false0001720580NONE00017205802024-05-102024-05-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): May 10, 2024 |
Adicet Bio, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-38359 |
81-3305277 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
131 Dartmouth Street, Floor 3 |
|
Boston, Massachusetts |
|
02116 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (650) 503-9095 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.0001 per share |
|
ACET |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On May 10, 2024, Adicet Bio, Inc. (the Company) presented at the 27th Annual Meeting of the American Society of Gene and Cell Therapy (ASGCT ) The Company’s presentation was posted to the “Presentations & Events” section of the Company’s website at investor.adicetbio.com and is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
ADICET BIO, INC. |
|
|
|
|
Date: |
May 10, 2024 |
By: |
/s/ Nick Harvey |
|
|
Name: Title: |
Nick Harvey
Chief Financial Officer |
ADI-270: An Armored Allogeneic Anti-CD70 CAR γδ T cell Therapy Candidate Designed for Multiple Solid and Hematological Cancer Indications 27th ASGCT Annual Meeting 2024�Baltimore, MD Shon Green, PhD VP, Nonclinical Development
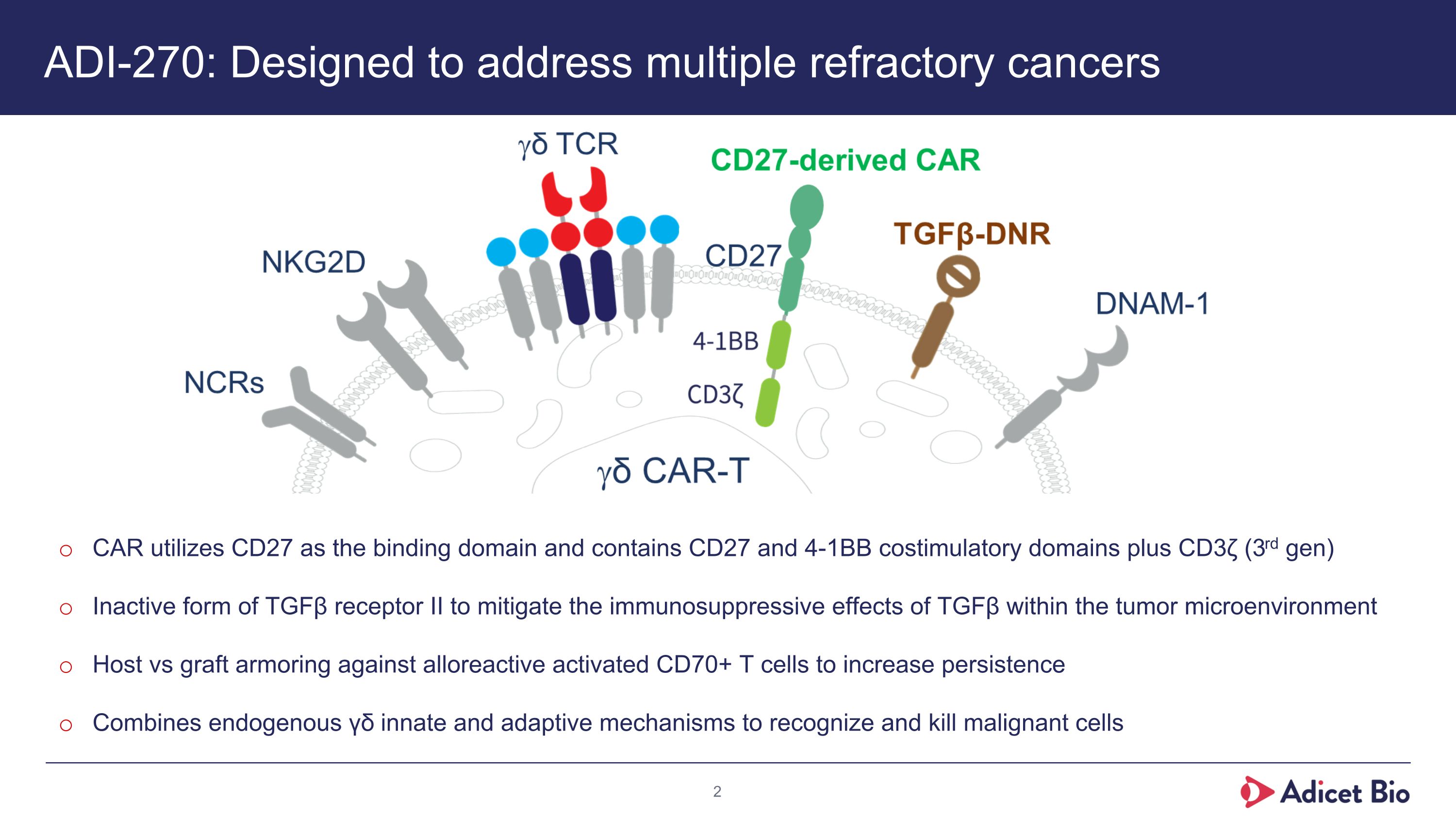
ADI-270: Designed to address multiple refractory cancers CAR utilizes CD27 as the binding domain and contains CD27 and 4-1BB costimulatory domains plus CD3ζ (3rd gen) Inactive form of TGFβ receptor II to mitigate the immunosuppressive effects of TGFβ within the tumor microenvironment Host vs graft armoring against alloreactive activated CD70+ T cells to increase persistence Combines endogenous γδ innate and adaptive mechanisms to recognize and kill malignant cells
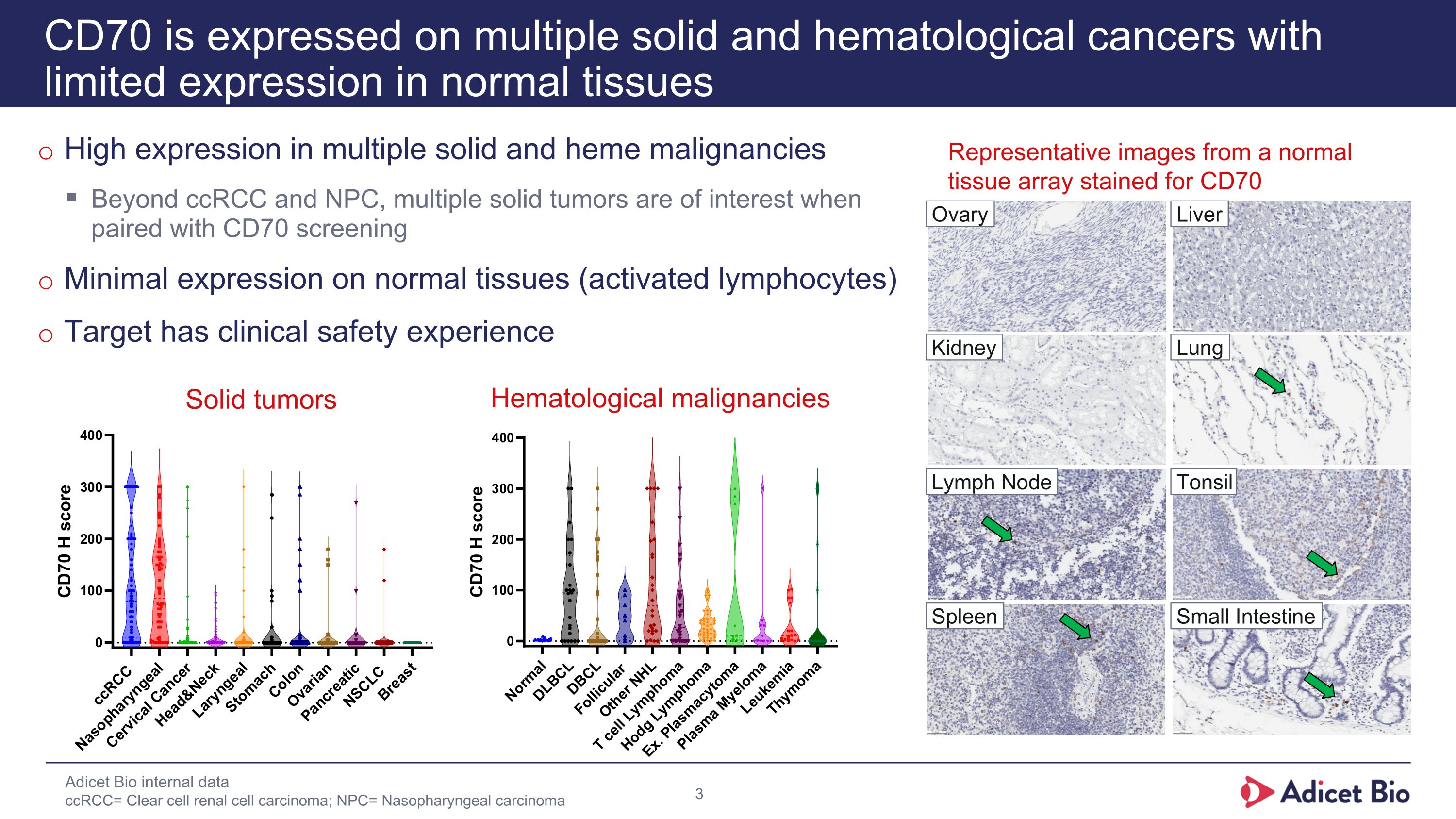
High expression in multiple solid and heme malignancies Beyond ccRCC and NPC, multiple solid tumors are of interest when paired with CD70 screening Minimal expression on normal tissues (activated lymphocytes) Target has clinical safety experience CD70 is expressed on multiple solid and hematological cancers with limited expression in normal tissues Representative images from a normal tissue array stained for CD70 Adicet Bio internal data ccRCC= Clear cell renal cell carcinoma; NPC= Nasopharyngeal carcinoma Lung Liver Kidney Ovary Lymph Node Tonsil Spleen Small Intestine Solid tumors Hematological malignancies
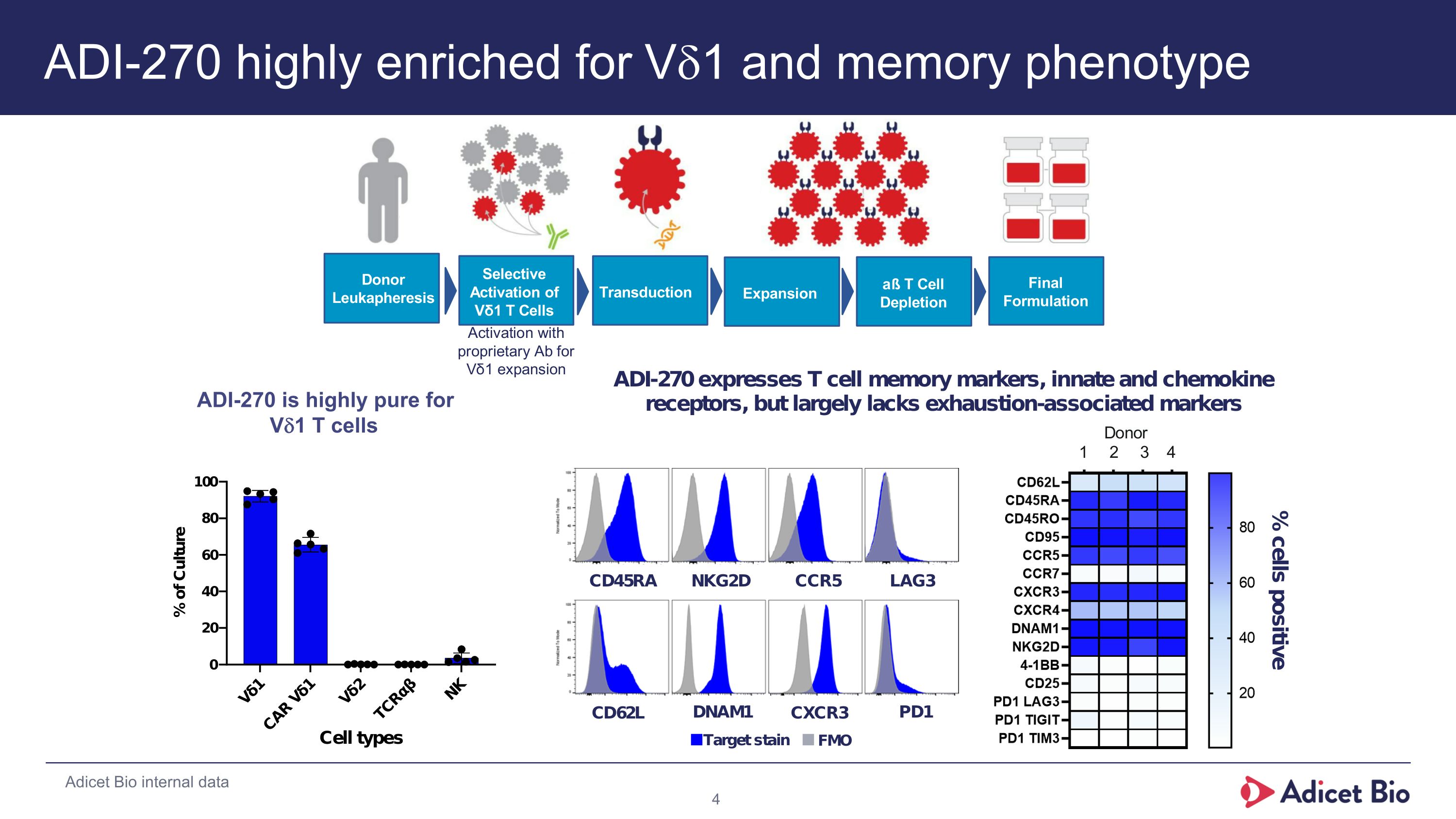
ADI-270 highly enriched for Vd1 and memory phenotype % cells positive D o n o r L e u k a p h e r e s i s T r a n s d u c t i o n E x p a n s i o n a ß T C e l l D e p l e t i o n F i n a l F o r m u l a t i o n S e l e c t i v e A c t i v a t i o n o f V δ 1 T C e l l s Adicet Bio internal data ADI-270 is highly pure for Vd1 T cells ADI-270 is highly pure for Vd1 T cells
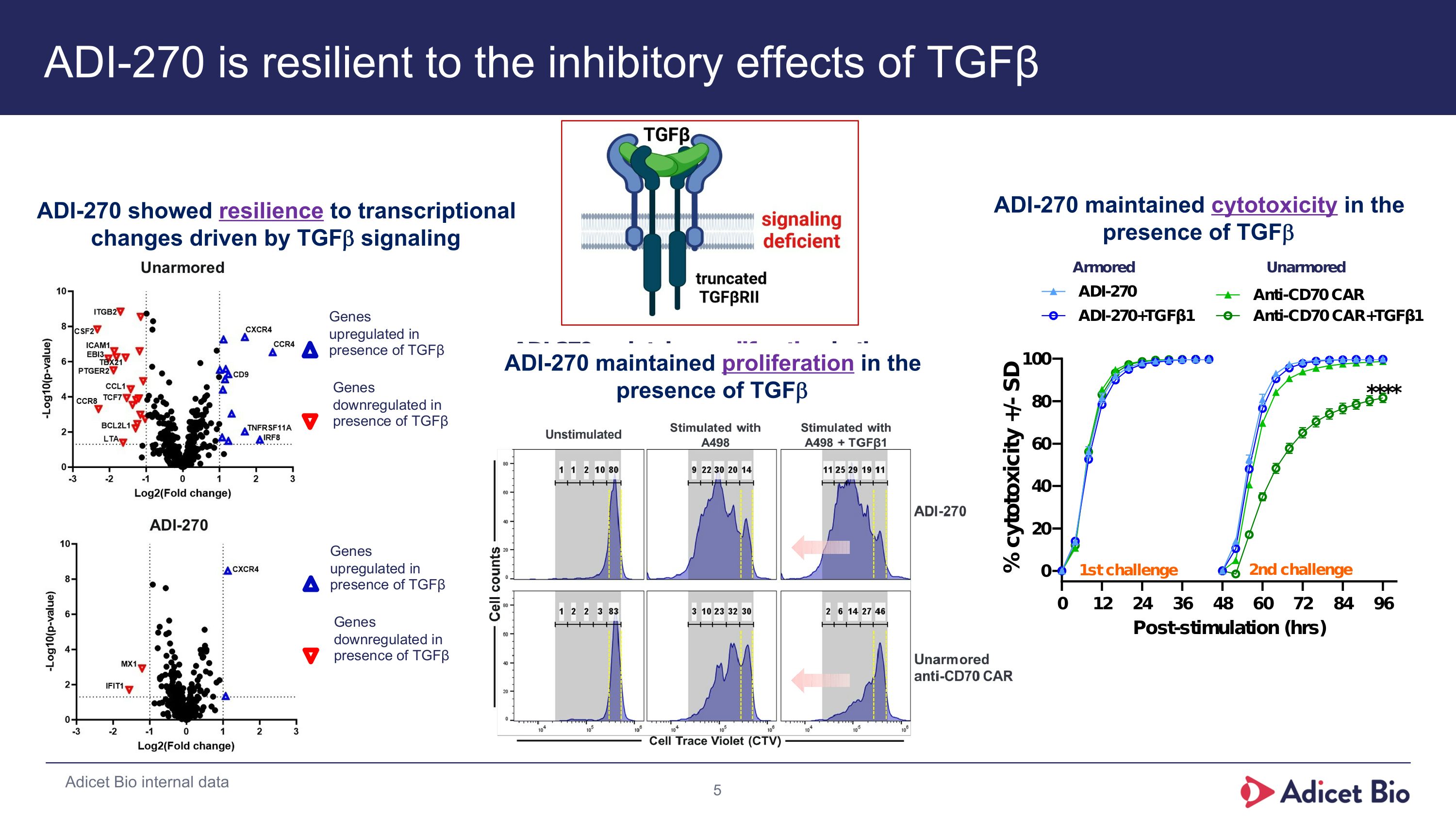
ADI-270 is resilient to the inhibitory effects of TGFβ Adicet Bio internal data ADI-270 showed resilience to transcriptional changes driven by TGFb signaling ADI-270 maintained proliferation in the presence of TGFb ADI-270 maintained cytotoxicity in the presence of TGFb
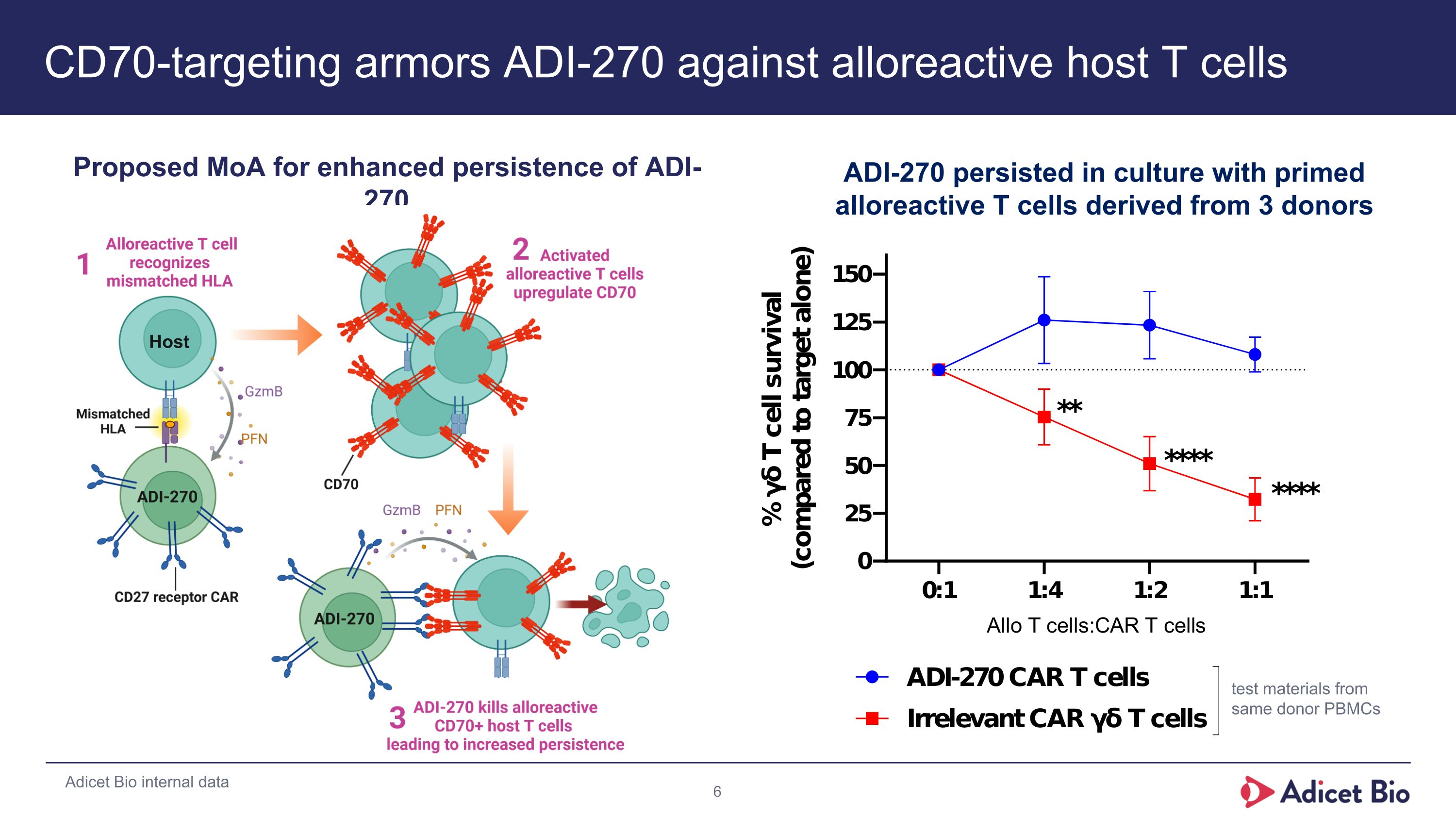
CD70-targeting armors ADI-270 against alloreactive host T cells Proposed MoA for enhanced persistence of ADI-270 Host Adicet Bio internal data test materials from same donor PBMCs ADI-270 persisted in culture with primed alloreactive T cells derived from 3 donors

ADI-270 exhibited potent in vitro cytotoxicity against a range of CD70 levels in a diverse set of solid and heme malignancies Adicet Bio internal data CD70 Ag density E:T ratio = 1:1

ADI-270 retained potent activity in the context of CD70-low tumors compared to clinically relevant CD70-targeting αβ CAR T cell benchmarks Adicet Bio internal data

ADI-270 contributed CAR-dependent and CAR-independent mechanisms of tumor targeting CD70+/- tumor mixture model Increased killing of CD70neg tumors within tumor mixture CAR T cell Ag activation A498 tumors and CD70 KO A498 E:T ratio = 1:1, N=2 donors, ****p<0.0001 Adicet Bio internal data test materials derived from same donor PBMCs

ADI-270 demonstrated higher innate cytolytic activity against CD70 negative tumor cells compared to CAR-T cell references ***p<0.001, ****p<0.0001 CD70 KO A498 tumors ADI-270 αβ CAR T references Higher killing Lower killing Adicet Bio internal data test materials derived from same donor PBMCs
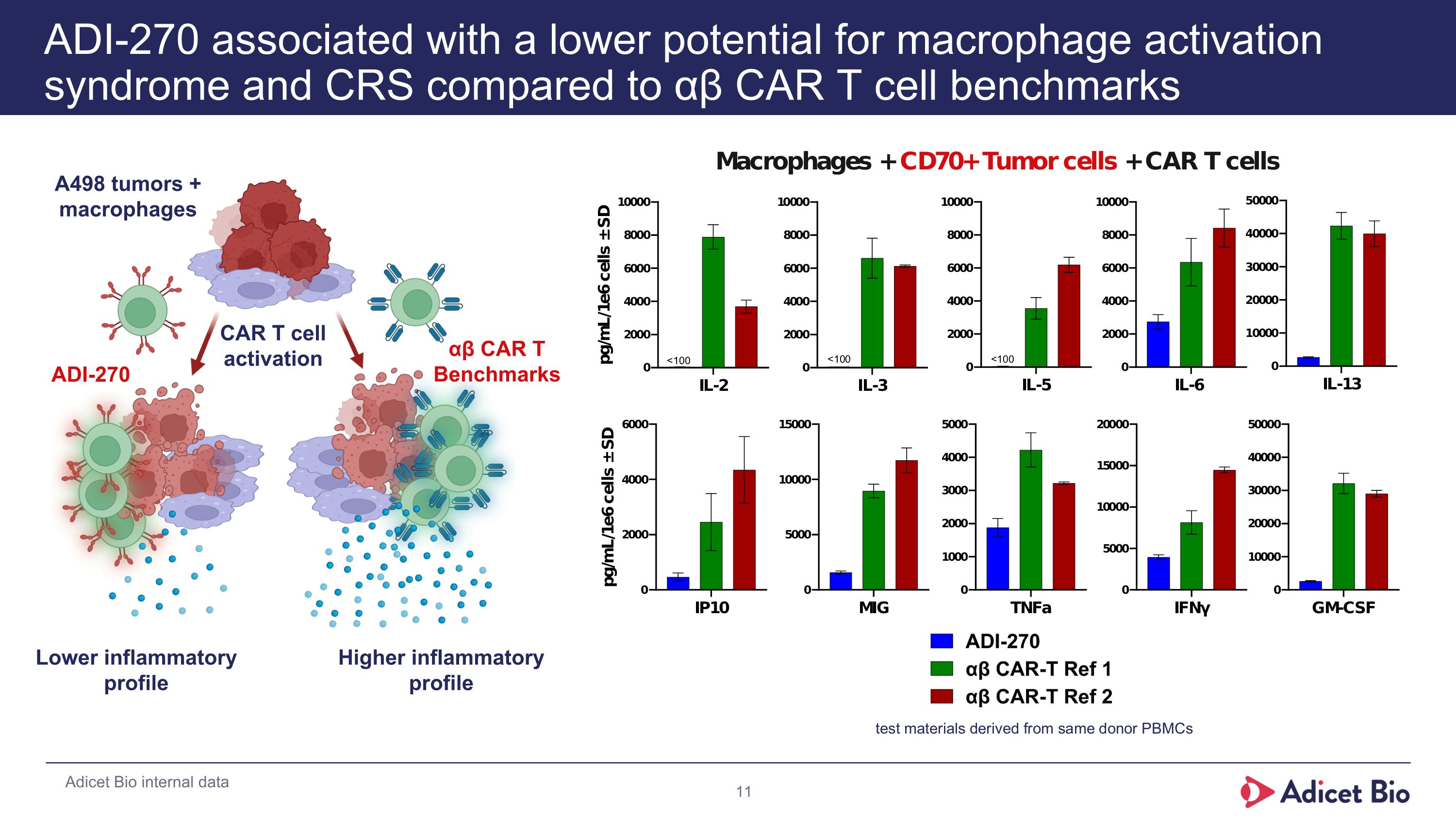
ADI-270 associated with a lower potential for macrophage activation syndrome and CRS compared to αβ CAR T cell benchmarks Lower inflammatory profile Higher inflammatory profile CAR T cell activation A498 tumors + macrophages ADI-270 αβ CAR T Benchmarks Adicet Bio internal data
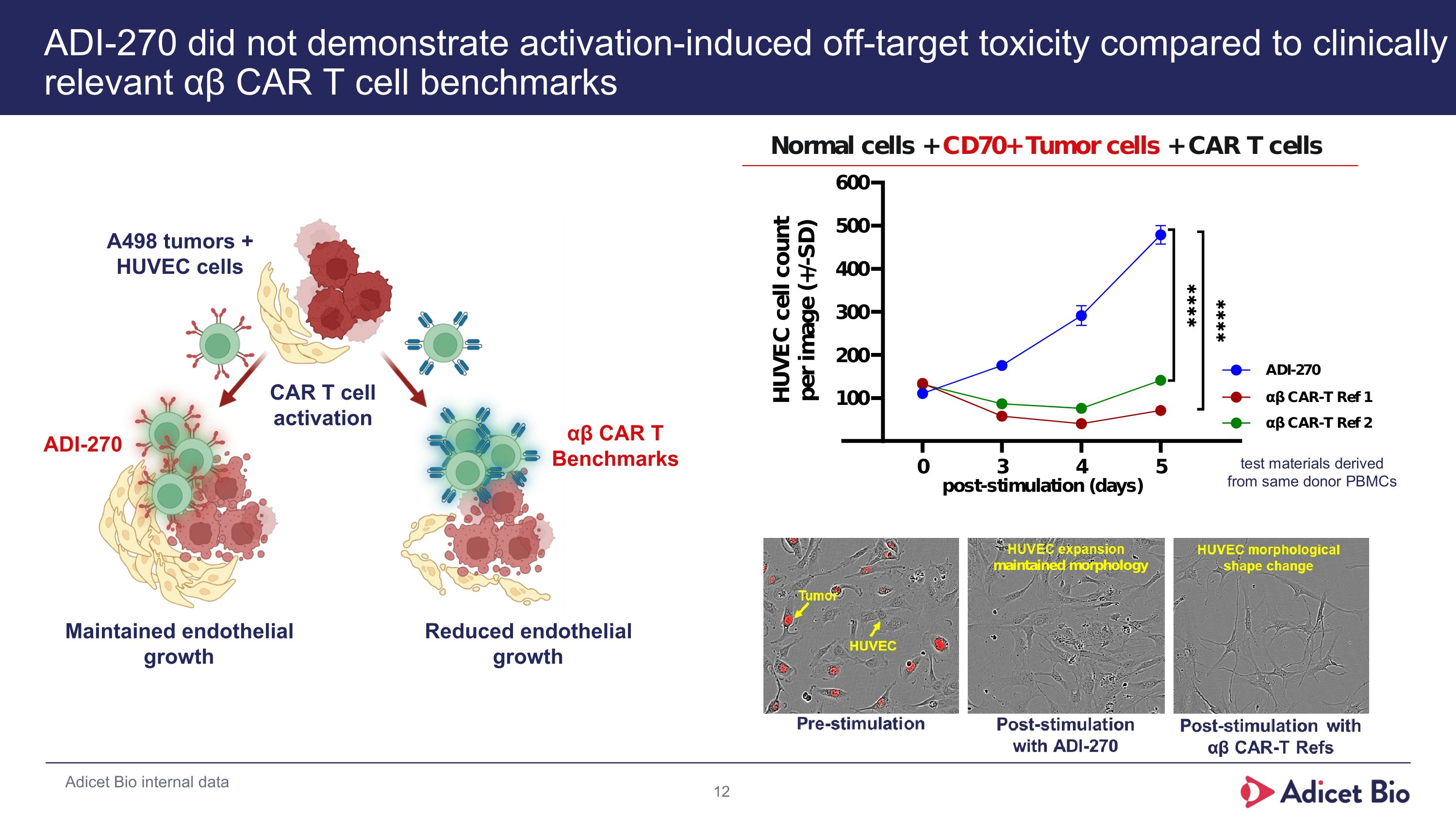
ADI-270 did not demonstrate activation-induced off-target toxicity compared to clinically relevant αβ CAR T cell benchmarks Maintained endothelial growth Reduced endothelial growth CAR T cell activation A498 tumors + HUVEC cells ADI-270 αβ CAR T Benchmarks Adicet Bio internal data

A single dose of ADI-270 showed potent regression and sustained systemic anti-tumor activity in ccRCC xenograft models ADI-270 (5, 10, or 15 x 106 I.V.) ADI-270 (5 or 10 x 106 I.V.) Small tumor ~100mm3 Medium tumor ~500mm3 Monitor tumor volume Monitor tumor volume Monitor tumor volume ****p<0.0001 Tx Tx Tumor regression (right flank) Rechallenge with tumor (left flank) 4 x 106 A498 cells Adicet Bio internal data Opposite Flank Tumor Rechallenge D18 post initial treatment 42 days post rechallenge
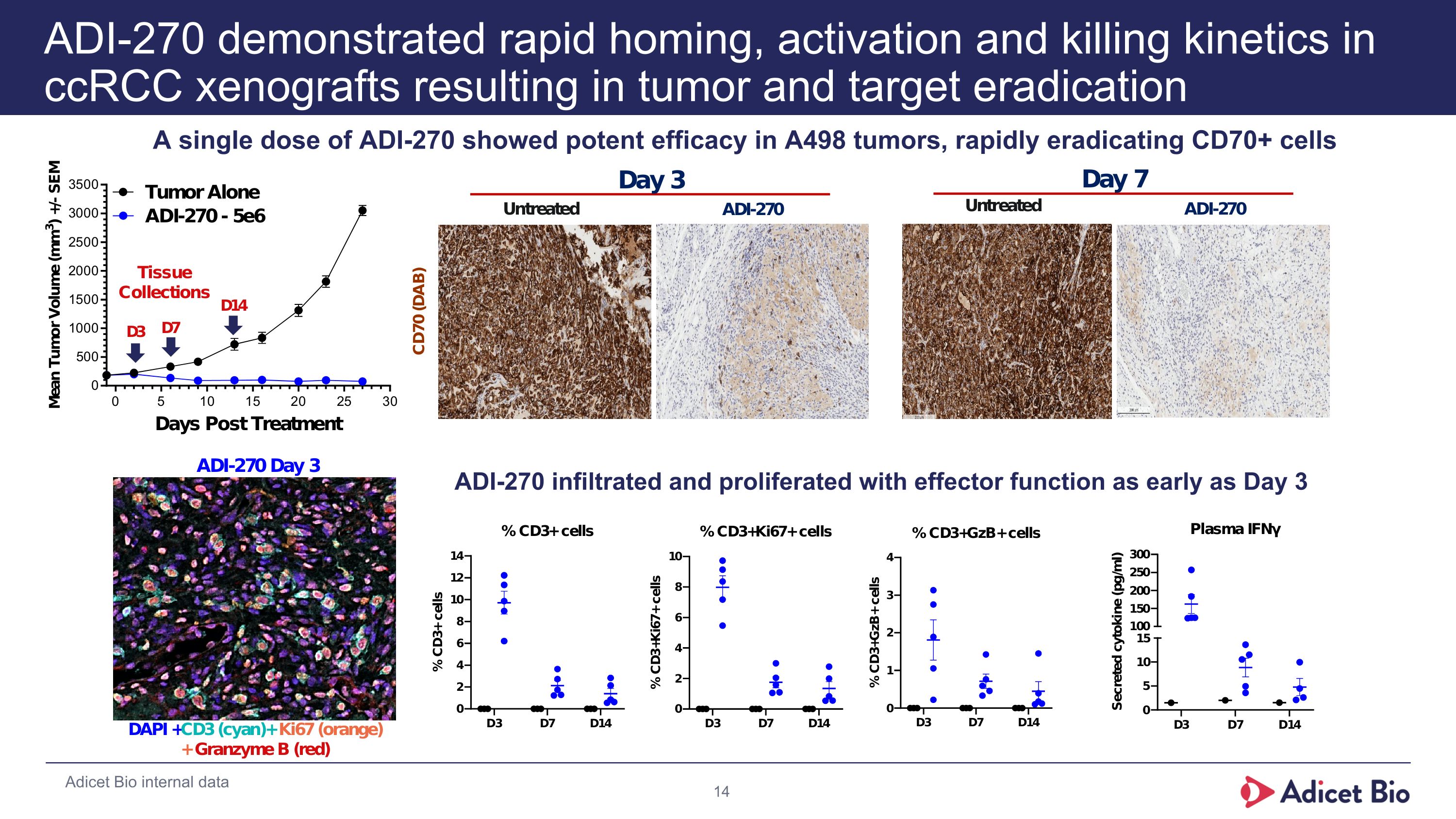
ADI-270 demonstrated rapid homing, activation and killing kinetics in ccRCC xenografts resulting in tumor and target eradication Adicet Bio internal data A single dose of ADI-270 showed potent efficacy in A498 tumors, rapidly eradicating CD70+ cells ADI-270 infiltrated and proliferated with effector function as early as Day 3

ADI-270 anti-tumor activity extended to multiple hematologic tumor xenografts associated with lower CD70 expression Adicet Bio internal data A single dose of ADI-270 was administered IV into NSG mice harboring SC tumor xenografts
Next steps: ADI-270 ADI-270 represents potential evolution of γδ CAR T-cell based therapeutics CD27-based 3rd gen CAR demonstrated significant potency advantages1,2,3,4 Armoring against TGFβ and alloreactive T cells confirmed and characterized preclinically Robust efficacy maintained across multiple relevant tumor models of varying stringency Desirable preclinical safety profile with lower potential for CRS and macrophage activation syndrome IND submission in ccRCC expected Q2 2024 1Shaffer et al., Blood 2011 2Acharya et al., Blood 2023 3Leick et al., Cancer Cell 2022 4Kasap et al., BioRxiv 2024
v3.24.1.1.u2
Document And Entity Information
|
May 10, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
May 10, 2024
|
| Entity Registrant Name |
Adicet Bio, Inc.
|
| Entity Central Index Key |
0001720580
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-38359
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
81-3305277
|
| Entity Address, Address Line One |
131 Dartmouth Street, Floor 3
|
| Entity Address, City or Town |
Boston
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02116
|
| City Area Code |
(650)
|
| Local Phone Number |
503-9095
|
| Entity Information, Former Legal or Registered Name |
Not applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
ACET
|
| Security Exchange Name |
NONE
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Adicet Bio (NASDAQ:ACET)
過去 株価チャート
から 4 2024 まで 5 2024
Adicet Bio (NASDAQ:ACET)
過去 株価チャート
から 5 2023 まで 5 2024