may be deducted from tax income. |
Under the Enterprise Income Tax Law (“EIT Law”), Foreign
Investment Enterprises (“FIEs”) and domestic companies are subject to Enterprise Income Tax (“EIT”) at a uniform
rate of 25%.
Huadong was granted a High and New Technology
Enterprise (“HNTE”) certificate and received a preferential tax rate of 15% for a three-year validity period from November
30, 2016 and the HNTE certificate was renewed on December 22, 2022 with a three-year validity period. Thus, Huadong will remain eligible
for a 15% preferential tax rate from January 1, 2016 through December 31, 2025.
The EIT Law also provides that an enterprise established
under the laws of a foreign country or region but whose “de facto management body” is located in the PRC be treated as a resident
enterprise for PRC tax purposes and consequently be subject to the PRC income tax at the rate of 25% for its global income. The Implementing
Rules of the EIT Law define the location of the “de facto management body” as “the place where the exercising, in substance,
of the overall management and control of the production and business operation, personnel, accounting, properties, etc., of a non-PRC
company is located.” Based on a review of surrounding facts and circumstances, the Company does not believe that it is likely that
its entities registered outside of the PRC should be considered as resident enterprises for the PRC tax purposes.
The EIT Law also imposes a withholding income
tax on dividends distributed by a FIE to its immediate holding company outside of the PRC. As a result, Kang Fu, which is the parent of
Huada, Yada and Huadong, is therefore subject to a maximum withholding tax of 10% on dividends distributed by Huada, Yada and Huadong.
In accordance with accounting guidance, all undistributed earnings are presumed to be transferred to the parent company and are subject
to the withholding taxes. The presumption may be overcome if the Company has sufficient evidence to demonstrate that the undistributed
dividends will be re-invested and the remittance of the dividends will be postponed indefinitely. As of June 30, 2023, the Company has
determined that the undistributed earnings in Huada, Yada and Huadong will be re-invested into the subsidiary for the expansion of the
Company’s business in mainland China and hence the remittance of dividends will be postponed indefinitely. Uncertain tax positions
The Company evaluates each uncertain tax position
(including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits
associated with the tax positions. As of June 30, 2023, and 2022, the Company did not have any significant unrecognized uncertain tax
positions.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments and Contingencies
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Commitments and Contingencies [Abstract] |
|
| Commitments and Contingencies |
11. Commitments and Contingencies
Operating lease
The Company has an operating lease to rent an
office space in Shanghai. The lease term is 12 months, with the option to renew annually. Rent expense was $5,856 and $5,856 and is included
in general and administrative expenses for the six months ended June 30, 2023 and 2022, respectively. The Company has renewed the same
operating lease with a term from January 1, 2023 to December 31, 2023, with all other lease terms remaining the same.
Other commitments
The Company did not have other significant commitments,
long-term obligations or guarantees as of June 30, 2023 and 2022.
Contingencies
The Company is subject to legal proceedings and
regulatory actions in the ordinary course of business. The results of such proceedings cannot be predicted with certainty, but the Company
does not anticipate that the final outcome arising out of any such matter will have a material adverse effect on our consolidated business,
financial position, cash flows or results of operations taken as a whole.
On February 4, 2022, Macias Gini & O’Connell,
LLP (“Plaintiff”) initiated a lawsuit in San Francisco Superior Court. Plaintiff, a certified public accounting firm based
in the U.S., was hired by Kang Fu International Medical Co, and subsequently by Meihua International Medical Technologies Co, Ltd. (collectively,
“Meihua”), to audit Meihua’s consolidated financial statements for the 2018 and 2019 calendar years. Plaintiff seeks
damages from Meihua for its alleged failure to pay for services rendered in the amount of $210,000, plus interest and attorneys’
fees. The case was dismissed with prejudice in August of 2023.
On August 29, 2023, Zhu Cheng initiated a lawsuit
against Yada, Huada, Huadong and Kang Fu in Yangzhou Economic Development Zone Court. Zhu Cheng seeks damages from the above entities
for service fee of approximately $2.3 million (RMB 17.0 million). The Company is preparing to file a motion to dismiss the case. No contingent
liability has been accrued since the Company has deemed the possibility of loss to be remote.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Statutory Surplus Reserves and Restricted Net Assets
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Statutory Surplus Reserves and Restricted Net Assets [Abstract] |
|
| Statutory Surplus Reserves and Restricted Net Assets |
12. Statutory Surplus Reserves and Restricted Net Assets
Pursuant to laws applicable to entities incorporated
in the PRC, the Company is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the
discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of
the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net
income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations
to the discretionary surplus reserve are made at the discretion of the Board of Directors. As of December 31, 2022 and June 30, 2023,
the Company did not have a discretionary surplus reserve. As of December 31, 2022, all of the Company’s PRC subsidiaries reserves
had reached 50% of their registered capital threshold and, as a result, the Company stopped being required to allocate after-tax profits
to this reserve.
As a result of these PRC laws and regulations
and the requirement that distributions by PRC entities can only be paid out of distributable profits computed in accordance with PRC GAAP,
the PRC entities are restricted from transferring a portion of their net assets to the Company. Amounts restricted include paid-in capital
and the statutory reserves of the Company’s PRC subsidiaries. The aggregate amounts of capital and statutory reserves restricted
which represented the amount of net assets of the relevant subsidiaries in the Company not available for distribution was $15,665,860
as of June 30, 2023 and December 31, 2022.
Under PRC laws and regulations, statutory surplus
reserves are restricted to set-off against losses, expansion of production and operation and increasing registered capital of the respective
company and are not distributable other than upon liquidation. The reserves are not allowed to be transferred to the Company in terms
of cash dividends, loans or advances, nor allowed for distribution except under liquidation.
|
| X |
- References
+ Details
| Name: |
mhua_StatutorySurplusReservesAndRestrictedNetAssetsAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350/tableOfContent
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Related Party Transactions and Balances
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Related Party Transactions and Balances [Absract] |
|
| Related Party Transactions and Balances |
13. Related Party Transactions and Balances
Related Parties:
| Name of related parties |
|
Relationship with the Company |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
| Yangzhou Meihua Import and Export Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
Related Party Sales
The Company sells products to its related parties
and the sales amount from related parties for the six months end 2023 and 2022 are as follows:
Sales:
| | |
For the Six Months ended
June 30, | |
| Name of related party | |
2023 | | |
2022 | |
| Yangzhou Meihua Import and Export Co., Ltd. | |
$ | 11,751 | | |
$ | 18,849 | |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. | |
| - | | |
| 10,818 | |
| Total | |
$ | 11,751 | | |
$ | 29,667 | |
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Subsequent Events
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
14. Subsequent Events
The Company has evaluated the impact of events
that have occurred subsequent to June 30, 2023, through the issuance date of the consolidated financial statements, and concluded that
no subsequent events have occurred that would require recognition in the consolidated financial statements or disclosure in the notes
to the consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Accounting Policies, by Policy (Policies)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Summary of Significant Accounting Policies [Abstract] |
|
| Basis of Presentation |
Basis of Presentation The accompanying unaudited condensed consolidated
financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S.
GAAP”) pursuant to the rules and regulations of the Securities Exchange Commission (“SEC”). The interim results of operations
are not necessarily indicative of results to be expected for any other interim period or for a full year. In the opinion of management,
all adjustments, consisting only of normal recurring adjustments, considered necessary for a fair presentation of its financial position
and operating results have been included. These financial statements should be read in conjunction with the Company’s audited consolidated
financial statements and the related notes thereto for the fiscal years ended December 31, 2022 and 2021.
|
| Use of Estimates |
Use of Estimates The preparation of unaudited condensed consolidated
financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the amounts
reported and disclosed in the consolidated financial statements and related notes. The most significant estimates and judgments include
allowance for bad debts and the valuation of inventory. Actual amounts could differ from those estimates.
|
| Non-controlling interests |
Non-controlling interests Non-controlling interest represents the portion
of the net assets of subsidiaries attributable to interests that are not owned or controlled by the Company. The non-controlling interest
is presented in the consolidated balance sheets, separately from equity attributable to the shareholders of the Company. Non-controlling
interest’s operating results are presented on the face of the consolidated statements of income and comprehensive income as an allocation
of the total income for the year between non-controlling shareholders and the shareholders of the Company. As of June 30, 2023, non-controlling
interests represent one non-controlling shareholders’ proportionate share of equity interests in Hainan Guoxie.
|
| Functional Currency and Foreign Currency Translation |
Functional Currency and Foreign Currency
Translation The Company’s reporting currency is the
United States dollar (“US$”). The Company’s operations are principally conducted through the PRC subsidiaries where
the local currency is the functional currency. Therefore, the functional currency of Kang Fu is the Hong Kong dollar and the functional
currency of Huada, Yada, Huadong and Guanghui is the Renminbi (“RMB”). Transactions denominated in currencies other than
the functional currencies are translated into the functional currency of the entity at the exchange rates prevailing on the transaction
dates. Monetary assets and liabilities denominated in currencies other than the applicable functional currency are translated into the
functional currency at the prevailing rates of exchange at the balance date. The resulting exchange differences are reported in the consolidated
statements of income and comprehensive income. The assets and liabilities of the Company are
translated at the exchange spot rate at the balance sheet date, stockholders’ equity is translated at the historical rates and the
revenues and expenses are translated at the average exchange rates for the periods, except that the exchange rate used for translation
from Hong Kong dollar to US$ was 7.8000, a pegged rate determined by the linked exchange rate system in Hong Kong. This pegged rate was
used to translate Kang Fu’s balance sheets, income statement items and cash flow items for both the six months ended June 30, 2023
and 2022. The resulting translation adjustments are reported under other comprehensive income in the consolidated statements of income
and comprehensive income in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification
(“ASC”) 220, Comprehensive Income. The following are the exchange rates that were used in translating the Company’s
PRC subsidiaries’ financial statements into the consolidated financial statements:
| | |
June 30, 2023 | |
December 31, 2022 | |
June 30, 2022 |
| | |
| |
| |
|
| Period-end spot rate | |
US$1=RMB 7.2513 | |
US$1=RMB 6.8972 | |
US$1=RMB 6.6981 |
| | |
| |
| |
|
| Average rate | |
US$1=RMB 6.9283 | |
US$1=RMB 6.7290 | |
US$1=RMB 6.4791 |
|
| Certain Risks and Concentration |
Certain Risks and Concentration The Company’s financial instruments that
potentially subject the Company to significant concentrations of credit risk consist primarily of cash and receivables. As of six months
ended June 30, 2023 and December 31, 2022 substantially all the Company’s cash were held in major financial institutions located
in Hong Kong and the PRC, which management considers to be of high credit quality. For the six months ended June 30, 2023, two customers
accounted for approximately 18.18% and 10.01% of the Company’s total revenues. For the six months ended June 30, 2022, two customers
accounted for approximately 32.53% and 11.25% of the Company’s total revenues. As of June 30, 2023, two customers accounted for
approximately 30.92% and 21.31% of the Company’s accounts receivable. As of December 31, 2022, two customers accounted for approximately
27.73% and 13.14% respectively, of the Company’s accounts receivable. For the six months ended June 30, 2023, one supplier
accounted for approximately 14.73% of the Company’s total purchases. As of six months ended June 30, 2022, there was no supplier
that individually represented greater than 10% of the total purchase of the Company’s total purchases.
|
| Fair Value Measurement |
Fair Value Measurement Fair value is the price that would be received
from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When
determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers
the principal or most advantageous market in which it would transact, and it considers assumptions that market participants would use
when pricing the asset or liability. The Company adopted the guidance of Accounting
Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods
for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| |
Level 1: |
Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| |
|
|
| |
Level 2: |
Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| |
|
|
| |
Level 3: |
Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The Company’s financial instruments include
cash, accounts receivable, bank acceptance receivables, due from related parties, accounts payable, other liabilities and accrued expenses
and short-term bank borrowings. The carrying amounts approximate their fair values due to their short maturities as of June 30, 2023 and
December 31, 2022. The Company noted no transfers between levels
during any of the periods presented. The Company did not have any instruments that were measured at fair value on a recurring or non-recurring
basis as of June 30, 2023 and December 31, 2022.
|
| Cash |
Cash Cash consists of petty cash on hand and cash held
in banks, which are highly liquid and are unrestricted as to withdrawal or use.
|
| Bank Acceptance Receivables |
Bank Acceptance Receivables Bank acceptance receivables are issued by bank
under the request of the Company’s customers, to pay for the purchased goods. The Company can choose to hold acceptance notes until
maturity and receive the face value payment from the bank, or sell (exchange) the acceptance notes at a discount to another party willing
to wait until maturity to receive the bank’s promised payment. The maturity date of the receivables is all within one year of the
original issuance date and carried at face value. The Company is not lending money, it just sells goods to the customers (customers can
pay the purchased goods by cash, accounts receivable or bank acceptance receivables). The receivables mature within one year, and are
non-interest bearing. As bank acceptance receivables are issued by the banks and payments are guaranteed. The Company has not discounted
any bank acceptances and there were no endorsed bank acceptances that are unmatured as of June 30, 2023. The Company collected approximately
$6.0 million as of August 31, 2023.
|
| Accounts Receivable and Allowance for Doubtful Accounts |
Accounts Receivable and Allowance for Doubtful
Accounts Accounts receivable represent trade receivables
and are recognized initially at fair value and subsequently adjusted for any allowance for doubtful accounts or impairment. The Company follows the guidance under ASC 326,
Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Instruments. The standard uses a new forward-looking
“methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information
to inform credit loss estimates.” The Company has adopted the loss rate methodology to estimate historical losses on accounts receivables.
The Company has adopted the aging methodology to estimate the credit losses on accounts receivables. The historical data is adjusted to
account for forecasted changes in the macroeconomic environment in order to calculate the current expected credit loss. The provision
is recorded against accounts receivables balances, with a corresponding charge recorded in the consolidated statements of operations and
comprehensive income. The Company historically has not had material
bad debts in accounts receivable. There were no bad debt expenses related to accounts receivable for six months ended June 30, 2023 and
2022, and there was no provision for doubtful accounts as of June 30, 2023 and December 31, 2022.
|
| Inventories |
Inventories Inventories are valued using the lower of cost
or net realizable value. Cost is principally determined using the weighted-average method. Manufactured inventories included cost of materials,
labor and overhead expenses. The Company records adjustments to inventory for excess quantities, obsolescence, or impairment, when appropriate,
to reflect inventory at net realizable value. These adjustments are based upon a combination of factors including current sales volume,
market conditions, lower of cost or market analysis and expected realizable value of the inventory. There were no write-downs recognized of inventories
as of June 30, 2023 and December 31, 2022.
|
| Prepayment and other current assets |
Prepayment and other current assets As of June 30, 2023 and December 31, 2022, prepayment
and other current assets were $15,329,511 and $16,428,779. Prepayment and other assets primarily consist
of prepayments for land use right and property, refundable tax credits and receivables, security deposits made to customers, advances
to employees, which are presented net of allowance for doubtful accounts. These balances are unsecured and are reviewed periodically to
determine whether their carrying value has become impaired. The Company considers the balances to be impaired if the utilization or refund
of the balances becomes doubtful. The Company uses the aging method to estimate the allowance for uncollectible balances. The allowance
is also based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical trends
of collections and utilizations. Actual amounts received or utilized may differ from management’s estimate of credit worthiness
and the economic environment. Delinquent account balances are written off against allowance for doubtful accounts after management has
determined that the likelihood of collection is not probable. The allowance for doubtful accounts amounted to nil as of June 30, 2023
and December 31, 2022.
|
| Property, Plant and Equipment |
Property, Plant and Equipment Property, plant and equipment items are recorded
at their historic cost, less accumulated depreciation and impairment losses. The Company calculates depreciation using the straight-line
method, after consideration of the estimated residual values, over the following estimated useful lives:
| Category | |
Useful lives | |
Estimated
residual
value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
Major improvements are capitalized and expenditures
for maintenance and repairs are expensed as incurred. Construction in progress represents property, plant and equipment under construction
or being installed. Costs include original cost, installation, construction and other direct costs. Interest expenses directly related
to construction in progress would be capitalized. Construction in progress is transferred to the appropriate fixed asset account and depreciation
commences when the asset has been substantially completed and placed in service.
|
| Intangible Assets |
Intangible Assets Intangible assets are non-monetary assets without
physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and
impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives.
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful lives, which is as
follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
|
| Impairment of Long-Lived Assets |
Impairment of Long-Lived Assets The Company accounts for impairment of long-lived
assets in accordance with Accounting Standards Codification (“ASC”) 360, Property, Plant and Equipment. (“ASC
360”). Long-lived assets consist primarily of property, plant and equipment, and intangible assets. In accordance with ASC 360,
the Company evaluates the carrying value of long-lived assets when it determines a triggering event has occurred, or whenever events or
changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When indicators exist, recoverability of
assets is measured by a comparison of the carrying value of the asset group to the estimated undiscounted future net cash flows expected
to be generated by the asset. Examples of such triggering events include a significant disposal of a portion of such assets, and adverse
change in the market involving the business employing the related assets. If such assets are determined not to be recoverable, the Company
performs an analysis of the fair value of the asset group and will recognize an impairment loss when the fair value is less than the carrying
amounts of such assets. The fair value, based on reasonable and supportable assumptions and projections, require subjective judgments.
Depending on the assumptions and estimates used, the appraised fair value projected in the evaluation of long-lived assets can vary within
a range of outcomes. The Company considers the likelihood of possible outcomes in determining the best estimate for the fair value of
the assets. The Company did not record any impairment charges for the six months ended June 30, 2023 and 2022. There can be no assurance
that future events will not have impact on company revenue or financial position which could result in impairment in the future.
|
| Investment |
Investment In accordance with Financial Accounting Standards
Board (“FASB”) ASC 321, “Investment-Equity Securities,” the Company accounts for non-marketable securities on
a prospective basis. Equity investments that do not have readily determinable fair values and do not qualify for the net asset value practical
expedient are eligible for the measurement alternative. On March 3, 2011, Yada invested in Yangzhou Juyuan
Guarantee Co., Ltd (“Juyuan”) and obtained 12% equity interest of Juyuan. For the Company’s passive and without significant
influence or control equity investment in private company which do not have readily determinable fair values, the Company has elected
the measurement alternative defined as cost, less impairment, plus or minus adjustments resulting from observable price changes in orderly
transactions for the identical or a similar investment of the Company. The investment is reviewed periodically to determine if its value
has been impaired and adjustments are recorded as necessary in profit or loss for the period. On January 5, 2023, majority shareholder
of Juyuan purchased 5% equity interest of Juyuan from Yada for a consideration of $360,839 (RMB 2.5 million). Investments in entities in which the Company can
exercise significant influence but does not own a majority equity interest or control are accounted for using the equity method of accounting
in accordance with ASC 323, Investments-Equity Method and Joint Ventures (“ASC 323”). Under the equity method, the Company
initially records its investment at cost and the difference between the cost of the equity investee and the amount of the underlying equity
in the net assets of the equity investee is accounted for as if the investee were a consolidated subsidiary. The share of earnings or
losses of the investee are recognized in the consolidated statements of comprehensive loss. Equity method adjustments include the Company’s
proportionate share of investee income or loss, adjustments to recognize certain differences between the Company’s carrying value
and its equity in net assets of the investee at the date of investment, impairments, and other adjustments required by the equity method.
The Company assesses its equity investment for other-than-temporary impairment by considering factors as well as all relevant and available
information including, but not limited to, current economic and market conditions, the operating performance of the investees including
current earnings trends, the general market conditions in the investee’s industry or geographic area, factors related to the investee’s
ability to remain in business, such as the investee’s liquidity, debt ratios, and cash burn rate and other company-specific information. Investments in equity securities without readily
determinable fair values are measured at cost minus impairment adjusted by observable price changes in orderly transactions for the identical
or a similar investment of the same issuer. These investments are measured at fair value on a nonrecurring basis when there are events
or changes in circumstances that may have a significant adverse effect. An impairment loss is recognized in the consolidated statements
of comprehensive loss equal to the amount by which the carrying value exceeds the fair value of the investment. Prior to the adoption
of ASU 2016-01 on January 1, 2019, these investments were accounted for using the cost method of accounting, measured at cost less other-than-temporary
impairment. On December 1, 2022, Huadong invested RMB 40 million
into Jiangsu Zhongxiangxin International Science and Technology Innovation Park Co., Ltd. (“Zhongxiangxin”), and obtained
25% ownership interest of Zhongxiangxin. Zhongxiangxin manufactures and sells medical materials in the PRC. The Company accounted for
the investments using equity method, because the Company has significant influence but does not own a majority equity interest or otherwise
control over the equity investee. Under the equity method, the Company adjusts the carrying amount of the investment and recognizes investment
income or loss for its share of the earnings or loss of the investee after the date of investment. When the Company’s share of losses
in the equity investee equals or exceeds its interest in the equity investee, the Company does not recognize further losses, unless the
Company has incurred obligations or made payments or guarantees on behalf of the equity investee. For the six months ended June 30, 2023
and 2022, the investment loss from Zhongxiangxin was $1,632 and nil. The Company continually reviews its investments
in equity investees to determine whether a decline in fair value below the carrying value is other-than-temporary. The primary factors
the Company considers in its determination include the financial condition, operating performance and the prospects of the equity investee;
other company specific information such as recent financing rounds; the geographic region, market and industry in which the equity investee
operates; and the length of time that the fair value of the investment is below its carrying value. If the decline in fair value is deemed
to be other-than-temporary, the carrying value of the equity investee is written down to fair value. For the for the six months ended June 30, 2023
and 2022, no impairment indicators were identified of its investment in the private company was no impairment recorded.
|
| Value-added Tax |
Value-added Tax Value-added taxes (“VAT”) collected
from customers relating to product sales and remitted to governmental authorities are presented on a net basis. VAT collected from customers
is excluded from revenue which is recorded in VAT payable. The Company is subject to a VAT rate of 13%. The VAT payable may be offset
by VAT paid by the Company on raw materials and other materials included in the cost of producing or acquiring its finished products.
|
| Related Parties |
Related Parties Parties are considered to be related to the Company
if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with
the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal
owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly
influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully
pursuing its own separate interests. The Company discloses all significant related party transactions.
|
| Revenue Recognition |
Revenue Recognition Based on the requirements of ASC Topic 606, the
Company recognizes revenue when control of the promised goods or services is transferred to the customers in an amount that reflects the
consideration the Company expects to be entitled to receive in exchange for those goods or services. The Company primarily sells its products
to hospitals and medical equipment companies. Revenue is recognized when the following 5-step revenue recognition criteria are met:
| 1) | Identify
the contract with a customer |
| 2) | Identify
the performance obligations in the contract |
| 3) | Determine
the transaction price |
| 4) | Allocate
the transaction price |
| 5) | Recognize
revenue when or as the entity satisfies a performance obligation |
Revenue from product sales is recognized at the
point in time control of the products is transferred, generally upon customer receipt based upon the standard contract terms. Shipping
and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered to
be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard quality policy and
returns are generally not significant. Payment terms for product sales are generally set at 90 to 180 days after customer acceptance of
the product.
|
| Revenue Disaggregation |
Revenue Disaggregation The Company’s disaggregated revenues are
represented by two categories which are type of goods and type of customers. Type of Goods
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 23,435,544 | | |
| 27,046,663 | |
| Resales of Sourced Disposable Medical Devices from Third Party Manufacturers | |
| 24,754,532 | | |
| 27,786,184 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
Type of Customers
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Direct sales | |
| 4,305,506 | | |
| 4,582,321 | |
| Distributors | |
| 43,884,570 | | |
| 50,250,526 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
|
| Earnings per Ordinary Share |
Earnings per Ordinary Share Earnings (loss) per ordinary share is calculated
in accordance with ASC 260, Earnings per Share. Basic earnings (loss) per ordinary share is computed by dividing the net income
(loss) attributable to shareholders of the Company by the weighted average number of ordinary shares outstanding during the year. Diluted
earnings per ordinary share is computed in accordance with the treasury stock method and based on the weighted average number of ordinary
shares and dilutive ordinary share equivalents. Dilutive ordinary share equivalents are excluded from the computation of diluted earnings
per ordinary share if their effects would be anti-dilutive. There is no ordinary share equivalent issued to date.
|
| Comprehensive Income (Loss) |
Comprehensive Income (Loss) ASC 220, Comprehensive Income (“ASC 220”)
establishes rules for reporting and display of comprehensive income and its components. ASC 220 requires that unrealized gains and losses
on the Company’s foreign currency translation adjustments be included in comprehensive income (loss).
|
| Advertising Costs |
Advertising Costs The Company’s advertising costs are expensed
as incurred. Advertising expenses are included in selling expenses in the accompanying consolidated statements of income and comprehensive
income. Advertising expenses were $8,275, and $7,603 for the six months ended June 30, 2023, and 2022, respectively
|
| Research and Development Costs |
Research and Development Costs Research and development expenses are expensed
as incurred. Research and development expenses were $1,460,376 and $1,642,204 for the six months ended June 30, 2023, and 2022, respectively.
|
| Income Tax |
Income Tax Current income taxes are provided on the basis
of net profit for financial reporting purposes, adjusted for income and expense items which are not assessable or deductible for income
tax purposes, in accordance with the regulations of the relevant tax jurisdictions. Deferred income taxes are recognized for temporary
differences between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements, net operating
loss carry forwards and credits. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more
likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided in accordance
with the laws of the relevant taxing authorities. Deferred tax assets and liabilities are measured using enacted rates expected to apply
to taxable income in which temporary differences are expected to be reversed or settled. The effect on deferred tax assets and liabilities
of changes in tax rates is recognized in the statement of comprehensive income in the period of the enactment of the change. The Company considers positive and negative evidence
when determining whether a portion or all of its deferred tax assets will more likely than not be realized. This assessment considers,
among other matters, the nature, frequency and severity of current and cumulative losses, forecasts of future profitability, the duration
of statutory carry-forward periods, its experience with tax attributes expiring unused, and its tax planning strategies. The ultimate
realization of deferred tax assets is dependent upon its ability to generate sufficient future taxable income within the carry-forward
periods provided for in the tax law and during the periods in which the temporary differences become deductible. When assessing the realization
of deferred tax assets, the Company has considered possible sources of taxable income including (i) future reversals of existing
taxable temporary differences, (ii) future taxable income exclusive of reversing temporary differences and carryforwards, (iii) future
taxable income arising from implementing tax planning strategies, and (iv) specific known trend of profits expected to be reflected
within the industry. The Company recognizes a tax benefit associated
with an uncertain tax position when, in its judgment, it is more likely than not that the position will be sustained upon examination
by a taxing authority. For a tax position that meets the more-likely-than-not recognition threshold, the Company initially and subsequently
measures the tax benefit as the largest amount that the Company judges to have a greater than 50% likelihood of being realized upon ultimate
settlement with a taxing authority. The Company’s liability associated with unrecognized tax benefits is adjusted periodically due
to changing circumstances, such as the progress of tax audits, case law developments and new or emerging legislation. Such adjustments
are recognized entirely in the period in which they are identified. The Company’s effective tax rate includes the net impact of
changes in the liability for unrecognized tax benefits and subsequent adjustments as considered appropriate by management. The Company
classifies interest and penalties recognized on the liability for unrecognized tax benefits as income tax expense.
|
| Segment Reporting |
Segment Reporting FASB ASC 280, “Segment Reporting,”
establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational
structure as well as information of the Company’s business segments, geographical areas, segments and major customers. The Company
uses the “management approach” in determining reportable operating segments. The management approach considers the internal
organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance
as the source for determining the Company’s reportable segments. The chief operating decision maker is the Company’s president
and Chief Executive Officer (“CEO”). Management, including the chief operating decision maker, reviews operating results of
different products at revenue level with no allocation of operating costs. Consequently, based on management’s assessment, the Company
has determined that it has only one operating segment as defined by FASB ASC 280. The Company has disclosed the type of revenue by government category
as follows.
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 3,561,156 | | |
| 4,462,704 | | |
| 8,023,860 | | |
| 3,812,092 | | |
| 3,845,205 | | |
| 7,657,297 | |
| Class II | |
| 17,313,377 | | |
| 17,761,970 | | |
| 35,075,347 | | |
| 20,185,052 | | |
| 19,712,006 | | |
| 39,897,058 | |
| Class III | |
| 524,802 | | |
| 873,575 | | |
| 1,398,377 | | |
| 416,595 | | |
| 1,168,221 | | |
| 1,584,816 | |
| Others | |
| 2,036,209 | | |
| 1,656,283 | | |
| 3,692,492 | | |
| 2,632,924 | | |
| 3,060,752 | | |
| 5,693,676 | |
| Total | |
| 23,435,544 | | |
| 24,754,532 | | |
| 48,190,076 | | |
| 27,046,663 | | |
| 27,786,184 | | |
| 54,832,847 | |
Class I, II, and III medical devices are defined
by the National Medical Products Administration of China according to their risk levels under the Regulation on the Supervision and Administration
of Medical Devices (2021 Revision), Article 6 as follows:
| ● | “Class
I Medical Devices” means medical devices with low risks, whose safety and effectiveness can be ensured through routine administration. |
| ● | “Class
II Medical Devices” means medical devices with moderate risks, which shall be strictly controlled and administered to ensure their
safety and effectiveness. |
| ● | “Class
III Medical Devices” means medical devices with relatively high risks, which shall be strictly controlled and administered through
special measures to ensure their safety and effectiveness. |
Furthermore, the Company has disclosed revenue by major product type
included in each government category.
| | |
| |
June 30, 2023 | | |
June 30, 2022 | |
| Category | |
Products | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 1,073,853 | | |
| 1,346,164 | |
| | |
Oral medicine bottle | |
| 1,830,363 | | |
| 2,189,018 | |
| | |
Anal bag | |
| 849,099 | | |
| 395,292 | |
| | |
Other Class I | |
| 4,270,545 | | |
| 3,726,823 | |
| Subtotal-Class I | |
| |
| 8,023,860 | | |
| 7,657,297 | |
| Class II | |
Masks | |
| 47,946 | | |
| 211,468 | |
| | |
Identification tape | |
| 5,494,306 | | |
| 7,218,564 | |
| | |
Disposable medical brush | |
| 4,481,601 | | |
| 4,606,634 | |
| | |
Gynecological inspection kits | |
| 3,022,727 | | |
| 5,807,398 | |
| | |
Surgical kit | |
| 2,206,201 | | |
| 13,546,908 | |
| | |
Medical brush | |
| 2,809,448 | | |
| 2,586,945 | |
| | |
Medical kit | |
| 983,584 | | |
| 883,977 | |
| | |
Other Class II | |
| 16,029,534 | | |
| 5,035,164 | |
| Subtotal-Class II | |
| |
| 35,075,347 | | |
| 39,897,058 | |
| Class III | |
Electronic pump | |
| 138,751 | | |
| 67,866 | |
| | |
Anesthesia puncture kit | |
| 229,616 | | |
| 205,218 | |
| | |
Disposable infusion pump | |
| 113,335 | | |
| 78,453 | |
| | |
Infusion pump | |
| 178,461 | | |
| 90,036 | |
| | |
Electronic infusion pump | |
| 330 | | |
| 43,397 | |
| | |
Laparoscopic trocar | |
| 38 | | |
| 94,337 | |
| | |
Other Class III | |
| 737,846 | | |
| 1,005,509 | |
| Subtotal-Class III | |
| |
| 1,398,377 | | |
| 1,584,816 | |
| Others | |
| |
| 3,692,492 | | |
| 5,693,676 | |
| Total | |
| |
| 48,190,076 | | |
| 54,832,847 | |
For the six months ended June 30, 2023, and 2022,
revenues and assets within PRC contributed over 99.1% of the Company’s total revenues and assets.
|
| The Outbreak of COVID-19 |
The Outbreak of COVID-19 On January 30, 2020, the World Health Organization
declared the outbreak of the coronavirus disease (COVID-19) a “Public Health Emergency of International Concern,” and on March
11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” COVID-19 has had a severe and negative
impact on the Chinese and the global economy and such impact persists as of the date of this annual report. In fiscal year 2020, COVID-19 had a significant
impact on our business and results of operations as the sales volume of masks rose sharply while the sales of products other than masks
declined due to an overall decrease in market demand. In fiscal year 2021, with the stable control of the domestic epidemic in China,
the market of masks was no longer in urgent shortage compared to the same period in 2020, and the production of epidemic prevention products
resumed more normal production levels. In general, with the precise control of the epidemic in China, our production and operations have
recovered smoothly, and the demand for other products has increased gradually. After the initial outbreak of COVID-19, from time to time,
some instances of COVID-19 infections have emerged in various regions of China, including the infections caused by the Omicron variants
in 2022. For example, a wave of infections caused by the Omicron variants emerged in Shanghai in 2022, and a series of restrictions and
quarantines were implemented to contain the spread. Many of the restrictive measures previously adopted
by the PRC governments at various levels to control the spread of the COVID-19 virus have been revoked or replaced with more flexible
measures since December 2022. While the revocation or replacement of the restrictive measures to contain the COVID-19 pandemic could have
a positive impact on our normal operations, the extent of the impact on the Company’s future financial results will be dependent
on future developments such as the length and severity of the crisis, the potential resurgence of the crisis, future government actions
in response to the crisis and the overall impact of the COVID-19 pandemic on the global economy and capital markets, among many other
factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the
expected impact of the COVID-19 pandemic on its future operations, financial condition, liquidity and results of operations if the current
situation continues.
|
| Recently Issued Accounting Standards |
Recently Issued Accounting Standards In October 2021, the FASB issued ASU No. 2021-08,
“‘Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers”
(“ASU 2021-08”). This ASU requires entities to apply Topic 606 to recognize and measure contract assets and contract liabilities
in a business combination. The amendments improve comparability after the business combination by providing consistent recognition and
measurement guidance for revenue contracts with customers acquired in a business combination and revenue contracts with customers not
acquired in a business combination. The amendments are effective for the Company beginning after December 15, 2023, and are applied prospectively
to business combinations that occur after the effective date. The Company does not expect the adoption of ASU 2021-04 will have a material
effect on the consolidated financial statements. In June
2022, the FASB issued ASU 2022-03 Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject to Contractual
Sale Restrictions. The update clarifies that a contractual restriction on the sale of an equity security is not considered part of the
unit of account of the equity security and, therefore, is not considered in measuring fair value. The update also clarifies that an entity
cannot, as a separate unit of account, recognize and measure a contractual sale restriction. The update also requires certain additional
disclosures for equity securities subject to contractual sale restrictions. For public business entities, the amendments in this Update
are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities,
the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early
adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. As
an emerging growth company, the standard is effective for the Company for the year ended
December 31, 2025. The Company is in the process of evaluating the impact of the new guidance
on its consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
mhua_BankAcceptancesReceivablesPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_ImpairmentOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_NoncontrollingInterestsPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_OutbreakOfCOVID19PolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_PrepaymentsAndOtherCurrentAssetsPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_RelatedPartiesPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_RevenueDisaggregationPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_ValueaddedTaxPolicyTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for advertising cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 35
-Topic 720
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483406/720-35-50-1
| Name: |
us-gaap_AdvertisingCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480981/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for goodwill and intangible assets. This accounting policy also may address how an entity assesses and measures impairment of goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 30
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
| Name: |
us-gaap_GoodwillAndIntangibleAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482105/912-330-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//330/tableOfContent
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481569/310-20-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (b)
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-11B
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-1
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-6
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Subparagraph (d)
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-15
| Name: |
us-gaap_TradeAndOtherAccountsReceivablePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Organization and Principal Activities (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Organization and Principal Activities [Abstract] |
|
| Schedule of Principal Activities |
Entity Name | |
Registered
Location | |
Percentage
of
ownership | |
Date of
incorporation | |
Principal
activities |
| Meihua International Medical Technologies Co., Ltd. (“Meihua”) | |
Cayman | |
Parent | |
November 10, 2020 | |
Investment holding |
| | |
| |
| |
| |
|
康复国际医疗有限公司
Kang Fu International Medical Co., Limited (“Kang Fu”) | |
Hong Kong | |
100% by Meihua | |
October 13, 2015 | |
Investment holding |
| | |
| |
| |
| |
|
扬州华达医疗器械有限公司
Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) | |
Yangzhou | |
100% by Kang Fu | |
December 24, 2001 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏亚达科技集团有限公司
Jiangsu Yada Technology Group Co., Ltd. (“Yada”) | |
Yangzhou | |
100% by Huada | |
December 5, 1991 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
江苏华东医疗器械实业有限公司
Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) | |
Yangzhou | |
100% by Yada | |
November 18, 2000 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
扬州光辉医疗科技有限公司*
Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) | |
Yangzhou | |
100% by Huadong | |
December 22, 2020 | |
Medical Equipment Sales |
| | |
| |
| |
| |
|
海南国械医疗科技有限公司
Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) | |
Hainan | |
55% by Kang Fu | |
October 07, 2021 | |
Medical Equipment Sales |
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfPrincipalActivitiesTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Summary of Significant Accounting Policies [Abstract] |
|
| Schedule of Exchange Rates |
The following are the exchange rates that were used in translating the Company’s
PRC subsidiaries’ financial statements into the consolidated financial statements:
| | |
June 30, 2023 | |
December 31, 2022 | |
June 30, 2022 |
| | |
| |
| |
|
| Period-end spot rate | |
US$1=RMB 7.2513 | |
US$1=RMB 6.8972 | |
US$1=RMB 6.6981 |
| | |
| |
| |
|
| Average rate | |
US$1=RMB 6.9283 | |
US$1=RMB 6.7290 | |
US$1=RMB 6.4791 |
|
| Schedule of Estimated Useful Lives |
The Company calculates depreciation using the straight-line
method, after consideration of the estimated residual values, over the following estimated useful lives:
| Category | |
Useful lives | |
Estimated
residual
value | |
| Buildings | |
20 years | |
| 10 | % |
| Machinery and Equipment | |
10 years | |
| 10 | % |
| Motor vehicles | |
5 years | |
| 10 | % |
| Electronic Equipment | |
5 years | |
| 10 | % |
| Office Equipment | |
3 years | |
| 10 | % |
| Inspection Equipment | |
5 years | |
| 10 | % |
|
| Schedule of Amortization of Finite-Lived Intangible Assets |
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful lives, which is as
follows:
| Category | |
Useful lives |
| Land use rights | |
50 years |
| Patent | |
5 years |
| Trademark | |
10 years |
|
| Schedule of Types of Goods |
The Company’s disaggregated revenues are
represented by two categories which are type of goods and type of customers.
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Self-Manufactured Products | |
| 23,435,544 | | |
| 27,046,663 | |
| Resales of Sourced Disposable Medical Devices from Third Party Manufacturers | |
| 24,754,532 | | |
| 27,786,184 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
|
| Schedule of Type of Customers |
Type of Customers
| | |
For The Six Months Ended
June 30, | |
| | |
2023 | | |
2022 | |
| | |
US$ | | |
US$ | |
| Direct sales | |
| 4,305,506 | | |
| 4,582,321 | |
| Distributors | |
| 43,884,570 | | |
| 50,250,526 | |
| Total Revenue | |
| 48,190,076 | | |
| 54,832,847 | |
|
| Schedule of Disclosed the Type of Revenue by Government Category |
The Company has disclosed the type of revenue by government category
as follows.
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
US$ | | |
US$ | |
| Category | |
Produced | | |
Purchased | | |
Total | | |
Produced | | |
Purchased | | |
Total | |
| Class I | |
| 3,561,156 | | |
| 4,462,704 | | |
| 8,023,860 | | |
| 3,812,092 | | |
| 3,845,205 | | |
| 7,657,297 | |
| Class II | |
| 17,313,377 | | |
| 17,761,970 | | |
| 35,075,347 | | |
| 20,185,052 | | |
| 19,712,006 | | |
| 39,897,058 | |
| Class III | |
| 524,802 | | |
| 873,575 | | |
| 1,398,377 | | |
| 416,595 | | |
| 1,168,221 | | |
| 1,584,816 | |
| Others | |
| 2,036,209 | | |
| 1,656,283 | | |
| 3,692,492 | | |
| 2,632,924 | | |
| 3,060,752 | | |
| 5,693,676 | |
| Total | |
| 23,435,544 | | |
| 24,754,532 | | |
| 48,190,076 | | |
| 27,046,663 | | |
| 27,786,184 | | |
| 54,832,847 | |
|
| Schedule of Major Product Type Included in Each Government Category |
Furthermore, the Company has disclosed revenue by major product type
included in each government category.
| | |
| |
June 30, 2023 | | |
June 30, 2022 | |
| Category | |
Products | |
US$ | | |
US$ | |
| Class I | |
Eye drops bottle | |
| 1,073,853 | | |
| 1,346,164 | |
| | |
Oral medicine bottle | |
| 1,830,363 | | |
| 2,189,018 | |
| | |
Anal bag | |
| 849,099 | | |
| 395,292 | |
| | |
Other Class I | |
| 4,270,545 | | |
| 3,726,823 | |
| Subtotal-Class I | |
| |
| 8,023,860 | | |
| 7,657,297 | |
| Class II | |
Masks | |
| 47,946 | | |
| 211,468 | |
| | |
Identification tape | |
| 5,494,306 | | |
| 7,218,564 | |
| | |
Disposable medical brush | |
| 4,481,601 | | |
| 4,606,634 | |
| | |
Gynecological inspection kits | |
| 3,022,727 | | |
| 5,807,398 | |
| | |
Surgical kit | |
| 2,206,201 | | |
| 13,546,908 | |
| | |
Medical brush | |
| 2,809,448 | | |
| 2,586,945 | |
| | |
Medical kit | |
| 983,584 | | |
| 883,977 | |
| | |
Other Class II | |
| 16,029,534 | | |
| 5,035,164 | |
| Subtotal-Class II | |
| |
| 35,075,347 | | |
| 39,897,058 | |
| Class III | |
Electronic pump | |
| 138,751 | | |
| 67,866 | |
| | |
Anesthesia puncture kit | |
| 229,616 | | |
| 205,218 | |
| | |
Disposable infusion pump | |
| 113,335 | | |
| 78,453 | |
| | |
Infusion pump | |
| 178,461 | | |
| 90,036 | |
| | |
Electronic infusion pump | |
| 330 | | |
| 43,397 | |
| | |
Laparoscopic trocar | |
| 38 | | |
| 94,337 | |
| | |
Other Class III | |
| 737,846 | | |
| 1,005,509 | |
| Subtotal-Class III | |
| |
| 1,398,377 | | |
| 1,584,816 | |
| Others | |
| |
| 3,692,492 | | |
| 5,693,676 | |
| Total | |
| |
| 48,190,076 | | |
| 54,832,847 | |
|
| X |
- DefinitionTabular disclosure of disclosed the type of revenue by government category.
| Name: |
mhua_ScheduleOfDisclosedTheTypesOfRevenueByGovernmentCategoryTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of exchange rates.
| Name: |
mhua_ScheduleOfExchangeRateTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of major product type included in each government category.
| Name: |
mhua_ScheduleOfMajorProductTypesIncludedInEachGovernmentCategoryTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfTypeOfCustomersTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of public utility physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation expense and method used, including composite depreciation, and accumulated depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 980
-SubTopic 20
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481834/980-20-45-1
| Name: |
us-gaap_ScheduleOfPublicUtilityPropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Prepayments and Other Assets (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Schedule of Prepayments and Other Current Assets [Abstract] |
|
| Schedule of Prepayments and Other Current Assets |
Prepayments and other current assets consist of
the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Other receivable | |
| 159,817 | | |
| 239,148 | |
| Prepaid tax | |
| - | | |
| 250,410 | |
| Prepaid for land use right (1) | |
| 15,169,694 | | |
| 15,948,501 | |
| Prepaid for property (2) | |
| 11,856,920 | | |
| 12,323,842 | |
| Total | |
| 27,186,431 | | |
| 28,761,901 | |
| Less: non-current portion | |
| (11,856,920 | ) | |
| (12,333,122 | ) |
| Prepayments and other current assets | |
| 15,329,511 | | |
| 16,428,779 | |
| (1) | On
October 22, 2018, the Company signed a land use right agreement with the government of Touqiao Town, Yangzhou City and paid RMB 50 million
($6.9 million) and RMB 60 million ($8.27 million), respectively, in 2018 and 2019 according to the agreement. As a result of COVID-19,
the land use right had not been transferred to the Company as scheduled. Both parties agreed to cancel the transaction and the funds
prepaid for land use right will be returned to the Company before December 31, 2023. |
| (2) | On
April 20, 2020, the Company signed a factory building purchase agreement with Jiangsu Qionghua Group Co., Ltd. and paid deposit of RMB
85 million ($11.72 million). As a result of COVID-19, the factory building had not been completed as scheduled. Both parties agreed to
cancel the transaction and that the deposit for the building would be returned to the Company on or before December 31, 2025, with such
deposit accumulating interest at an annual interest rate of 3.5%. |
|
| X |
- References
+ Details
| Name: |
mhua_PrepaymentsandOtherAssetsTablesLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amounts of other current assets.
| Name: |
us-gaap_ScheduleOfOtherCurrentAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Inventories (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Inventories [Abstract] |
|
| Schedule of Inventories |
Inventories consist of the following:
| | |
June 30, 2023 | | |
December 31, 2021 | |
| | |
US$ | | |
US$ | |
| Raw material | |
| 493,143 | | |
| 177,474 | |
| Work-in-process | |
| 20,501 | | |
| 343,795 | |
| Finished goods | |
| 977,974 | | |
| 560,119 | |
| Goods in transit | |
| 95,814 | | |
| | |
| Low-value consumables | |
| 59,714 | | |
| 40,650 | |
| Total | |
| 1,647,146 | | |
| 1,122,038 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483489/210-10-50-1
| Name: |
us-gaap_ScheduleOfInventoryCurrentTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible Assets (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Intangible Assets [Abstract] |
|
| Schedule of Intangible Assets |
Intangible assets consisted of the following:
| | |
June 30, 2023 | | |
December 31, 2022 | |
| | |
US$ | | |
US$ | |
| Land use rights | |
| 4,134,521 | | |
| 752,887 | |
| Patents | |
| 27,582 | | |
| 28,997 | |
| Software | |
| 12,113 | | |
| 9,424 | |
| Trademarks | |
| 115,836 | | |
| 121,789 | |
| Total | |
| 4,290,052 | | |
| 913,097 | |
| Less: accumulated amortization | |
| 414,025 | | |
| 415,497 | |
| Intangible assets, net | |
| 3,876,027 | | |
| 497,600 | |
|
| Schedule of Company’s Amortization Expenses |
The following table sets forth the Company’s
amortization expenses for the twelve months ending December 31 of the following years:
| 2023 | |
$ | 41,870 | |
| 2024 | |
| 83,740 | |
| 2025 | |
| 83,740 | |
| 2026 | |
| 82,953 | |
| 2027 | |
| 82,690 | |
| Thereafter | |
| 3,501,034 | |
| | |
$ | 3,876,027 | |
|
| X |
- DefinitionTabular disclosure of amortization expense of assets, excluding financial assets, that lack physical substance, having a limited useful life.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of goodwill and intangible assets, which may be broken down by segment or major class. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
| Name: |
us-gaap_ScheduleOfIntangibleAssetsAndGoodwillTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Bank Borrowings (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Bank Borrowings [Abstract] |
|
| Schedule of Bank Borrowings are Working Capital Loans from Banks |
Bank borrowings are working capital loans from
banks in China. Short-term bank borrowings as of June 30, 2023 consisted of the following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
1/18/2023 | |
5/25/2024 | |
| 4,000,000 | | |
| 551,625 | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Agricultural Bank of China | |
Huadong | |
| 3.60 | % | |
8/12/2022 | |
7/12/2023 | |
| 9,000,000 | | |
| 1,241,157 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
1/30/2023 | |
2/15/2024 | |
| 5,000,000 | | |
| 689,532 | |
| Bank of China | |
Huadong | |
| 3.80 | % | |
3/10/2023 | |
3/9/2024 | |
| 10,000,000 | | |
| 1,379,063 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023 | |
| 10,000,000 | | |
| 1,379,063 | |
| Industrial and Commercial Bank of China* | |
Yada | |
| 3.45 | % | |
2/17/2022 | |
2/16/2024 | |
| 9,000,000 | | |
| 1,241,156 | |
| Total | |
| |
| | | |
| |
| |
| 52,000,000 | | |
| 7,171,128 | |
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 4,000,000 | | |
| 579,946 | |
| Agricultural Bank of China | |
Huadong | |
| 3.40 | % | |
12/8/2022 | |
12/7/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Jiangsu Yangzhou Rural Commercial Bank | |
Huadong | |
| 3.95 | % | |
2/17/2022 | |
3/2/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Bank of China | |
Huadong | |
| 3.55 | % | |
3/9/2022 | |
1/19/2023* | |
| 5,000,000 | | |
| 724,932 | |
| Agricultural Bank of China | |
Yada | |
| 3.60 | % | |
12/8/2022 | |
12/6/2023* | |
| 10,000,000 | | |
| 1,449,864 | |
| Industrial and Commercial Bank of China | |
Yada | |
| 3.70 | % | |
2/18/2022 | |
2/21/2023* | |
| 9,000,000 | | |
| 1,304,877 | |
| Total | |
| |
| | | |
| |
| |
| 42,000,000 | | |
| 6,089,428 | |
| * | These
loans were renewed upon maturity. |
|
| Schedule of Carrying Values of the Company’s Pledged Assets to Secure Short-Term Borrowings |
The carrying values of the Company’s pledged assets to secure
short-term borrowings by the Company are as follows:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| Buildings, net | |
| 3,432,150 | | |
| 2,777,379 | |
| Land use right, net | |
| 90,322 | | |
| 96,416 | |
| Total | |
| 3,522,472 | | |
| 2,873,795 | |
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfTheCarryingValuesOfTheCompanysPledgedAssetsToSecureShorttermBorrowingsTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of short-term debt arrangements (having initial terms of repayment within one year or the normal operating cycle, if longer) including: (1) description of the short-term debt arrangement; (2) identification of the lender or type of lender; (3) repayment terms; (4) weighted average interest rate; (5) carrying amount of funds borrowed under the specified short-term debt arrangement as of the balance sheet date; (6) description of the refinancing of a short-term obligation when that obligation is excluded from current liabilities in the balance sheet; and (7) amount of a short-term obligation that has been excluded from current liabilities in the balance sheet because of a refinancing of the obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfShortTermDebtTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShortTermBorrowingsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Long-Term Bank Loan (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Long-Term Bank Loan [Abstract] |
|
| Schedule of Long-Term Bank Loan |
Long-term bank borrowings as of December 31, 2022 consisted of the
following:
| Lender | |
Company | |
Rate | | |
Issuance
Date | |
Expiration
Date | |
Amount-
RMB | | |
Amount-
US$ | |
| Bank of Communications | |
Huadong | |
| 3.50 | % | |
11/3/2022 | |
4/25/2024 | |
| 5,000,000 | | |
| 724,932 | |
| Total | |
| |
| | | |
| |
| |
| 5,000,000 | | |
| 724,932 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_LongTermDebtAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of long-debt instruments or arrangements, including identification, terms, features, collateral requirements and other information necessary to a fair presentation. These are debt arrangements that originally required repayment more than twelve months after issuance or greater than the normal operating cycle of the entity, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-3
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-1A
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
Reference 9: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-8
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-6
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-7
| Name: |
us-gaap_ScheduleOfDebtInstrumentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Taxes Payable (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Taxes Payable [Abstract] |
|
| Schedule of Taxes Payable |
Taxes payable consisted of the following:
| | |
June 30, 2023 | | |
December 31,
2022 | |
| | |
US$ | | |
US$ | |
| VAT payable | |
| 368,195 | | |
| 380,926 | |
| Income tax payable | |
| 1,012,775 | | |
| 690,824 | |
| Other tax payable | |
| 71,348 | | |
| 59,526 | |
| Total | |
| 1,452,318 | | |
| 1,131,276 | |
|
| X |
- DefinitionTabular disclosure of other liabilities.
| Name: |
us-gaap_OtherLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TaxesPayableCurrentAndNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Income Taxes [Abstract] |
|
| Schedule of Provisions for Income Tax |
Provisions for income tax are as follows:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Provisions for current income tax | |
| 2,064,697 | | |
| 2,433,772 | |
| Provisions for deferred income tax | |
| - | | |
| - | |
| Total | |
| 2,064,697 | | |
| 2,433,772 | |
|
| Schedule of Total Income Tax Expense |
The following is a reconciliation of the Company’s
total income tax expense to the income before income taxes for the six months ended June 30, 2023 and 2022, respectively:
| | |
June 30, 2023 | | |
June 30,
2022 | |
| | |
US$ | | |
US$ | |
| Income before income tax provision | |
| 9,097,681 | | |
| 8,988,658 | |
| Tax at the PRC EIT tax rates | |
| 2,203,896 | | |
| 2,689,271 | |
| Change in valuation allowance | |
| 16,934 | | |
| - | |
| Tax effect of non-deductible expenses | |
| 208,961 | | |
| 157,007 | |
| Tax effect of R&D expenses additional deduction* | |
| (365,094 | ) | |
| (412,506 | ) |
| Income tax expense | |
| 2,064,697 | | |
| 2,433,772 | |
| * | According
to PRC tax regulations, an additional of 100% of current year R&D expenses may be deducted from tax income. |
|
| X |
- DefinitionTabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 9
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
| Name: |
us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Related Party Transactions and Balances (Tables)
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Related Party Transactions and Balances [Absract] |
|
| Schedule of Related Parties |
Related Parties:
| Name of related parties |
|
Relationship with the Company |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
| Yangzhou Meihua Import and Export Co., Ltd. |
|
An entity controlled by Kai Liu, son of Yongjun Liu |
|
| Schedule of Sales |
The Company sells products to its related parties
and the sales amount from related parties for the six months end 2023 and 2022 are as follows:
| | |
For the Six Months ended
June 30, | |
| Name of related party | |
2023 | | |
2022 | |
| Yangzhou Meihua Import and Export Co., Ltd. | |
$ | 11,751 | | |
$ | 18,849 | |
| Shanghai Xinya Pharmaceutical Hanjiang Co., Ltd. | |
| - | | |
| 10,818 | |
| Total | |
$ | 11,751 | | |
$ | 29,667 | |
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfRelatedPartiesSalesTableTextBlock |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of related party transactions. Examples of related party transactions include, but are not limited to, transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners and (d) affiliates.
| Name: |
us-gaap_ScheduleOfRelatedPartyTransactionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Organization and Principal Activities (Details)
|
Oct. 07, 2021
CNY (¥)
|
Jun. 30, 2023 |
Oct. 13, 2015
USD ($)
|
Oct. 13, 2015
HKD ($)
|
Dec. 24, 2001
USD ($)
|
Nov. 18, 2000
CNY (¥)
|
Dec. 05, 1991
CNY (¥)
|
| Organization and Principal Activities (Details) [Line Items] |
|
|
|
|
|
|
|
| Registered capital |
|
|
$ 6,911,771
|
$ 53,911,815
|
|
|
|
| Subsidiary percentage |
55.00%
|
|
|
|
|
|
|
| Kang Fu [Member] |
|
|
|
|
|
|
|
| Organization and Principal Activities (Details) [Line Items] |
|
|
|
|
|
|
|
| Registered capital | $ |
|
|
|
|
$ 17,193,021
|
|
|
| Subsidiary ownership percentage |
|
100.00%
|
|
|
|
|
|
| Huada [Member] |
|
|
|
|
|
|
|
| Organization and Principal Activities (Details) [Line Items] |
|
|
|
|
|
|
|
| Registered capital |
|
|
|
|
|
|
¥ 51,390,000
|
| Yada [Member] |
|
|
|
|
|
|
|
| Organization and Principal Activities (Details) [Line Items] |
|
|
|
|
|
|
|
| Registered capital |
|
|
|
|
|
¥ 50,000,000
|
|
| Hainan [Member] |
|
|
|
|
|
|
|
| Organization and Principal Activities (Details) [Line Items] |
|
|
|
|
|
|
|
| Registered capital |
¥ 100,000,000
|
|
|
|
|
|
|
| X |
- References
+ Details
| Name: |
mhua_OrganizationandPrincipalActivitiesDetailsLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNet amount applicable to investors of capital units or shares.
| Name: |
us-gaap_CapitalUnitsNetAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe parent entity's interest in net assets of the subsidiary, expressed as a percentage.
| Name: |
us-gaap_MinorityInterestOwnershipPercentageByParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_KangFuMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_HuadaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_YadaMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_HainanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Organization and Principal Activities (Details) - Schedule of Principal Activities
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Meihua International Medical Technologies Co., Ltd. (“Meihua”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
Meihua International Medical Technologies Co., Ltd. (“Meihua”)
|
| Registered Location |
Cayman
|
| Percentage of ownership |
Parent
|
| Date of Incorporation |
Nov. 10, 2020
|
| Principal activities |
Investment holding
|
| Kang Fu International Medical Co., Limited (“Kang Fu”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
康复国际医疗有限公司 Kang Fu International Medical Co., Limited (“Kang Fu”)
|
| Registered Location |
Hong Kong
|
| Percentage of ownership |
100% by Meihua
|
| Date of Incorporation |
Oct. 13, 2015
|
| Principal activities |
Investment holding
|
| Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
扬州华达医疗器械有限公司 Yangzhou Huada Medical Equipment Co., Ltd. (“Huada”)
|
| Registered Location |
Yangzhou
|
| Percentage of ownership |
100% by Kang Fu
|
| Date of Incorporation |
Dec. 24, 2001
|
| Principal activities |
Medical Equipment Sales
|
| Jiangsu Yada Technology Group Co., Ltd. (“Yada”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
江苏亚达科技集团有限公司 Jiangsu Yada Technology Group Co., Ltd. (“Yada”)
|
| Registered Location |
Yangzhou
|
| Percentage of ownership |
100% by Huada
|
| Date of Incorporation |
Dec. 05, 1991
|
| Principal activities |
Medical Equipment Sales
|
| Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
江苏华东医疗器械实业有限公司 Jiangsu Huadong Medical Device Industry Co., Ltd. (“Huadong”)
|
| Registered Location |
Yangzhou
|
| Percentage of ownership |
100% by Yada
|
| Date of Incorporation |
Nov. 18, 2000
|
| Principal activities |
Medical Equipment Sales
|
| Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
扬州光辉医疗科技有限公司* Yangzhou Guanghui Medical Technology Co., Ltd. (“Guanghui”)
|
| Registered Location |
Yangzhou
|
| Percentage of ownership |
100% by Huadong
|
| Date of Incorporation |
Dec. 22, 2020
|
| Principal activities |
Medical Equipment Sales
|
| Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”) [Member] |
|
| Schedule of Principal Activities [Abstract] |
|
| Entity Name |
海南国械医疗科技有限公司 Hainan GuoxieTechnology Group Co. Ltd. (“Hainan Guoxie”)
|
| Registered Location |
Hainan
|
| Percentage of ownership |
55% by Kang Fu
|
| Date of Incorporation |
Oct. 07, 2021
|
| Principal activities |
Medical Equipment Sales
|
| X |
- References
+ Details
| Name: |
mhua_OrganizationandPrincipalActivitiesDetailsScheduleofPrincipalActivitiesLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_MeihuaInternationalMedicalTechnologiesCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_KangFuInternationalMedicalCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_YangzhouHuadaMedicalEquipmentCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_JiangsuYadaTechnologyGroupCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_JiangsuHuadongMedicalDeviceIndustryCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_YangzhouGuanghuiMedicalTechnologyCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_OwnershipAxis=mhua_HainanGuoxieTechnologyGroupCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Summary of Significant Accounting Policies (Details)
¥ in Millions |
|
|
6 Months Ended |
12 Months Ended |
|
|
|
Jan. 05, 2023
USD ($)
|
Jan. 05, 2023
CNY (¥)
|
Dec. 01, 2022
CNY (¥)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Dec. 31, 2022
USD ($)
|
Aug. 31, 2023
USD ($)
|
Mar. 03, 2011 |
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Exchange translation rate (in Dollars) |
|
|
|
$ 7.8
|
|
|
|
|
| Number of supplier |
|
|
|
1
|
|
|
|
|
| Total purchase, percentage |
|
|
|
14.73%
|
10.00%
|
|
|
|
| Collected amount (in Dollars) |
|
|
|
|
|
|
$ 6,000,000
|
|
| Prepayments and other current assets (in Dollars) |
|
|
|
$ 15,329,511
|
|
$ 16,428,779
|
|
|
| Allowance for doubtful accounts (in Dollars) |
|
|
|
|
|
|
|
|
| Consideration amount |
$ 360,839
|
¥ 2.5
|
|
|
|
|
|
|
| Investment loss (in Dollars) |
|
|
|
$ 1,632
|
|
|
|
|
| Current vat rate percentage |
|
|
|
13.00%
|
|
|
|
|
| Advertising expenses (in Dollars) |
|
|
|
$ 8,275
|
7,603
|
|
|
|
| Research and development expense (in Dollars) |
|
|
|
$ 1,460,376
|
$ 1,642,204
|
|
|
|
| Tax benefit percentage |
|
|
|
50.00%
|
|
|
|
|
| Total revenues and assets percentage |
|
|
|
99.10%
|
99.10%
|
|
|
|
| Maximum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Payment terms |
|
|
|
180 days
|
|
|
|
|
| Minimum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Payment terms |
|
|
|
90 days
|
|
|
|
|
| Revenue [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Number of customers |
|
|
|
2
|
2
|
|
|
|
| Revenue [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Total revenue rate |
|
|
|
18.18%
|
32.53%
|
|
|
|
| Revenue [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Total revenue rate |
|
|
|
10.01%
|
11.25%
|
|
|
|
| Accounts Receivable [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Number of customers |
|
|
|
2
|
|
2
|
|
|
| Total revenue rate |
|
|
|
30.92%
|
|
|
|
|
| Accounts Receivable [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Total revenue rate |
|
|
|
21.31%
|
|
27.73%
|
|
|
| Accounts Receivable [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Total revenue rate |
|
|
|
|
|
13.14%
|
|
|
| Jiangsu Huadong Medical Device Industrial Co. Ltd. [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Other investments (in Yuan Renminbi) | ¥ |
|
|
¥ 40.0
|
|
|
|
|
|
| Zhongxiangxin [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Ownership interest rate |
|
|
25.00%
|
|
|
|
|
|
| Investment [Member] |
|
|
|
|
|
|
|
|
| Summary of Significant Accounting Policies (Details) [Line Items] |
|
|
|
|
|
|
|
|
| Equity interest |
5.00%
|
5.00%
|
|
|
|
|
|
12.00%
|
| X |
- DefinitionCurrent vat rate percentage.
| Name: |
mhua_CurrentVatRatePercentage |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of exchange translation rate.
| Name: |
mhua_ExchangeTranslationRate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPrepayments and other current assets.
| Name: |
mhua_PrepaymentsAndOtherCurrentAssets |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_SummaryofSignificantAccountingPoliciesDetailsLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal revenues and assets percentage.
| Name: |
mhua_TotalRevenuesAndAssetsPercentage |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount charged to advertising expense for the period, which are expenses incurred with the objective of increasing revenue for a specified brand, product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483406/720-35-50-1
| Name: |
us-gaap_AdvertisingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479344/326-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of equity in the acquiree held by the acquirer immediately before the acquisition date in a business combination.
| Name: |
us-gaap_BusinessCombinationStepAcquisitionEquityInterestInAcquireePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the carrying value and the sale price of an investment. A loss would be recognized when the sale price of the investment is less than the carrying value of the investment. This element refers to the Loss included in earnings and not to the cash proceeds of the sale. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(9)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_LossOnSaleOfInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investments classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash outflow, made soon after acquisition date of business combination, to settle contingent consideration liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (d)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentForContingentConsiderationLiabilityInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of remaining performance obligation to total remaining performance obligation not recognized as revenue. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)(1)
-SubTopic 10
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479806/606-10-50-13
| Name: |
us-gaap_RevenueRemainingPerformanceObligationPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of the Variable Interest Entity's (VIE) voting interest owned by (or beneficial interest in) the reporting entity (directly or indirectly). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 5A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-5A
| Name: |
us-gaap_VariableInterestEntityOwnershipPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=mhua_RevenueMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_AccountsReceivableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=mhua_InvestmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Exchange Rates
|
6 Months Ended |
12 Months Ended |
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2023
CNY (¥)
|
Jun. 30, 2022
USD ($)
|
Jun. 30, 2022
CNY (¥)
|
Dec. 31, 2022
USD ($)
|
Dec. 31, 2022
CNY (¥)
|
| Schedule of Exchange Rates [Abstract] |
|
|
|
|
|
|
| Period-end spot rate |
$ 1
|
¥ 7.2513
|
$ 1
|
¥ 6.6981
|
$ 1
|
¥ 6.8972
|
| Average rate |
$ 1
|
¥ 6.9283
|
$ 1
|
¥ 6.4791
|
$ 1
|
¥ 6.729
|
| X |
- DefinitionThe amount of average rate.
| Name: |
mhua_AverageRate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of period end spot rate.
| Name: |
mhua_PeriodendSpotRate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_SummaryofSignificantAccountingPoliciesDetailsScheduleofExchangeRatesLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Estimated Useful Lives
|
6 Months Ended |
Jun. 30, 2023 |
|---|
| Buildings [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
20 years
|
| Estimated residual value |
10.00%
|
| Machinery and Equipment [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
10 years
|
| Estimated residual value |
10.00%
|
| Motor vehicles [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
5 years
|
| Estimated residual value |
10.00%
|
| Electronic Equipment [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
5 years
|
| Estimated residual value |
10.00%
|
| Office Equipment [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
3 years
|
| Estimated residual value |
10.00%
|
| Inspection Equipment [Member] |
|
| Public Utility, Property, Plant and Equipment [Line Items] |
|
| Useful lives |
5 years
|
| Estimated residual value |
10.00%
|
| X |
- References
+ Details
| Name: |
mhua_EstimatedResidualValue |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PublicUtilityPropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_BuildingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_MachineryAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_VehiclesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ElectricGenerationEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=mhua_InspectionEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionUseful life of finite-lived intangible assets, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_FiniteLivedIntangibleAssetUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
| Name: |
us-gaap_FiniteLivedIntangibleAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_CopyrightsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_PatentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_TrademarksMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Types of Goods - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Schedule of Types of Goods [Abstract] |
|
|
| Total Revenue |
$ 48,190,076
|
$ 54,832,847
|
| Self-Manufactured Products [Member] |
|
|
| Schedule of Types of Goods [Abstract] |
|
|
| Total Revenue |
23,435,544
|
27,046,663
|
| Resales of Sourced Disposable Medical Devices from Third Party Manufacturers [Member] |
|
|
| Schedule of Types of Goods [Abstract] |
|
|
| Total Revenue |
$ 24,754,532
|
$ 27,786,184
|
| X |
- DefinitionAmount of revenue that is not accounted for under Topic 606. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_RevenueNotFromContractWithCustomer |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_SelfManufacturedProductsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ResalesOfSourcedDisposableMedicalDevicesFromThirdPartyManufacturersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Type of Customers - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Schedule of Type of Customers [Abstract] |
|
|
| Direct sales |
$ 4,305,506
|
$ 4,582,321
|
| Distributors |
43,884,570
|
50,250,526
|
| Total Revenue |
$ 48,190,076
|
$ 54,832,847
|
| X |
- References
+ Details
| Name: |
mhua_SummaryofSignificantAccountingPoliciesDetailsScheduleofTypeofCustomersLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category [Line Items] |
|
|
| Produced |
$ 23,435,544
|
$ 27,046,663
|
| Purchased |
24,754,532
|
27,786,184
|
| Total revenue |
48,190,076
|
54,832,847
|
| Class I [Member] |
|
|
| Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category [Line Items] |
|
|
| Produced |
3,561,156
|
3,812,092
|
| Purchased |
4,462,704
|
3,845,205
|
| Total revenue |
8,023,860
|
7,657,297
|
| Class II [Member] |
|
|
| Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category [Line Items] |
|
|
| Produced |
17,313,377
|
20,185,052
|
| Purchased |
17,761,970
|
19,712,006
|
| Total revenue |
35,075,347
|
39,897,058
|
| Class III [Member] |
|
|
| Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category [Line Items] |
|
|
| Produced |
524,802
|
416,595
|
| Purchased |
873,575
|
1,168,221
|
| Total revenue |
1,398,377
|
1,584,816
|
| Other [Member] |
|
|
| Summary of Significant Accounting Policies (Details) - Schedule of Disclosed the Type of Revenue by Government Category [Line Items] |
|
|
| Produced |
2,036,209
|
2,632,924
|
| Purchased |
1,656,283
|
3,060,752
|
| Total revenue |
$ 3,692,492
|
$ 5,693,676
|
| X |
- References
+ Details
| Name: |
mhua_SummaryofSignificantAccountingPoliciesDetailsScheduleofDisclosedtheTypeofRevenuebyGovernmentCategoryLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=mhua_ClassIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=mhua_ClassIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=mhua_ClassIIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=mhua_OtherMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Summary of Significant Accounting Policies (Details) - Schedule of Major Product Type Included in Each Government Category - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Others |
$ 3,692,492
|
$ 5,693,676
|
| Total |
48,190,076
|
54,832,847
|
| Eye drops bottle [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
1,073,853
|
1,346,164
|
| Oral medicine bottle [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
1,830,363
|
2,189,018
|
| Anal bag [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
849,099
|
395,292
|
| Other Class I [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
4,270,545
|
3,726,823
|
| Class I [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
8,023,860
|
7,657,297
|
| Masks [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
47,946
|
211,468
|
| Identification tape [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
5,494,306
|
7,218,564
|
| Disposable medical brush [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
4,481,601
|
4,606,634
|
| Gynecological inspection kits [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
3,022,727
|
5,807,398
|
| Surgical kit [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
2,206,201
|
13,546,908
|
| Medical brush [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
2,809,448
|
2,586,945
|
| Medical kit [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
983,584
|
883,977
|
| Other Class II [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
16,029,534
|
5,035,164
|
| Class II [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
35,075,347
|
39,897,058
|
| Electronic pump [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
138,751
|
67,866
|
| Anesthesia puncture kit [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
229,616
|
205,218
|
| Disposable infusion pump [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
113,335
|
78,453
|
| Infusion pump [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
178,461
|
90,036
|
| Electronic infusion pump One [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
330
|
43,397
|
| Laparoscopic trocar [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
38
|
94,337
|
| Other Class III [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
737,846
|
1,005,509
|
| Class III [Member] |
|
|
| Schedule of Major Product Type Included in Each Government Category [Abstract] |
|
|
| Products |
$ 1,398,377
|
$ 1,584,816
|
| X |
- References
+ Details
| Name: |
mhua_SummaryofSignificantAccountingPoliciesDetailsScheduleofMajorProductTypeIncludedinEachGovernmentCategoryLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of operating expense for products and services of regulated operation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.2(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_UtilitiesOperatingExpenseProductsAndServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_EyeDropsBottleMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_OralMedicineBottleMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_AnalBagMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_OtherClassIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ClassIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_MasksMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_IdentificationTapeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_DisposableMedicalBrushMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_GynecologicalInspectionKitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_SurgicalKitMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_MedicalBrushMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_MedicalKitMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_OtherClassIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ClassIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ElectronicPumpMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_AnesthesiaPunctureKitMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_DisposableInfusionPumpMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_InfusionPumpMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ElectronicInfusionPumpOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_LaparoscopicTrocarMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_OtherClassIIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=mhua_ClassIIIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionLand use right agreement.
| Name: |
mhua_LandUseRightAgreement |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is the amount of cash advanced as security in return for borrowing securities from another party. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480848/942-470-50-3
| Name: |
us-gaap_DepositsPaidForSecuritiesBorrowedAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRate of interest on investment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherAssetsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Prepayments and Other Assets (Details) - Schedule of Prepayments and Other Current Assets - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Schedule of Prepayments and Other Current Assets [Abstract] |
|
|
|
| Other receivable |
|
$ 159,817
|
$ 239,148
|
| Prepaid tax |
|
|
250,410
|
| Prepaid for land use right |
[1] |
15,169,694
|
15,948,501
|
| Prepaid for property |
[2] |
11,856,920
|
12,323,842
|
| Total |
|
27,186,431
|
28,761,901
|
| Less: non-current portion |
|
(11,856,920)
|
(12,333,122)
|
| Prepayments and other current assets |
|
$ 15,329,511
|
$ 16,428,779
|
|
|
| X |
- DefinitionPrepaid for land use right.
| Name: |
mhua_PrepaidForLandUseRight |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfPrepaymentsAndOtherCurrentAssetsAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance, of receivables classified as other, due within one year or the operating cycle, if longer.
| Name: |
us-gaap_OtherReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed after one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for income and other taxes that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidTaxes |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
Inventories (Details) - Schedule of Inventories - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2021 |
| Schedule of Inventories [Abstract] |
|
|
| Raw material |
$ 493,143
|
$ 177,474
|
| Work-in-process |
20,501
|
343,795
|
| Finished goods |
977,974
|
560,119
|
| Goods in transit |
95,814
|
|
| Low-value consumables |
59,714
|
40,650
|
| Total |
$ 1,647,146
|
$ 1,122,038
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfInventoriesAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of completed merchandise or goods expected to be sold within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryFinishedGoods |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of raw materials expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryRawMaterials |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGross amount of unprocessed materials to be used in manufacturing or production process and supplies that will be consumed. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryRawMaterialsAndSupplies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of merchandise or goods in the production process expected to be completed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryWorkInProcess |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of other inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGross amount of merchandise or supplies to which the entity holds the title but does not hold physical possession because the goods are currently being transported. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherInventoryInTransit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount of an option or options to acquire real property.
| Name: |
us-gaap_PurchaseOptionsLand |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
Intangible Assets (Details) - Schedule of Intangible Assets - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2023 |
Dec. 31, 2022 |
| Intangible Assets (Details) - Schedule of Intangible Assets [Line Items] |
|
|
| Intangible assets |
$ 4,290,052
|
$ 913,097
|
| Less: accumulated amortization |
414,025
|
415,497
|
| Intangible assets, net |
3,876,027
|
497,600
|
| Land use rights [Member] |
|
|
| Intangible Assets (Details) - Schedule of Intangible Assets [Line Items] |
|
|
| Intangible assets |
4,134,521
|
752,887
|
| Patents [Member] |
|
|
| Intangible Assets (Details) - Schedule of Intangible Assets [Line Items] |
|
|
| Intangible assets |
27,582
|
28,997
|
| Software [Member] |
|
|
| Intangible Assets (Details) - Schedule of Intangible Assets [Line Items] |
|
|
| Intangible assets |
12,113
|
9,424
|
| Trademarks [Member] |
|
|
| Intangible Assets (Details) - Schedule of Intangible Assets [Line Items] |
|
|
| Intangible assets |
$ 115,836
|
$ 121,789
|
| X |
- References
+ Details
| Name: |
mhua_IntangibleAssetsDetailsScheduleofIntangibleAssetsLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of intangible assets, net.
| Name: |
mhua_IntangibleAssetsNet |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of decrease in accumulated depreciation, depletion and amortization as a result of reclassifications from intangible assets.
| Name: |
mhua_LessAccumulatedAmortization |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 928
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483147/928-340-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=mhua_LandUseRightsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_PatentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=mhua_SoftwareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_TrademarksMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Intangible Assets (Details) - Schedule of Company’s Amortization Expenses
|
Dec. 31, 2022
USD ($)
|
| Schedule of Company’s Amortization Expenses [Abstract] |
|
| 2023 |
$ 41,870
|
| 2024 |
83,740
|
| 2025 |
83,740
|
| 2026 |
82,953
|
| 2027 |
82,690
|
| Thereafter |
3,501,034
|
| Total |
$ 3,876,027
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfCompanySAmortizationExpensesAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the next rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextRollingTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized after the fifth rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the fifth rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the fourth rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the third rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the second rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
Investment (Details)
¥ in Millions |
|
6 Months Ended |
|
|
|
Jan. 05, 2023
USD ($)
|
Jan. 05, 2023
CNY (¥)
|
Jun. 30, 2023
USD ($)
|
Jun. 30, 2022
USD ($)
|
Dec. 01, 2022
CNY (¥)
|
Mar. 03, 2011
CNY (¥)
|
| Investment (Details) [Line Items] |
|
|
|
|
|
|
| Consideration amount |
$ 360,839
|
¥ 2.5
|
|
|
|
|
| Carrying value investment amount | $ |
|
|
$ 500,000
|
|
|
|
| Ownership interest, percentage |
|
|
|
|
25.00%
|
|
| Investment loss | $ |
|
|
$ 1,632
|
|
|
|
| Business Combination [Member] |
|
|
|
|
|
|
| Investment (Details) [Line Items] |
|
|
|
|
|
|
| Equity interest |
5.00%
|
5.00%
|
|
|
|
12.00%
|
| Yangzhou Juyuan Guarantee Co., Ltd. [Member] |
|
|
|
|
|
|
| Investment (Details) [Line Items] |
|
|
|
|
|
|
| Investment amount (in Yuan Renminbi) | ¥ |
|
|
|
|
|
¥ 6.0
|
| Jiangsu Huadong Medical Device Industrial Co. Ltd. [Member] |
|
|
|
|
|
|
| Investment (Details) [Line Items] |
|
|
|
|
|
|
| Investment amount (in Yuan Renminbi) | ¥ |
|
|
|
|
¥ 40.0
|
|
| X |
- References
+ Details
| Name: |
mhua_InvestmentDetailsLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_OwnershipInterestPercentage |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of equity in the acquiree held by the acquirer immediately before the acquisition date in a business combination.
| Name: |
us-gaap_BusinessCombinationStepAcquisitionEquityInterestInAcquireePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of the entity's equity method investment which has been sold.
| Name: |
us-gaap_EquityMethodInvestmentSoldCarryingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all investments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1)(h))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
| Name: |
us-gaap_Investments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the carrying value and the sale price of an investment. A loss would be recognized when the sale price of the investment is less than the carrying value of the investment. This element refers to the Loss included in earnings and not to the cash proceeds of the sale. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(b)(9)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_LossOnSaleOfInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow, made soon after acquisition date of business combination, to settle contingent consideration liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (d)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentForContingentConsiderationLiabilityInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=us-gaap_SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense for debt. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_InterestExpenseDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShortTermBorrowingsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Bank Borrowings (Details) - Schedule of Bank Borrowings are Working Capital Loans from Banks
|
6 Months Ended |
12 Months Ended |
|
|
|
Jun. 30, 2023
USD ($)
|
Dec. 31, 2022
USD ($)
|
Jun. 30, 2023
CNY (¥)
|
Dec. 31, 2022
CNY (¥)
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Amount |
|
$ 7,171,128
|
|
$ 6,089,428
|
|
¥ 52,000,000
|
|
¥ 42,000,000
|
| Bank of Communications [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Huadong
|
|
Huadong
|
|
|
|
|
| Rate |
|
3.55%
|
|
3.55%
|
|
3.55%
|
|
3.55%
|
| Issuance Date |
|
Jan. 18, 2023
|
|
Mar. 09, 2022
|
|
|
|
|
| Expiration Date |
|
May 25, 2024
|
|
Jan. 19, 2023
|
[1] |
|
|
|
| Amount |
|
$ 551,625
|
|
$ 579,946
|
|
¥ 4,000,000
|
|
¥ 4,000,000
|
| Bank of Communications [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Huadong
|
|
|
|
|
|
|
| Rate |
|
3.50%
|
|
|
|
3.50%
|
|
|
| Issuance Date |
|
Nov. 03, 2022
|
|
|
|
|
|
|
| Expiration Date |
|
Apr. 25, 2024
|
|
|
|
|
|
|
| Amount |
|
$ 689,532
|
|
|
|
¥ 5,000,000
|
|
|
| Agricultural Bank of China [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Huadong
|
|
Huadong
|
|
|
|
|
| Rate |
|
3.60%
|
|
3.40%
|
|
3.60%
|
|
3.40%
|
| Issuance Date |
|
Aug. 12, 2022
|
|
Dec. 08, 2022
|
|
|
|
|
| Expiration Date |
|
Jul. 12, 2023
|
|
Dec. 07, 2023
|
[1] |
|
|
|
| Amount |
|
$ 1,241,157
|
|
$ 1,304,877
|
|
¥ 9,000,000
|
|
¥ 9,000,000
|
| Jiangsu Yangzhou Rural Commercial Bank [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Huadong
|
|
Huadong
|
|
|
|
|
| Rate |
|
3.95%
|
|
3.95%
|
|
3.95%
|
|
3.95%
|
| Issuance Date |
|
Jan. 30, 2023
|
|
Feb. 17, 2022
|
|
|
|
|
| Expiration Date |
[1] |
Feb. 15, 2024
|
|
Mar. 02, 2023
|
|
|
|
|
| Amount |
|
$ 689,532
|
|
$ 724,932
|
|
¥ 5,000,000
|
|
¥ 5,000,000
|
| Bank of China [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Huadong
|
|
Huadong
|
|
|
|
|
| Rate |
|
3.80%
|
|
3.55%
|
|
3.80%
|
|
3.55%
|
| Issuance Date |
|
Mar. 10, 2023
|
|
Mar. 09, 2022
|
|
|
|
|
| Expiration Date |
[1] |
Mar. 09, 2024
|
|
Jan. 19, 2023
|
|
|
|
|
| Amount |
|
$ 1,379,063
|
|
$ 724,932
|
|
¥ 10,000,000
|
|
¥ 5,000,000
|
| Agricultural Bank of China [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Yada
|
|
Yada
|
|
|
|
|
| Rate |
|
3.60%
|
|
3.60%
|
|
3.60%
|
|
3.60%
|
| Issuance Date |
|
Dec. 08, 2022
|
|
Dec. 08, 2022
|
|
|
|
|
| Expiration Date |
|
Dec. 06, 2023
|
|
Dec. 06, 2023
|
[1] |
|
|
|
| Amount |
|
$ 1,379,063
|
|
$ 1,449,864
|
|
¥ 10,000,000
|
|
¥ 10,000,000
|
| Industrial and Commercial Bank of China [Member] |
|
|
|
|
|
|
|
|
| Short-Term Debt [Line Items] |
|
|
|
|
|
|
|
|
| Company |
|
Yada
|
[1] |
Yada
|
|
|
|
|
| Rate |
|
3.45%
|
[1] |
3.70%
|
|
3.45%
|
[1] |
3.70%
|
| Issuance Date |
|
Feb. 17, 2022
|
[1] |
Feb. 18, 2022
|
|
|
|
|
| Expiration Date |
[1] |
Feb. 16, 2024
|
|
Feb. 21, 2023
|
|
|
|
|
| Amount |
|
$ 1,241,156
|
[1] |
$ 1,304,877
|
|
¥ 9,000,000
|
[1] |
¥ 9,000,000
|
|
|
| X |
- DefinitionShort term Bank Borrowings Company.
| Name: |
mhua_ShortTermBankBorrowingsCompany |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionReflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer.
| Name: |
mhua_ShortTermBorrowings1 |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDate the credit facility terminates, in YYYY-MM-DD format. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityExpirationDate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDate the credit facility first became available, in YYYY-MM-DD format. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityInitiationDate1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ShortTermDebtLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of the carrying amount of short-term borrowings outstanding as of the balance sheet date which accrues interest at a set, unchanging rate.
| Name: |
us-gaap_ShortTermDebtPercentageBearingFixedInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.3
Bank Borrowings (Details) - Schedule of Carrying Values of the Company’s Pledged Assets to Secure Short-Term Borrowings - USD ($)
|
Jun. 30, 2023 |
Dec. 31, 2022 |
| Schedule of Carrying Values of the Company’s Pledged Assets to Secure Short-Term Borrowings [Abstract] |
|
|
| Buildings, net |
$ 3,432,150
|
$ 2,777,379
|
| Land use right, net |
90,322
|
96,416
|
| Total |
$ 3,522,472
|
$ 2,873,795
|
| X |
- References
+ Details
| Name: |
mhua_ScheduleOfCarryingValuesOfTheCompanySPledgedAssetsToSecureShortTermBorrowingsAbstract |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe total amount of cash and securities held by third party trustees pursuant to terms of debt instruments or other agreements as of the date of each statement of financial position presented, which can be used by the trustee only to pay the noncurrent portion of specified obligations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrust |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation of building structures held for productive use including addition, improvement, or renovation to the structure, including, but not limited to, interior masonry, interior flooring, electrical, and plumbing. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_BuildingsAndImprovementsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation and depletion of additions or improvements to real estate held for productive use. Examples include, but are not limited to, walkways, driveways, fences, and parking lots.
| Name: |
us-gaap_LandImprovements |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- References
+ Details
| Name: |
mhua_FloatingInterestRate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
mhua_LoanAgreement |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LongTermDebtAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Long-Term Bank Loan (Details) - Schedule of Long-Term Bank Loan - 12 months ended Dec. 31, 2022
|
USD ($) |
CNY (¥) |
| Schedule of Long-Term Bank Loan [Abstract] |
|
|
| Total |
$ 724,932
|
¥ 5,000,000
|
| Bank of Communications [Member] |
|
|
| Schedule of Long-Term Bank Loan [Abstract] |
|
|
| Company |
Huadong
|
Huadong
|
| Rate |
3.50%
|
3.50%
|
| Issuance Date |
Nov. 03, 2022
|
Nov. 03, 2022
|
| Expiration Date |
Apr. 25, 2024
|
Apr. 25, 2024
|
| Total |
$ 724,932
|
¥ 5,000,000
|
| X |
- References
+ Details
| Name: |
mhua_CompanyName |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_ExpirationDate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_IssuanceDate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
mhua_LongTermBankLoanRate |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLong term bank loan total.
| Name: |
mhua_LongTermsBankLoanTotal |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtInstrumentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=mhua_BankOfCommunicationsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Taxes Payable (Details) - Schedule of Taxes Payable - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2023 |
Dec. 31, 2022 |
| Schedule of Taxes Payable [Abstract] |
|
|
| VAT payable |
$ 368,195
|
$ 380,926
|
| Income tax payable |
1,012,775
|
690,824
|
| Other tax payable |
71,348
|
59,526
|
| Total |
$ 1,452,318
|
$ 1,131,276
|
| X |
- References
+ Details
| Name: |
mhua_TaxesPayableDetailsScheduleofTaxesPayableLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred through that date and payable for statutory sales and use taxes, including value added tax. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_SalesAndExciseTaxPayableCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of tax expense classified as other.
| Name: |
us-gaap_TaxesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for statutory income, sales, use, payroll, excise, real, property and other taxes. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(15)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_TaxesPayableCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Income Taxes (Details) - HKD ($)
$ in Millions |
|
6 Months Ended |
|
Dec. 29, 2017 |
Jun. 30, 2023 |
Nov. 30, 2016 |
| Income Taxes [Line Items] |
|
|
|
| Income tax, percentage |
|
16.50%
|
|
| Assessable profits (in Dollars) |
$ 2.0
|
|
|
| Lower tax, percentage |
8.25%
|
|
|
| Remaining taxable income, percentage |
16.50%
|
|
|
| R&D expenses, percentage |
|
100.00%
|
|
| Enterprise income tax, percentage |
|
25.00%
|
|
| Preferential tax, percentage |
|
15.00%
|
15.00%
|
| PRC income tax, percentage |
|
25.00%
|
|
| Dividends distributed percentage |
|
10.00%
|
|
| X |
- DefinitionPreferential tax percentage.
| Name: |
mhua_PreferentialTaxPercentage |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionRemaining taxable income percentage.
| Name: |
mhua_RemainingTaxableIncomePercentage |
| Namespace Prefix: |
mhua_ |
| Data Type: |
dtr:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Details) - Schedule of Provisions for Income Tax - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Schedule of Provisions for Income Tax [Abstract] |
|
|
| Provisions for current income tax |
$ 2,064,697
|
$ 2,433,772
|
| Provisions for deferred income tax |
|
|
| Total |
$ 2,064,697
|
$ 2,433,772
|
| X |
- DefinitionAmount of other deferred income tax expense (benefit) pertaining to income (loss) from continuing operations. For example, but not limited to, acquisition-date income tax benefits or expenses recognized from changes in the acquirer's valuation allowance for its previously existing deferred tax assets resulting from a business combination and adjustments to beginning-of-year balance of a valuation allowance because of a change in circumstance causing a change in judgment about the realizability of the related deferred tax asset in future periods. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
| Name: |
us-gaap_DeferredOtherTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Details) - Schedule of Total Income Tax Expense - USD ($)
|
6 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Schedule of Total Income Tax Expense [Abstract] |
|
|
|
| Income before income tax provision |
|
$ 9,097,681
|
$ 8,988,658
|
| Tax at the PRC EIT tax rates |
|
2,203,896
|
2,689,271
|
| Change in valuation allowance |
|
16,934
|
|
| Tax effect of non-deductible expenses |
|
208,961
|
157,007
|
| Tax effect of R&D expenses additional deduction* |
[1] |
(365,094)
|
(412,506)
|
| Income tax expense |
|
$ 2,064,697
|
$ 2,433,772
|
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for shareholder services. Includes, but is not limited to, fee and expense for transfer and dividend disbursing agent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
| Name: |
us-gaap_InvestmentCompanyShareholderServiceFeeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of rent expense incurred for leased assets, including but not limited to, furniture and equipment, that is not directly or indirectly associated with the manufacture, sale or creation of a product or product line.
| Name: |
us-gaap_LeaseAndRentalExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash outflow for fees classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForFees |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
mhua_StatutorySurplusReservesandRestrictedNetAssetsDetailsLineItems |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe percentage points added to the reference rate to compute the variable rate on the lessor's capital lease (sales-type and direct financing leases).
| Name: |
us-gaap_CapitalLeasesOfLessorContingentRentalsBasisSpreadOnVariableRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe net amount of all regulatory assets less all regulatory liabilities as of the end of the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 980
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481878/980-340-50-1
| Name: |
us-gaap_NetRegulatoryAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
| X |
- References
+ Details
| Name: |
mhua_RelationshipWithTheCompany |
| Namespace Prefix: |
mhua_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=mhua_ShanghaiXinyaPharmaceuticalHanjiangCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=mhua_YangzhouMeihuaImportAndExportCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=mhua_YangzhouMeihuaImportAndExportCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=mhua_ShanghaiXinyaPharmaceuticalHanjiangCoLtdMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Meihua International Med... (NASDAQ:MHUA)
過去 株価チャート
から 4 2024 まで 5 2024

Meihua International Med... (NASDAQ:MHUA)
過去 株価チャート
から 5 2023 まで 5 2024
