GE, Lantheus Say Heart Disease Tracer Trial Met Key Endpoints
2022年9月13日 - 10:16PM
Dow Jones News
By Dean Seal
General Electric Co.'s healthcare business and Lantheus Holdings
Inc. said Tuesday that the Phase 3 clinical trial for their
investigational radiotracer met co-primary endpoints in the
detection of coronary artery disease.
The companies said the trial for the radiotracer, dubbed
[18F]flurpiridaz, met the co-primary endpoints of exceeding a 60%
threshold for both sensitivity and specificity for detecting heart
disease.
The trial also met its first key secondary endpoint by
demonstrating that [18F]flurpiridaz Positron Emission Tomography
has higher diagnostic efficacy for patients with suspected coronary
artery disease when compared with the predominant procedure used in
nuclear cardiology today.
GE Healthcare has led the funding and development of the
investigational agent and will have global commercialization rights
to the imaging agent if it is approved. Lantheus has collaborated
on the development and will work with GE on a potential
commercialization through a joint steering committee, with
entitlement to royalties based on commercial sales.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
September 13, 2022 09:01 ET (13:01 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
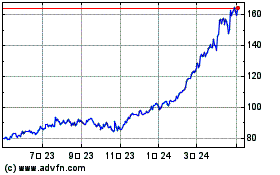
GE Aerospace (NYSE:GE)
過去 株価チャート
から 3 2024 まで 4 2024
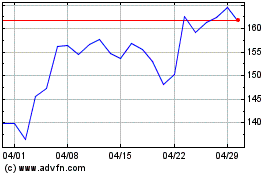
GE Aerospace (NYSE:GE)
過去 株価チャート
から 4 2023 まで 4 2024