UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934
For
the month of May 2024
Commission
File Number: 001-38064
Aeterna
Zentaris Inc.
(Translation
of registrant’s name into English)
c/o
Norton Rose Fulbright Canada, LLP, 222 Bay Street, Suite 3000, PO Box 53, Toronto ON M5K 1E7
(Address
of principal executive office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):
Explanatory
Note
On June 3, 2024, Aeterna Zentaris Inc.
(“Aeterna”, “we”, “our” or the “Company”) and Ceapro Inc. (“Ceapro”)
closed the previously announced business combination transaction (the “Arrangement”) pursuant to the Arrangement
Agreement, dated as of December 13, 2023 (as amended by the Amendment Agreement, dated January 16, 2024, and as may be further
amended, supplemented or otherwise modified from time to time, the “Arrangement Agreement”). Pursuant to the Arrangement
Agreement, at the effective time of the Arrangement (the “Effective Time”), Aeterna acquired all of the issued and
outstanding common shares in the capital of Ceapro in a company-approved Plan of Arrangement (the “Plan of Arrangement”)
under the Canada Business Corporations Act such that at the Effective Time Ceapro became a wholly-owned subsidiary of Aeterna.
Aeterna will continue the operations of Aeterna and Ceapro on a combined basis. In connection with the closing of the Arrangement,
Aeterna issued a news release on June 3, 2024, a copy of which is attached hereto as Exhibit 99.1 and is incorporated herein by
reference.
Also on June 3, 2024, Aeterna filed with
the Canadian Securities Regulatory Authorities on SEDAR+ a material change report relating to the closing of the Arrangement, a copy
of which is attached hereto as Exhibit 99.2 and is incorporated herein by reference.
On the same date, Aeterna also filed on
SEDAR+ the executed Warrant Agreement, dated as of May 31, 2024, between Aeterna and Computershare Trust Company of Canada, governing
the terms of the common share purchase warrants issued in accordance
with the Plan of Arrangement, a copy of which is attached hereto as Exhibit 99.3 and is incorporated herein by reference.
On
the same date, Aeterna also filed on SEDAR+ its report on modern slavery
pursuant to the Fighting Against Forced Labour and Child Labour in Supply Chains Act for the financial year ended December 31, 2023,
a copy of which is attached hereto as Exhibit 99.4 and is incorporated herein by reference.
On
May 29, 2024, Aeterna issued a news release announcing that it had applied to extend the date for its annual meeting of shareholders.
A copy of the news release is attached hereto as Exhibit 99.5 and is incorporated herein by reference.
On
June 3, 2024, Aeterna issued a Notice of Meeting and Record Date to NASDAQ and to Canadian Securities Regulatory Authorities. Copies
of the Notices of Meeting and Record Date are attached hereto as Exhibits 99.6 and 99.7 and are incorporated herein by
reference.
Exhibit
99.1 included with this Report on Form 6-K is hereby incorporated by reference into Aeterna’s Registration Statements on
Forms S-8 (No. 333-224737, No. 333-210561, No. 333-200834 and No. 333-279844) and Form F-3 (No. 333-254680)
(collectively, the “Registration Statements”) and shall be deemed to be a part thereof from the date on which this
Report on Form 6-K is furnished, to the extent not superseded by documents or reports subsequently filed or furnished. The
information contained on any websites referenced in Exhibit 99.1 included with this Report on Form 6-K is not incorporated by
reference or deemed to be a part of this Report on Form 6-K or any of the Registration Statements.
Aeterna
has filed a Registration Statement on Form F-1 (including a prospectus) (File No. 333-277115) (the “Form F-1 Registration Statement”)
with the U.S. Securities and Exchange Commission (the “SEC”) for the issuance of common share purchase warrants and Common
Shares issuable upon exercise thereof in connection with the Plan of Arrangement. Before you invest in any Common Shares, you should
read the prospectus in the Form F-1 Registration Statement and the other documents incorporated by reference therein for more complete
information about Aeterna, Ceapro, the Arrangement and the common share purchase warrant offering.
You
may get copies of the Form F-1 Registration Statement for free by visiting EDGAR on the SEC website at www.sec.gov or at SEDAR+ at www.sedarplus.ca.
Alternatively, you may obtain copies of them by contacting the following:
Media
Contact
Joel Shaffer
FGS
Longview
joel.shaffer@fgslongview.com
416-670-6468
No
Offer or Solicitation
This
Report on Form 6-K and the exhibit attached hereto and incorporated by reference herein, and the information contained herein and therein
are not, and do not, constitute an offer to sell any securities or a solicitation of an offer to buy any securities in the United States
or any other state or jurisdiction, nor shall any securities of Aeterna be offered or sold in any jurisdiction in which such an offer,
solicitation or sale would be unlawful. Neither the SEC nor any state securities commission has approved or disapproved of the transactions
described herein or determined if this communication is truthful or complete. Any representation to the contrary is a criminal offense.
You
should not construe the contents of this Report on Form 6-K or the exhibit attached hereto and incorporated herein by reference as legal,
tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal
and related matters concerning the matters described herein.
Forward-Looking
Statements
The
information in this Report on Form 6-K and the exhibit attached hereto and incorporated herein by reference include forward-looking statements
within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, specifically Section 27A of the U.S. Securities Act
of 1933, as amended, and Section 21E of the U.S. Securities Exchange Act of 1934, as amended. These forward-looking statements involve
a number of known and unknown risks, uncertainties and other factors that could cause actual results and outcomes to be materially
different from historical results or from any future results expressed or implied by such forward-looking statements.
Forward-looking
statements include, but are not limited to, those relating to Aeterna’s expectations regarding the anticipated benefits and
synergies as well as the assets, cost structure, financial position, cash flows and growth prospects of the combined company.
Risks
and factors that could cause actual results or outcomes to differ materially from expectations include, among others, the following:
| ● |
Aeterna’s
ability to raise capital and obtain financing to continue its currently planned operations; |
| ● |
Aeterna’s
ability to maintain compliance with the continued listing requirements of the NASDAQ and to maintain the listing of its common shares
on the NASDAQ; |
| ● |
Aeterna’s
ability to continue as a going concern, which is dependent, in part, on its ability to transfer cash from Aeterna Zentaris GmbH to
Aeterna and its U.S. subsidiary and to secure additional financing; |
| ● |
Aeterna’s
now heavy dependence on the success of Macrilen™ (macimorelin) and related out-licensing arrangements and the continued availability
of funds and resources to successfully commercialize the product, including its heavy reliance on the success of the license and
assignment agreement with Novo Nordisk A/S; |
| ● |
Aeterna’s
ability to enter into out-licensing, development, manufacturing, marketing and distribution agreements with other pharmaceutical
companies and keep such agreements in effect; |
| ● |
Aeterna’s
reliance on third parties for the manufacturing and commercialization of Macrilen™ (macimorelin); |
| ● |
potential
disputes with third parties, leading to delays in or termination of the manufacturing, development, out-licensing or commercialization
of Aeterna’s product candidates, or resulting in significant litigation or arbitration; |
| ● |
uncertainties
related to the regulatory process; |
| ● |
unforeseen
global instability, including the instability due to the global pandemic of the novel coronavirus; |
| ● |
Aeterna’s
ability to efficiently commercialize or out-license Macrilen™ (macimorelin); |
| ● |
Aeterna’s
reliance on the success of the pediatric clinical trial in the European Union (“E.U.”) and U.S. for Macrilen™ (macimorelin); |
| ● |
the
degree of market acceptance of Macrilen™ (macimorelin); |
| ● |
Aeterna’s
ability to obtain necessary approvals from the relevant regulatory authorities to enable it to use the desired brand names for its
product; |
| ● |
Aeterna’s
ability to successfully negotiate pricing and reimbursement in key markets in the E.U. for Macrilen™ (macimorelin); |
| ● |
any
evaluation of potential strategic alternatives to maximize potential future growth and shareholder value may not result in any such
alternative being pursued, and even if pursued, may not result in the anticipated benefits; |
| ● |
Aeterna’s
ability to protect its intellectual property; and |
| ● |
the
potential of liability arising from shareholder lawsuits and general changes in economic conditions. |
Additional
risk factors that could cause actual results to differ materially include those risks identified in Item 3. “Key Information –
Risk Factors” contained in Aeterna’s most recent Annual Report on Form 20-F filed with the SEC and its other filings and
submissions from time to time, including those containing its quarterly and annual results, with the SEC, which are available on Aeterna’s
website located at www.aeterna.com.
Many
of these risks and factors are beyond Aeterna’s control. Aeterna cautions you not to place undue reliance on these forward-looking
statements. All written and oral forward-looking statements attributable to Aeterna and/or Ceapro, or persons acting on their behalf,
are qualified in their entirety by these cautionary statements. Moreover, unless required by law to update these statements, Aeterna
will not necessarily update any of these statements after the date hereof, either to conform them to actual results or to changes in
their expectation.
DOCUMENTS
INDEX
| Exhibit |
|
Description |
| 99.1 |
|
News Release, dated June 3, 2024 |
| 99.2 |
|
Material Change Report |
| 99.3 |
|
Warrant Agreement between the Registrant and Computershare Trust Company of Canada, as Warrant Agent, dated as of May 31, 2024 |
| 99.4 |
|
Aeterna Zentaris Inc. Modern Slavery Report |
| 99.5 |
|
News Release, dated May 29, 2024 |
| 99.6 |
|
Notice of Meeting and Record Date, dated June 3, 2024 (NASDAQ) |
| 99.7 |
|
Notice of Meeting and Record Date, dated June 3, 2024 (Canadian Securities Regulatory Authorities) |
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
AETERNA
ZENTARIS INC. |
| |
|
|
| Date:
June 10, 2024 |
By: |
/s/
Giuliano La Fratta |
| |
|
Giuliano
La Fratta |
| |
|
Chief
Financial Officer |
Exhibit
99.1
Aeterna
Zentaris and Ceapro Complete Merger Transaction
TORONTO
and EDMONTON, June 3, 2024 – Aeterna Zentaris Inc. (NASDAQ: AEZS) (TSX: AEZS) (“Aeterna” or the “Company”)
and Ceapro Inc. (TSX-V: CZO) (OTCQX: CRPOF) (“Ceapro”), two innovative biopharmaceutical development companies, are pleased
to announce the successful completion and closing of their all-stock merger of equals transaction (the “Transaction”), which
was previously announced by Aeterna and Ceapro in their joint press release of December 14, 2023.
“This
is an important day for shareholders of both companies as Aeterna and Ceapro have now officially come together to create a diversified
business that is expected to create value for many years to come,” said Ronald W. Miller, Chair of the Company. “With the
successful completion of this merger, we are now optimized to bring value-driving, transformational products to the market.”
Gilles
Gagnon, Chief Executive Officer of the Company, said: “with the shared benefits, additional competencies and resources resulting
from this merger, we are now poised to push forward exciting development programs in selected areas while continuing to grow our revenue
generating base business and constantly looking for strategic growth opportunities”.
“We
would like to thank Aeterna shareholders for their support for this transaction,” said Carolyn Egbert, former Chair of the Company.
“We also look forward to working with the Ceapro team to build a long-term, sustainable business”.
| |
●
|
Greater
potential for stable cash flow to support R&D of potentially higher return pharmaceutical products. The Company currently
generates revenues from two main active ingredients, oat beta glucan and avenanthramides, extracted and purified using its proprietary
technology. Cash from these products is planned to be used along with the Company’s revenue from the commercialization or licensing
of its macimorelin product to support the development of exciting, high potential-return products, ideally creating growing and sustainable
revenue for the Company and investors. |
| |
|
|
| |
●
|
Greater
diversification of commercial and development product pipeline lowers risk. The Company is expected to benefit from an extensive
and diversified pipeline of innovative products in development, including quicker-to-market biotechnology products and exciting potentially
higher return, but longer-horizon, products. With this pipeline rejuvenation, the Company is anticipated to boast: |
| |
●
|
more
products in the pipeline that are closer to potential commercialization; |
| |
●
|
an
enhanced ability to strategically focus financial and company resources in a manner that provides the most value to the company and
shareholders; and |
| |
●
|
a
more compelling value proposition and lower risk profile. |
| |
●
|
Expanded
pharmaceutical research and development capabilities. The Company’s talented team brings deep expertise and knowledge that
are expected to play a key role in advancing the Company and its development pipeline. The Company has the infrastructure to support
development activities and potentially offer improved efficiencies, in addition to cost savings. It now also has an expanded development
pipeline of products which its leadership is committed to prioritizing as they evaluate what will provide the best overall potential
for the Company, shareholders, and consumers. |
| |
●
|
Compelling
North American + European combination. The Company now has an operational presence in North America and Europe. While the Company
expects to continue to maintain some presence in Europe, its leadership believes that it needs to re-focus operations within the
North American biotechnology market, to provide optimal exposure to potential new investors, business development opportunities and
talent. |
| |
|
|
| |
●
|
Expertise
and efficiencies. The Company can now leverage the combined experience and expertise of its predecessor companies, including
navigating the conduct of human clinical trials and the crucial regulatory approval process required to bring pharmaceutical products
to market. The Company plans to leverage this expertise with the higher value pharmaceutical opportunities being advanced for its
active ingredients and technologies. |
Following
the closing of the Transaction, Aeterna’s board of directors now consists of eight directors: Ronald W. Miller (Chair), Carolyn
Egbert, Gilles Gagnon, Ulrich Kosciessa, Geneviève Foster, William Li, Dennis Turpin and Peter Edwards. The executive leadership
team now consists of Gilles Gagnon, President and Chief Executive Officer, and Giuliano La Fratta, Senior Vice President and Chief Financial
Officer.
A
new name for the combined company is expected to be announced in the coming weeks and will be put forward for shareholder approval at
the upcoming annual meeting of shareholders, details of which are expected to be announced shortly.
Full
details of the Transaction and certain other matters are set out in the respective information circulars filed by Aeterna and Ceapro
which are available under each company’s profile on SEDAR+ at www.sedarplus.ca or, as regards Aeterna, its reports on Form
6-K and other filings on EDGAR at www.sec.gov.
Aeterna
is an “Eligible Interlisted Issuer” as such term is defined in the TSX Company Manual. As an Eligible Interlisted Issuer,
the Company has relied on an exemption pursuant to Section 602.1 of the TSX Company Manual, the effect of which is that the Company was
not required to comply with certain requirements relating to the issuance of securities in connection with the Transaction.
Information
for Ceapro Shareholders
The
shares of Ceapro are expected to be delisted from the TSX Venture Exchange within five business days. Ceapro is also in the process of
applying to cease to be a reporting issuer under applicable Canadian securities laws.
Pursuant
to the Transaction, former Ceapro shareholders are entitled to receive 0.02360 of an Aeterna Zentaris share for each Ceapro share held.
In order to receive Aeterna Zentaris shares in exchange for Ceapro shares, Ceapro registered shareholders must complete, sign, date and
return (together with the certificate or DRS statement representing their shares) the letter of transmittal that was mailed to them prior
to closing of the Transaction. The letter of transmittal is also available under Ceapro’s profile on SEDAR+ at www.sedarplus.ca
and by contacting Computershare Investor Services Inc., the depositary, by telephone at 1-514-982-7555 or toll-free in North America
at 1-800-564-6253 or by email at corporateactions@computershare.com.
For
those shareholders of Ceapro whose shares are registered in the name of a broker, investment dealer, bank, trust company or other intermediary
or nominee, they should contact such intermediary or nominee for assistance in depositing their Ceapro shares and should follow the instructions
of such intermediary or nominee.
About
Aeterna Zentaris Inc.
Aeterna
is a specialty biopharmaceutical company engaged in the development and commercialization of a diverse portfolio of pharmaceutical and
diagnostic products, including those focused on areas of significant unmet medical need. One of Aeterna’s lead products is macimorelin
(Macrilen; Ghryvelin), the first and only U.S. FDA and European Commission approved oral test indicated for the diagnosis of adult growth
hormone deficiency (AGHD). Aeterna is also engaged in the development of therapeutic assets and proprietary extraction technology, which
is applied to the production of active ingredients from renewable plant resources currently used in cosmeceutical products (i.e., oat
beta glucan and avenanthramides which are found in leading skincare product brands like Aveeno and Burt’s Bees formulations) and
being developed as potential nutraceuticals and/or pharmaceuticals.
The
company is listed on the NASDAQ Capital Market and the Toronto Stock Exchange, and trades on both exchanges under the ticker symbol “AEZS”.
For more information, please visit Aeterna’s website at www.zentaris.com.
Forward-Looking
Statements
The
information in this news release has been prepared as of June 3, 2024. Certain statements in this news release, referred to herein
as “forward-looking statements”, constitute “forward-looking statements” within the meaning of the United
States Private Securities Litigation Reform Act of 1995, as amended, and “forward-looking information” under the
provisions of Canadian securities laws. All statements, other than statements of historical fact, that address circumstances,
events, activities, or developments that could or may or will occur are forward-looking statements. When used in this news release,
words such as “anticipate”, “assume”, “believe”, “could”, “expect”,
“forecast”, “future”, “goal”, “guidance”, “intend”,
“likely”, “may”, “would” or the negative or comparable terminology as well as terms usually used
in the future and the conditional are generally intended to identify forward-looking statements, although not all forward-looking
statements include such words. Forward-looking statements in this news release include, but are not limited to, statements relating to
the future business and operations of the combined Company, including cash flow, research and development, and costs.
Forward-looking
statements are necessarily based upon a number of factors and assumptions that, while considered reasonable by the Company as of the
date of such statements, are inherently subject to significant business, economic, operational and other risks, uncertainties, contingencies
and other factors, including those described below, which could cause actual results, performance or achievements of the combined Company
to be materially different from results, performance or achievements expressed or implied by such forward-looking statements and, as
such, undue reliance must not be placed on them.
Forward-looking
statements involve known and unknown risks and uncertainties which include, among others: the combined Company’s present and future
business strategies; operations and performance within expected ranges; anticipated future cash flows; local and global economic conditions
and the environment in which the combined Company operates; anticipated capital and operating costs; uncertainty in product development
and related clinical trials and validation studies, including our reliance on the success of the pediatric clinical trial in the European
Union and U.S. for Macrilen™ (macimorelin); the commencement of the DETECT-trial may be delayed or we may not obtain regulatory
approval to initiate that study; we may be unable to enroll the expected number of subjects in the DETECT-trial and the result of the
DETECT-trial may not support receipt of regulatory approval in child-onset growth hormone deficiency; results from ongoing or planned
pre-clinical studies of macimorelin by the University of Queensland or for our other products under development may not be successful
or may not support advancing the product to human clinical trials; our ability to raise capital and obtain financing to continue our
currently planned operations; our now heavy dependence on the success of Macrilen™ (macimorelin) and related out-licensing arrangements
and the continued availability of funds and resources to successfully commercialize the product; the ability to secure strategic partners
for late stage development, marketing, and distribution of our products, including our ability to enter into a new license agreement
or similar arrangement following the termination of the license agreement with Novo Nordisk AG; our ability to enter into out-licensing,
development, manufacturing, marketing and distribution agreements with other pharmaceutical companies and keep such agreements in effect;
our ability to protect and enforce our patent portfolio and intellectual property; and our ability to continue to list our common shares
on the NASDAQ Capital Market.
Investors
should consult our quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks
and uncertainties, including those discussed in our Annual Report on Form 20-F and MD&A filed under the Company’s profile on
SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. We disclaim any obligation to update any such risks or uncertainties
or to publicly announce any revisions to any of the forward-looking statements contained herein to reflect future results, events or
developments, unless required to do so by a governmental authority or applicable law.
No
securities regulatory authority has either approved or disapproved of the contents of this news release. The Toronto Stock Exchange accepts
no responsibility for the adequacy or accuracy of this news release.
For
Further Information
Aeterna
Investor Contact:
Aeterna,
Investor Relations
AZinfo@aezsinc.com
+1
843-900-3223
Aeterna
Media Contact:
Joel
Shaffer
FGS
Longview
joel.shaffer@fgslongview.com
416-670-6468
Exhibit 99.2


Exhibit
99.3










































































Exhibit
99.4

Aeterna
Zentaris Modern Slavery Report
Introduction
This
report on modern slavery (“Report”) is made pursuant to the Fighting Against Forced Labour and Child Labour in Supply Chains
Act (“Act”). This Report is made by Aeterna Zentaris Inc. (“Aeterna”, the “Company” or “we”)
for the financial year ended December 31, 2023 (“Reporting Period”). It describes the risks of forced labour and child labour
in our operations and supply chains and the steps we have taken to identify, manage and mitigate those risks.
Steps
taken during the Reporting Period to prevent and reduce risks of forced labour and child labour
At
Aeterna, we have a long-standing commitment to corporate responsibility. This responsibility includes respecting human rights and ensuring
that modern slavery, in all of its forms, is not tolerated within our business or supply chain.
In
general terms, the Company took the following steps during the Reporting Period to prevent and reduce the risk that forced labour or
child labour is used in our business or supply chains:
| |
● |
completed
a mapping of the first tier of our supply chains; |
| |
● |
conducted
an internal risk assessment of there being any forced labour or child labour in our operations; |
| |
● |
reviewed
our Code of Conduct and Business Ethics (“Code”) to assess against international standards, especially
with respect to human rights; and |
| |
● |
engaged
external counsel to provide training and advice in respect of the issues of forced and child labour and the requirements of the Act. |
Structure,
activities and supply chains
Overview
and Activities
Aeterna
is a specialty biopharmaceutical company commercializing and developing therapeutics and diagnostic tests. The Company’s lead product,
Macrilen® (“macimorelin”), is the first and only U.S. Food and Drug Administration and European Medicines Agency
approved oral test indicated for the diagnosis of patients with adult growth hormone deficiency. Macimorelin is currently marketed under
the tradename Ghryvelin™ in the European Economic Area and under the tradename “Macimorelin 60 mg granules for oral suspension
in sachet” in the United Kingdom through an exclusive licensing agreement with Pharmanovia. The Company’s several other license
and commercialization partners have already received or are seeking approval for commercialization of macimorelin in Israel and the Palestinian
Authority, the Republic of Korea, Turkey and several non-European Union Balkan countries.
The
Company is also dedicated to the development of therapeutic assets and has established a pre-clinical development pipeline to potentially
address unmet medical needs across a number of indications, with a focus on rare and/or orphan indications, including, Neuromyelitis
Optica Spectrum Disorder, Parkinson’s Disease, Chronic Hypoparathyroidism and Amyotrophic Lateral Sclerosis (commonly known as
Lou Gehrig’s Disease).
Corporate
Structure
Aeterna
is incorporated under the Canada Business Corporations Act. Our common shares are listed for trading on both the Nasdaq Capital
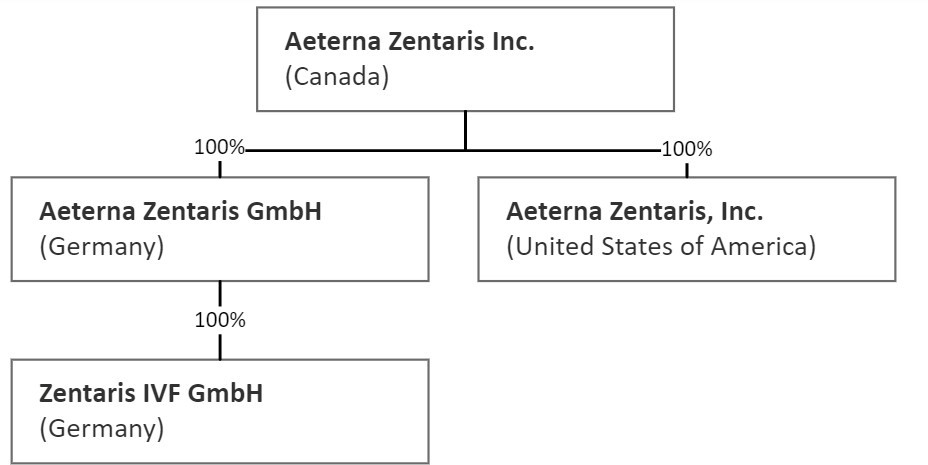
Market and the Toronto Stock Exchange under the trading symbol “AEZS”. We have three wholly-owned subsidiaries (direct and
indirect): Aeterna Zentaris GmbH (“Aeterna Germany”), which is based in Frankfurt am Main, Germany and incorporated
under the laws of Germany; Zentaris IVF GmbH, a direct wholly-owned subsidiary of Aeterna Germany, which is based in Frankfurt am Main,
Germany and incorporated under the laws of Germany; and Aeterna Zentaris, Inc., which is based in Charleston, South Carolina, U.S. and
incorporated under the laws of the State of Delaware.
As
of December 31, 2023, the Company had a total of 21 active employees, three of which were based in Canada, 17 in Germany and one in the
U.S.
Supply
Chain
The
Company’s supply chain operates primarily in the European Economic Area, with a smaller footprint in North America and Asia, and
is broadly divided into two categories:
| |
● |
Commercial
operations – relating to the manufacture and supply of active pharmaceutical ingredients for the Company’s lead product,
Macrilen®; and |
| |
● |
Clinical
research and development – relating to the supply of pharmaceutical and other products used in the Company’s clinical
DETECT-trial and the Company’s preclinical research on our four pipeline projects. |
| |
● |
Our
top tier suppliers and their manufacturing operations are based in the European Economic Area, namely Italy, France and Germany. |
Policies
and due diligence processes related to forced labour and child labour
Aeterna
is committed to conducting our operations in accordance with the highest degree of integrity and in compliance with all applicable laws
in the regions in which we operate.
Code
of Conduct and Business Ethics
Our
Code, which applies to all employees, contractors, officers and directors of Aeterna and its subsidiaries, sets forth our code of ethics
and expectations regarding responsible business conduct. The Code includes a statement on supply chains, which reinforces the Company’s
expectation that our vendors, suppliers and customers will obey all laws and regulations in providing and purchasing products from the
Company. They are also contractually encouraged to adhere to the spirit of the Code in their operations.
In
addition, our suppliers are required to comply with the terms and conditions of the contracts that they enter into with Aeterna. Such
contracts require suppliers to comply with all applicable laws and regulations in their performance of the contracts. The Company’s
vendor selection process also includes an assessment of the supplier’s reputation in their industry, the geographic location of
their operations and where the supplier sources services and materials from.
Code
of Business Conduct and Ethics for Members of the Board of Directors
In
addition to our Code, this ancillary code ensures that directors comply, and oversee compliance by employees, officers and other directors,
with laws, rules and regulations applicable to the Company and its subsidiaries. Directors are required to promote ethical behaviour
by, among other things, encouraging employees to report ethical misconduct. To that end, the Company maintains a Whistleblower Protection
Policy which encourages employees to report any misconduct, including ethical concerns, through our free and confidential on-line reporting
hotline.
Ongoing
monitoring
We
are committed to continually enhancing various measures, including the terms outlined in our suppliers’ contracts and our Code
to address and mitigate any risks of forced labour or child labour.
Areas
of risk for forced labour and child labour
Operations
During
the Reporting Period, Aeterna completed an internal risk assessment of forced labour and child labour in our operations and assessed
the risk to be low. All of our employees are highly skilled and require specific skill sets for their respective roles. They are based
in Canada, the U.S. and Germany and are hired in accordance with group-wide standard policies. As mentioned above, Aeterna has a comprehensive
Code that outlines the expected behavior of individuals doing work for the Company.
Supply
Chain
While
we have a global footprint, the majority of our supply chains are centered in the European Economic Area, Canada and the U.S. The materials
purchased by the Company, and its suppliers, include chemical compounds and active pharmaceutical ingredients used in the manufacturing
of finished drug products. The Company completed a mapping of the first tier of our supply chain, which enabled us to evaluate critical
suppliers, group and prioritize them, identify potential vulnerabilities and assess the controls in place. In assessing risks we considered
the geographic location of suppliers, the complexity of the supply chains, especially those relating to areas known for forced or child
labour, industry-specific risks linked to human rights and labour practices, the duration of our supply relationships and overall spend.
The
Company did not identify any instances of forced labour or child labour and concluded that there is a low risk for human rights violations,
including forced and child labour, in our supply chain based on:
| |
● |
the
geographic location of our first-tier suppliers, which are considered low risk; |
| |
● |
the
pharmaceutical industry in which we operate and the types of products being supplied, which are considered low risk; and |
| |
● |
the
type of labour employed by the Company and our manufacturers, suppliers and research partners, who are highly skilled and reputable
in their respective industries. |
Remediation
measures
As
mentioned above, our Whistleblower Protection Policy offers a reporting mechanism for employees to report ethical or legal violations,
among other concerns. The management of Aeterna is committed to remedying any non-compliance identified by employees, suppliers and other
stakeholders. As a result of the Company not identifying any forced labour or child labour in our operations or supply chains, we have
not had to take any measures to remediate any forced labour or child labour or to remediate any loss of income.
Training
for the prevention of forced labour and child labour
Certain
members of Aeterna’s management received informal training during the Reporting Period, provided by external counsel, regarding
the risks of forced labour and child labour and the requirements of the Act. The Company also engaged external counsel to provide formal
training for all employees of Aeterna and its subsidiaries. The training, which took place shortly after the end of the Reporting Period,
covered a range of topics including the purpose and scope of the Act, international human rights frameworks, statistics related to modern
slavery, the meaning and indicators of forced labour and child labour and measures to prevent and reduce modern slavery risks in the
supply chain, including an overview of Aeterna’s governance framework and policies.
In
addition, all employees of Aeterna and its subsidiaries are required to annually review the Code and certify their compliance with it.
Assessing
effectiveness
We
understand that we have a responsibility to assess and mitigate the risks of modern slavery in our operations and supply chains over
the long term. The Board of Directors of Aeterna has overall responsibility for the strategy around modern slavery. During the Reporting
Period, Aeterna put in place a number of measures meant to prevent and reduce the risk that forced labour or child labour is used in
our activities and supply chains. We have not yet taken any actions to assess the effectiveness of those actions.
Approval
and attestation
This
Report was approved pursuant to subparagraph 11(4)(a) of the Act by the Board of Directors of Aeterna. In accordance with the requirements
of the Act, and in particular section 11 thereof, I attest that I have reviewed the information contained in this Report for the entity
listed above. Based on my knowledge, and having exercised reasonable diligence, I attest that the information in this Report is true,
accurate and complete in all material respects, for the purposes of the Act, for the Reporting Period.
I
make the above attestation in my capacity as a director of the Board of Directors of Aeterna for and on behalf of the Board.
I
have the authority to bind Aeterna Zentaris Inc.
| /s/
Carolyn Egbert |
|
/s/
Dennis Turpin |
| Carolyn
Egbert, Chair of the Board |
|
Dennis
Turpin, Director |
May
15th, 2024
Exhibit
99.5

Aeterna
Zentaris Provides Update on Timing for Annual Meeting of Shareholders and Due Bill Redemption Date
TORONTO,
ONTARIO, May 29, 2024 – Aeterna Zentaris Inc. (NASDAQ: AEZS) (TSX: AEZS) (“Aeterna” or the “Company”),
a specialty biopharmaceutical company developing and commercializing a diversified portfolio of pharmaceutical and diagnostic products,
today announced that it is making an application for an order pursuant to section 133(3) of the Canada Business Corporations Act
extending the time for the Company to call and hold its annual meeting of its shareholders to a date that is not later than July 31,
2024 (the “Application”). The Application is scheduled to be heard before a judge of the Ontario Superior Court of Justice,
Commercial List (the “Court”) on June 3, 2024, at 11:00 a.m. via videoconference. There can be no assurance that the
order sought by Aeterna will be granted.
In
support of the Application, the Company will file an application record, a factum and a draft order with the Court. These materials will
also be available on the Company’s website at www.zentaris.com. Any shareholder that wishes to attend the hearing may request
a link by emailing the Company at AZinfo@aezsinc.com.
Aeterna
is seeking the extension from the Court to provide sufficient time to complete the previously announced transaction (the “Transaction”)
with Ceapro Inc. (“Ceapro”) in advance of the annual meeting so as to permit former Ceapro shareholders to attend and vote
at the annual meeting. At the annual meeting, shareholders will be asked to vote on the new name and director nominees for the combined
company. As previously disclosed, subject to obtaining all required approvals and satisfying all required conditions, the Transaction
is expected to close on or about June 3, 2024.
The
Toronto Stock Exchange has also granted the Company an extension permitting the Company to hold its annual meeting on or before July
31, 2024.
The
Company would also like to clarify that the redemption date for the due bills attaching to the Company’s common shares as a result
of the previously announced warrant issuance is June 3, 2024.
About
Aeterna Zentaris Inc.
Aeterna
is a specialty biopharmaceutical company developing and commercializing a diversified portfolio of pharmaceutical and diagnostic products
focused on areas of significant unmet medical need. Aeterna’s lead product, macimorelin (Macrilen; Ghryvelin), is the first and
only U.S. FDA and European Commission approved oral test indicated for the diagnosis of adult growth hormone deficiency (AGHD). Aeterna
is leveraging the clinical success and compelling safety profile of macimorelin to develop it for the diagnosis of childhood-onset growth
hormone deficiency (CGHD), an area of significant unmet need.

Aeterna
is also dedicated to the development of its therapeutic assets and has established a pre-clinical development pipeline to potentially
address unmet medical needs across a number of indications, including neuromyelitis optica spectrum disorder (NMOSD), Parkinson’s
disease (PD), hypoparathyroidism and amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease).
For
more information, please visit www.zentaris.com and connect with the Company on Twitter, LinkedIn and Facebook.
Forward-Looking
Statements
This
press release contains statements that may constitute forward-looking statements within the meaning of U.S. and Canadian securities legislation
and regulations, and such statements are made pursuant to the safe-harbor provision of the U.S. Securities Litigation Reform Act of 1995.
Forward-looking statements are frequently, but not always, identified by words such as “expects,” “aiming”, “anticipates,”
“believes,” “intends,” “potential,” “possible,” and similar expressions. Such statements,
based as they are on current expectations of management, inherently involve numerous risks, uncertainty and assumptions, known and unknown,
many of which are beyond our control.
Forward-looking
statements in this press release include, but are not limited to, those relating to Aeterna’s expectations regarding: the potential
obtaining of an order from the Court via the Application; the timing for the Company’s annual meeting; the timing and location
for the hearing of the Application; the ability of Aeterna and Ceapro to complete the Transaction on the terms described herein, or at
all; the anticipated timeline for the completion of the Transaction; and receipt of final regulatory and stock exchange approvals with
respect to the Transaction (including approval of the continued listing of the Common Shares on the Nasdaq and the TSX).
Forward-looking
statements involve known and unknown risks and uncertainties, and other factors which may cause the actual results, performance or achievements
stated herein to be materially different from any future results, performance or achievements expressed or implied by the forward-looking
information. Such risks and uncertainties include, among others, our reliance on the success of the DETECT clinical trial in the European
Union and U.S. for Macrilen™ (macimorelin) in CGHD; results from our ongoing or planned pre-clinical studies and our DETECT clinical
trial under development may not be successful or may not support advancing the product further in pre-clinical studies, to human clinical
trials or regulatory approval; our ability to raise capital and obtain financing to continue our currently planned operations; our now
heavy dependence on the success of Macrilen™ (macimorelin) and related out-licensing arrangements and the continued availability
of funds and resources to successfully commercialize the product; the global instability due to the global pandemic of COVID-19 and the
war in the Ukraine, and their unknown potential effect on our planned operations; our ability to enter into out-licensing, development,
manufacturing, marketing and distribution agreements with other pharmaceutical companies and keep such agreements in effect; our ability
to continue to list our common shares on the NASDAQ; and the availability and timing of required stock exchange, regulatory and other
approvals for the completion of the Transaction with Ceapro. Investors should consult our quarterly and annual filings with the Canadian
and U.S. securities commissions for additional information on risks and uncertainties, including those risks discussed in our Annual
Report on Form 20-F under the caption “Risk Factors”. Given the uncertainties and risk factors, readers are cautioned not
to place undue reliance on these forward-looking statements. We disclaim any obligation to update any such factors or to publicly announce
any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, unless required
to do so by a governmental authority or applicable law.
For
a more detailed discussion of such risks and other factors that may affect Aeterna’s and ability to achieve the expectations set
forth in the forward-looking statements contained in this news release, see Aeterna’s Annual Report on Form 20-F and MD&A filed
under Aeterna’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov, as well as Aeterna’s other
filings with the Canadian securities regulators and the Securities and Exchange Commission.
No
securities regulatory authority has either approved or disapproved of the contents of this news release. The Toronto Stock Exchange accepts
no responsibility for the adequacy or accuracy of this release.
Investor
Contact:
Investor
Relations
AZinfo@aezsinc.com
+1
843-900-3223
Exhibit 99.6

Exhibit 99.7

Aeterna Zentaris (NASDAQ:AEZS)
過去 株価チャート
から 10 2024 まで 11 2024
Aeterna Zentaris (NASDAQ:AEZS)
過去 株価チャート
から 11 2023 まで 11 2024