AUSTIN,
Texas, July 1, 2024 /PRNewswire/ -- Paradromics
Inc., a leading developer of brain-computer interfaces (BCI), today
announced its acceptance into the U.S. Food and Drug
Administration's newest program for innovative devices, the Total
Product Life Cycle Advisory Program. According to the FDA, the TAP
accelerator program was launched to "help spur more rapid
development and more rapid and widespread patient access to safe,
effective, high-quality medical devices of public health
importance."
The TAP program was created exclusively for devices designated
by the FDA as Breakthrough Devices. The Paradromics Connexus®
Direct Data Interface has received two such designations from the
FDA: one recognizing its potential to help patients communicate
again after losing the ability to speak and a second for its
ability to help patients with severe loss of movement to control
computer devices. The FDA Breakthrough Device Designations provide
an expedited review process for transformative medical devices with
the potential to treat irreversible, debilitating conditions. TAP
further accelerates the review process by providing additional
opportunities for rapid communication between regulators and
companies.
Matthew Willsey, MD, PhD, a
leading neurosurgeon at the University of
Michigan Medical School states, "I'm excited to see BCI
technologies, such as the Connexus device, entering the clinical
phase. This is one step closer to restoring movement to people with
paralysis, or speech to those who have lost this function."
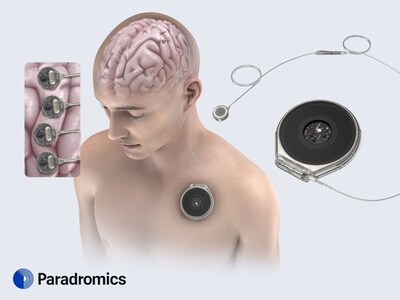
Paradromics is one of two companies with fully implantable BCIs
capable of recording from single neurons, and the only company to
achieve this using durable materials and packaging. Those qualities
provide a sizable advantage: the ability to obtain very
high-resolution data over long periods of time. This
high-resolution data can enable complex applications like decoding
intended speech. The first use of the technology will be for
individuals living with ALS, spinal cord injury, and stroke who
have lost their ability to communicate. Future applications of the
technology will be in treatment-resistant mental health, such as
depression.
"We are happy to partner more closely with the FDA" said
Paradromics CEO, Matt Angle.
"Building a device that senses neural signals with single neuron
resolution and works reliably in the body for years was
challenging. There are easier approaches, but they aren't as good
for patients. We want to deliver the best possible device on the
safest possible timeline, and so we appreciate access to the TAP
program."
In preparation for its clinical trial in 2025, Paradromics is
also announcing the launch of the Paradromics Patient Registry,
where patients can submit their interest in participating in the
trials when they begin. Establishing this line of communication
with potential patients further supports Paradromics' efforts to
ensure that innovative technologies are brought to patients as
swiftly and safely as possible.
About Paradromics Inc.
Paradromics (www.paradromics.com), headquartered in Austin, Texas, is bringing to market a high
data-rate brain-computer interface (BCI). The first application of
the Connexus® Direct Data Interface is a BCI-enabled assistive
communication device for severely motor-impaired people. This
technology could revolutionize the treatment of neurological and
brain-related conditions ranging from sensory deficits to mood
disorders—allowing millions of people to live happier, healthier,
and more enriching lives.
Media Contact:
media@paradromics.com
Gavin Mathis
Prime Movers Lab
gavin@primemoverslab.com
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/paradromics-accepted-to-fda-regulatory-accelerator-program-and-announces-new-patient-registry-302186953.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/paradromics-accepted-to-fda-regulatory-accelerator-program-and-announces-new-patient-registry-302186953.html
SOURCE Paradromics