UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT
REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
July 28, 2023 (July 28, 2023)
FORBION
EUROPEAN ACQUISITION CORP.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Cayman Islands |
|
001-41148 |
|
N/A |
| (State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
|
|
|
|
|
| 4001 Kennett Pike, Suite 302
Wilmington, Delaware |
|
19807 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: +1 732
838-4533
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of
the registrant under any of the following provisions:
| ☒ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17
CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Class A ordinary shares, par value $0.0001 per share |
|
FRBN |
|
The Nasdaq Stock Market LLC |
| Redeemable warrants, each whole warrant exercisable for one Class A ordinary share at an exercise price of $11.50 |
|
FRBNW |
|
The Nasdaq Stock Market LLC |
| Units, each consisting of one Class A ordinary share and one-third of one redeemable warrant |
|
FRBNU |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of
1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an
emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange
Act. ☐
On July 27, 2023, Anthony T. Cheung, Chief Technology Officer of enGene, Inc. participated in the Controlled Release Society Meeting and
Expo held in Las Vegas and delivered a presentation entitled “Development of a Non-Viral Gene Therapy Platform for Mucosal Tissues” at 3:00 p.m. Pacific Time (the
“Presentation”).
A Copy of the Presentation is furnished as Exhibit 99.1, to this Current Report.
The information in this Item 8.01 and Exhibit 99.1 attached hereto shall not be deemed “filed” for purposes of Section 18 of
the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the U.S. Securities Act of 1933 (as
amended) or the Exchange Act, except as expressly set forth by specific reference in such filing.
Forward-Looking Statements
The information in this Current Report includes “forward-looking statements” within the meaning of the “safe harbor”
provisions of the United States Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, statements regarding FEAC’s management team’s expectations, hopes, beliefs, intentions or
strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words
“anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,”
“predict,” “project,” “should,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking
statements in this Current Report may include, for example, statements regarding FEAC’s ability to consummate the proposed Business Combination.
All forward-looking statements are based on estimates and assumptions that, while considered reasonable by FEAC and its management are
inherently uncertain and are inherently subject to risks, variability and contingencies, many of which are beyond FEAC’s control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and
should not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual
events and circumstances are beyond the control of FEAC. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or
implied by such forward-looking statements. These risks and uncertainties include the occurrence of any event, change or other circumstances that could give rise to the termination of the definitive agreements with respect to the proposed Business
Combination; the outcome of any legal proceedings that may be instituted against FEAC following this Current Report; the inability to complete the proposed Business Combination due to the failure to obtain approval of the shareholders of FEAC, or to
satisfy other conditions to closing; changes to the proposed structure of the proposed Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the
proposed Business Combination; the ability of the combined company to meet stock exchange listing standards following the consummation of the proposed Business Combination, the combined company’s ability to raise additional capital to fund its
produce development activity, and its ability to maintain key relationships and to attract and retain talented personnel; costs related to the proposed Business Combination; changes in applicable laws or regulations; the possibility that the
combined company may be adversely affected by changes in domestic and foreign business, market, financial, political, legal conditions and laws and regulations; the inability of the parties to successfully or timely consummate the proposed Business
Combination, including the risk that any regulatory approvals are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the combined company or the expected benefits of the proposed Business Combination; or
other risks and uncertainties set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements and Risk Factor Summary” in FEAC’s Annual Report on Form
10-K for the fiscal year ended December 31, 2022, or other documents filed or to be filed from time to time by FEAC with the SEC.
Any forward-looking statement speaks only as of the date on which it was made. FEAC anticipates that subsequent events and developments will
cause FEAC’s assessments to change. While FEAC may elect to update these forward-looking statements at some point in the future, FEAC specifically disclaims any obligation to do so, unless required by applicable law. Nothing in this Current
Report should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue
reliance on forward-looking statements, which speak only as of the date they are made.
FEAC disclaims any and all liability for any loss
or damage (whether foreseeable or not) suffered or incurred by any person or entity as a result of anything contained or omitted from this Current Report and such liability is expressly disclaimed.
Participants in the Solicitation
FEAC
and its directors, managers, executive officers, other members of management and employees may be deemed participants in the solicitation of proxies from FEAC’s shareholders with respect to the proposed Business Combination under the rules of
the SEC. FEAC’s investors and security holders may obtain more detailed information regarding the names and interests in the proposed Business Combination of FEAC’s directors and officers, without charge, in FEAC’s filings with the
SEC, including, when filed with the SEC, the preliminary proxy statement/prospectus and the amendments thereto, the definitive proxy statement/prospectus, and other documents filed with the SEC.
No Offer or Solicitation
This Current
Report is not a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Business Combination and does not constitute an offer to sell or the solicitation of an offer to buy any securities, or a
solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such
jurisdiction.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
| Exhibit
No. |
|
Description |
|
|
| 99.1 |
|
Presentation entitled “Development of a Non-Viral Gene Therapy Platform for Mucosal Tissues” |
|
|
| 104 |
|
Cover Page Interactive Data File - the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on
its behalf by the undersigned hereunto duly authorized.
Date: July 28, 2023
|
|
|
| FORBION EUROPEAN ACQUISITION CORP. |
|
|
| By: |
|
/s/ Jasper Bos |
|
|
Jasper Bos |
|
|
Chief Executive Officer |

Exhibit 99.1 DEVELOPMENT OF A NON-VIRAL GENE THERAPY PLATFORM FOR
MUCOSAL TISSUES enGene Controlled Release Society 2023 Meeting and Expo Las Vegas, July 2023

enGene: organ-localized non-viral gene therapies for mucosal tissues
Mucosal tissues are Mucosal tissues are attractive underserved by gene therapy target tissues for gene therapy: 1. Ease of accessibility Nasopharynx Carcinoma 2. Mucosal epithelium are vast tissues Rhinitis Mucosal vaccines 3. Provide localized
delivery Respiratory Tract 4. Minimize systemic exposure & toxicity Cystic Fibrosis Asthma COPD Lung cancer Gastrointestinal Tract Colorectal cancer Inflammatory Bowel Disease Short Bowel Syndrome Familial Adenomatous Polyposis Bladder Cancer
Interstitial cystitis 2 | © 2023 | enGene Schematic created using BioRender.com

Presentation Outline 1. Solving for mucosal organ delivery: Development
of the DDX platform 2. Transfection of the bladder epithelium and development of a potent immuno-oncology approach for bladder cancer: Preclinical MoA 3. LEGEND Clinical Trial: PoM and PoC demonstrated in human clinical trial 3 | © 2023 |
enGene

DEVELOPMENT OF THE DDX PLATFORM enGene

Mucus is a formidable barrier to transfection of mucosal epithelium
Filtering by interactions Filtering by size Traps cationic and Excludes large particles hydrophobic particles (>200-400 nm) Direction of flow Direction of flow Mucus Mucus 5 | © 2023 | enGene

® DDX is the delivery vehicle that enables non-viral gene therapies
for mucosal tissues Dually Derivatized Oligochitosan (DDX®): the mucosal tissue gene delivery vehicle Hydrophilic surface Minimize trapping by mucin layer Short-chain poly-D-glucosamine (chitosan) Cationic surface charge For efficient cellular
uptake 100nm Gluconic Acid Mono-Arginine High packaging efficiency Captured payload at >99% efficiency Stable in wide pH range Stable in mucosal tissue environment 6 | © 2023 | enGene

Reversible coating of nanoparticles with PEG-b-polyglutamic acid
(PEG-b-PLE) diblock copolymers PEG Plasmid - - - - DNA - PGA + + peptide tail + In-line Mixing + In-line Mixing + + + DDX® polymer DDX®/DNA Reversibly PEGylated DDX®/DNA Nanoparticle Nanoparticle This novel reversible PEGylation
technique presents multiple levers for optimization 7 | © 2023 | enGene

® Reversible coating of DDX nanoparticles with PEG improves mucus
penetration while preserving potency enGene’s novel reversible PEGylation of DDX® strongly improves Reversible PEGylation resolves the “PEG Dilemma” associated with mucus penetration conventional permanent PEGylation Transwell
12 DDX NP Fluorescent-tagged NP Reversibly PEG DDX NP suspension Pig mucin Crosslinked PEG DDX NP 1 9 Crosslinked PEG DDX NP 2 70 Max level attainable 60 6 50 40 30 3 20 10 0 0 50 100 Polystyrene PEG-DDX® PS-NH+ DDX NP PEG-DDX NP DDX® DNA
(ng) (hydrophobic cationic) (reversibly PEGylated) enGene’s reversible PEGylation of DDX nanoparticles improves mucus penetration without affecting their transfection efficiency 8 | © 2023 | enGene % Diffusion mg protein/mg total cellular
protein

PRECLINICAL MOA: TRANSFECTION OF THE BALDDER EPITHELIUM &
DEVELOPMENT OF A POTENT IMMUNO- ONCOLOGY APPROACH FOR BLADDER CANCER enGene

FDA guidance: single-arm registrational study for BCG-unresponsive
NMIBC • The Agency’s position is that no therapy exists for BCG-unresponsive NMIBC • Clearly defined patient population and entry criteria for BCG-unresponsive NMIBC • Approval is based on complete response rate, durability,
and safety FDA guideline: use of a single-arm trial is appropriate for approval in BCG-unresponsive NMIBC 10 | © 2023 | enGene

Background: Non-muscle invasive bladder cancer (NMIBC) NMIBC is a clear
unmet medical need Initial EG-70 pt. population Tis (carcinoma in situ) 2 Highest cost of all cancers to treat >550,000 new cases of bladder Ta (urothelium) 1 cancer per year T1 (lamina propria) BCG is the only approved first line treatment
non-muscle invasive disease T2 (muscle) 50% of patients fail BCG >75% of cases are non-muscle invasive disease T3 (fat layer) Radical cystectomy is the standard to prevent muscle invasion after BCG failure Global BCG shortage has T4 (other organs
around the bladder, such as prostate, uterus, vagina, pelvis or abdomen) created a public health crisis FDA guidance: goal of therapy in BCG-unresponsive NMIBC is to avoid cystectomy Prostate (male only) Market: over 60,000 newly diagnosed
BCG-unresponsive NMIBC patients globally per year 1 2 11 | © 2023 | enGene Richters et al., World Journal of Urology 2020; Mossanen and Gore, Curr Opin Urol 2014; Schematic created using BioRender.com

EG-70 (detalimogene voraplasmid) : dual-immune activator designed to
produce meaningful efficacy in NMIBC RIG-I agonists: • Stimulate T cell recruitment and neo-antigen presentation • NK cell stimulation and suppressor cell attenuation promotes tumor killing environment • Clinical data with innate
immune activators support biological rationale IL-12: • Polarization, proliferation, and activation of T cells increases tumor killing • Promotes immunological memory EG-70 is an easy-to-use lyophilized drug product • Strong
efficacy in oncology with dose-limiting toxicities when systemically administered – localized and reconstituted in urology clinics at point of circumscribed IL-12 is required administration 12 | © 2023 | enGene Schematic created using
BioRender.com
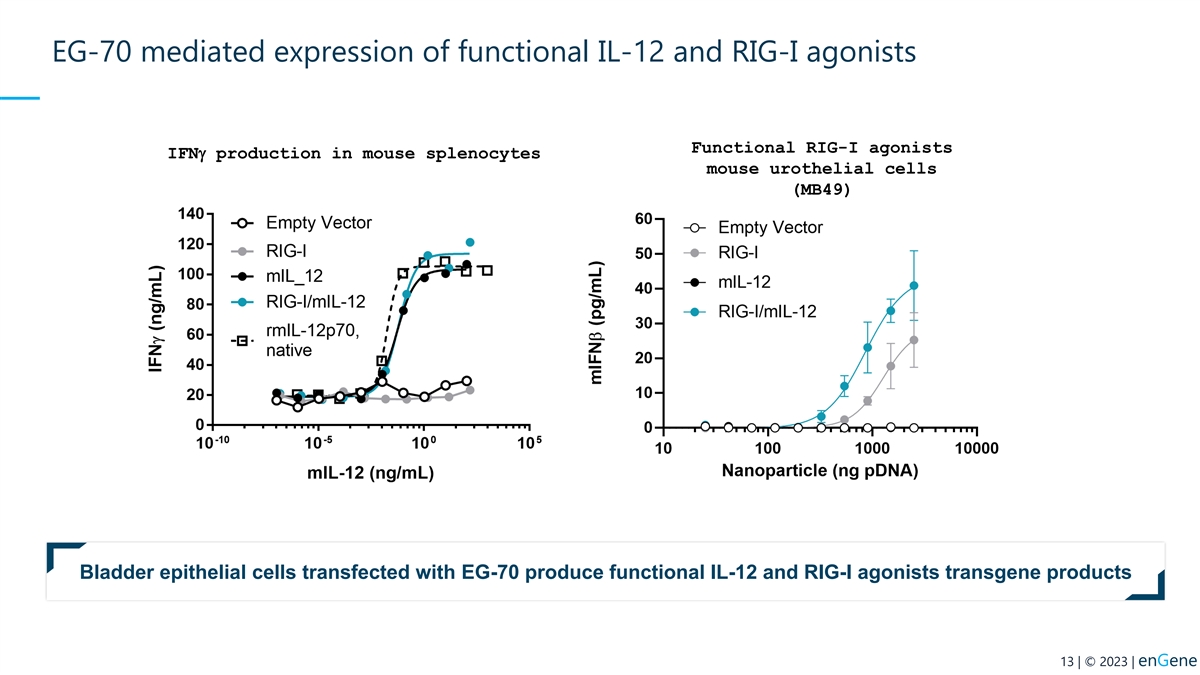
EG-70 mediated expression of functional IL-12 and RIG-I agonists
Functional RIG-I agonists IFNg production in mouse splenocytes mouse urothelial cells (MB49) Bladder epithelial cells transfected with EG-70 produce functional IL-12 and RIG-I agonists transgene products 13 | © 2023 | enGene
INT02-348

Transgenes expression in mouse bladder following single intravesical
administration of mEG-70 A. mIL -12 prot ein B. eRNA11 RNA VA1 RNA C. Transgene products show dose-dependent expression in mouse bladders Each data point represents expression in a single mouse bladder. n=8/group; data are presented with geometric
mean ± 95% CI; *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001; Mann Whitney test. There was no mIL-12 protein detected in 14 | © 2023 | enGene INT02-348 plasma of mEG-70-treated animals.

EG-70 induces dose-dependent target engagement in bladders of
tumor-bearing mice Ifnb Ifna2 Ddx58 Irf7 Isg15 Cxcl10 Ifit1 Ccl5 Tnfa Ifng Strong dose-dependent induction of RIG-I and IL-12 signaling in bladders of tumor-bearing mice treated with EG-70 n=8/group; data are mean +/- SEM; Mann Whitney t-test; 15 |
© 2023 | enGene INT02-348 tumor bearing mice treated with indicated doses of mEG-70; bladder harvested 24h post-administration for Taqman qPCR of bladder tissue lysates

Multiple MoA of EG-70 result in profound antitumor efficacy
Bioluminescence Experimental Design Survival (bladder tumor luciferase) Induction – 9 days Treatment Monitoring ns 10 10 Sham – 1% Mannitol 9 10 8 10 mEG-70 (5µg DNA/mL) 7 10 Luc-MB49 Cells 6 10 mEG-70 (20µg DNA/mL) 5 10
Instilled to induce orthotopic bladder 4 10 cancer mEG-70 (80µg DNA/mL) 3 10 2 10 1 10 0 Day 1: Day 10: Day 17: Day 28: 10 Luc-MB49 instillation IVI Tx IVI Tx Bioluminescence Naive Sham 5 20 80 mEG-70 (mg pDNA) Naïve Control Animals mEG-70
reduces tumor burden and mediates long-term survival in an orthotopic murine model bladder cancer – extends preclinical validation to advanced disease Bioluminescence: n=16-19/group; bioluminescence data are geometric mean +/- 95% CI; One-way
ANOVA; representative images from IVIS imaging; Survival: n=12/group, Log-rank (Mantel-Cox) IVI: intravesical 16 | © 2023 | enGene Bioluminescence Total Flux [p/s]

EG-70 mechanism of action: increased NK cell and reduced MDSC and TAM
ratios in bladder tissue MDSC Myeloid NK cells Macrophage *** **** *** **** 10 80 40 40 60 30 30 5 40 20 20 20 10 10 0 0 0 0 Sham mEG-70 Sham mEG-70 Sham mEG-70 Sham mEG-70 NK cells activation/maturation Cytokine profile CD69+/KLRG1+ CD11b+/CD27+
IFNγ Granzyme B CXCL10 TNFα IL-4 **** ** 40 25 250 15 0.25 6 60 20 200 0.20 30 10 15 150 0.15 4 40 20 10 100 0.10 5 2 20 10 5 50 0.05 ND 0 0 0 0.00 0 0 0 Sham mEG-70 Sham mEG-70 Sham mEG-70 Sham mEG70 Sham mEG70 Sham mEG70 Sham mEG70
mEG-70 promotes a less suppressive tumor microenvironment in the bladder NK: natural killer frequency, activation and cytokine profiling assessed on Day 13 (3 days post first dose); mean ± SD MDSC: myeloid-derived suppressive cell and TAM:
tumor-associated macrophage assessed on Day 20 (3 days post second dose); mean ± SD 17 | © 2023 | enGene Cytokine profile on Day 13 is shown; mean ± SD % CD69+KLRG+ % NK1.1+ % CD11b+CD27+ % CD11b+ pg IFNg/ mg total protein pg Granzyme
B/ mg total protein %Ly6G+Ly6C+ pg IP-10/ mg total protein %CD11b+Ly6C+F480hi pg TNFα/ mg total protein pg IL-4/ mg total protein

EG-70 mechanism of action: T-cell recruitment and infiltration into
tumor microenvironment Lymph node Bladder CD4 CD8 CD4 CD8 **** **** 15 25 30 30 *** **** 20 10 20 20 15 10 5 10 10 5 0 0 0 0 Sham mEG-70 Sham mEG-70 Sham mEG-70 Sham mEG-70 H&E CD3 CD4 CD8 Sham mEG-70 mEG-70 promotes an anti-tumor adaptive
immune cell response 18 | © 2023 | enGene T cells assessed on Day 23 (6 days post second dose), mean ± SD; IHC performed on bladder tissue collected on Day 22 (5 days post second dose) % CD4+ % CD8+ % CD4+ % CD8+

Depletion of helper T cells results in loss of anti-tumor activity
Study MB49-Luc IVI IVI Termination instillation Tx1 Tx2 FACS to confirm depletion (blood) Study timeline -3 -2 -1 1 3 6 10 13 17 20 24 27 29 Anti-mouse CD4/CD8α intraperitoneal injection CD4 depleted animals Bladder weight Survival Depletion of
CD4 T cells results in loss of mEG-70-mediated anti-tumor activity 19 | © 2023 | enGene B INT02 INT02 ladder we - -ig 429 409 ht data: Mean ± SEM, *p<0.05, unpaired t test; Survival: *p<0.05, Log-rank (Mantel-Cox) test

EG-70 cured animals are protected from re-challenge: demonstrates
generation of anti-tumor immunity Bioluminescence after local intravesical rechallenge 85-days post-cure Naive control re-challenge 10 13 days post rechallenge 10 mEG-70-cured re-challenge Naïve re-challenge 8 10 6 10 4 10 mEG-70-cured
rechallenge 2 10 0 10 7 13 21 28 Days post-rechallenge Days after re-challenge Treatment with mEG-70 results in long-lasting anti-tumor response as demonstrated by protection from local tumor re-challenge 20 | © 2023 | enGene INT02-388
Quantifiable total flux signal below background radiance Total Flux [log p/s] Total Flux [photons/sec]

EG-70 systemic and antigen-specific protection Opens advanced disease:
MIBC and Metastatic Disease Re-challenge MB49-Luc IVI IVI IVIS IVIS IVIS IVIS IVIS instillation Tx1 Tx2 MB49-Luc Study Day 1 10 17 73 80 86 94 101 108 mEG-70-cured : MB49-Luc SC re-challenge mEG-70 Disease induction mEG-70-cured : B16-F10 SC
re-challenge Naïve : MB49-Luc SC re-challenge Naïve : B16-F10 SC re-challenge Tumor volume after distal subcutaneous rechallenge MB49-Luc s.c. re-challenge B16-F10 s.c. re-challenge Re-challenge Naïve Re-challenge Naïve 2500 2500
2500 2500 2000 2000 2000 2000 1500 1500 1500 1500 1000 1000 1000 1000 500 500 500 500 0 0 0 0 0 7 14 21 28 35 42 49 0 7 14 21 28 35 42 49 0 7 14 21 28 35 42 49 0 7 14 21 28 35 42 49 Days post-rechallenge Days post-rechallenge Days post-rechallenge
Days post-rechallenge Tumor growth : 0/16 (0%) 12/12 (100%) 11/15 (73%) 12/12 (100%) Treatment with mEG-70 results in systemic anti-tumor immunity that is antigen-specific 21 | © 2023 | enGene INT02-388 3 Tumor Volume (mm )

EG-70 induces strong target engagement of RIG-I and IL-12 signaling:
biomarkers translate across species Ifng Ifnb1 Irf7 Tnfa Mouse Non-human primate Empty EG-70 Empty EG-70 Empty EG-70 Empty EG-70 Vector Vector Vector Vector Mouse data: n=8/group; data are mean +/- SEM; Mann Whitney t-test; tumor bearing mice;
bladders collected 24h post-administration for Taqman qPCR of bladder tissue lysates NHP data: EG-70 or empty vector control; intravesival administration; tissue collected 48 h post-administration; mean ± SEM, fold change vs empty vector
control; 22 | © 2023 | enGene INT02 Tbp used a-s in 348 ternal control gene; representative of 1 animal per treatment group, with 5 pieces of bladder tissue per animal

EG-70: strong efficacy coupled to a clean safety profile Toxicology
studies did not reveal a maximum tolerated dose (MTD) • Mice - no adverse findings attributable to mEG-70 • Monkeys - very well-tolerated with no systemic findings; • Local findings limited to transient mild inflammation consistent
with intravesical administrations • All changes showed reversibility Biodistribution - payloads are circumscribed to bladder as intended and designed • Bladder: plasmid DNA found at sustained levels in bladder tissue and urine •
Blood: Transient plasmid DNA in the NHP blood 24 to 48 hours after dosing; regressed to zero rapidly • Non-target tissues: no significant levels detected No Adverse Events Limit (NOAEL) was the highest dose tested – maximum feasible dose
• Enabled pharmacologically active dose in mice and in NHPs to be the starting dose for Phase 1 EG-70 is a powerful anti-tumor agent with an exquisite safety profile; well-tolerated, circumscribed payloads, with no MTD 23 | © 2023 |
enGene

EG-70 IN NMIBC PATIENTS WHO ARE BCG-UNRESPONSIVE AND HIGH-RISK NMIBC
PATIENTS WHO HAVE BEEN INCOMPLETELY TREATED WITH BCG OR ARE BCG-NAÏVE NCT04752722 enGene

Phase 1/2 combined registrational study design enables speed to pivotal
data and registration Phase 1 – completion 2H2022 Phase 2 – interim data 1H2024 N = <24 patients N ~100 patients Patients: High-risk NMIBC failed BCG, with Cis Patients: High-risk NMIBC failed BCG, with Cis EG-70 Dosing: 2 or 4 doses
in 12-week cycle EG-70 Dosing: RP2D for 4 cycles Cohorts: 3+3 dose escalation (4 dose levels) Cohorts: Single-arm, open label Endpoints: 1° - Safety; 2° - Identify RP2D*; Efficacy Endpoints: 1° - CR** rate at 12-months; 2° -
safety and durability Fast Track Designation granted: rapid path to BLA submission in 1H2025 25 | © 2023 | enGene *RP2D is Recommended Phase 2 Dose; ** CR is complete response; Expected timelines and milestones are for illustrative purposes
only

Phase 1 study schema - design, dosing, and analysis Overview Cohorts,
dosing, and analysis Patients: High-risk NMIBC with CIS – failed BCG Dose-level 1 (N=3) - 250µg DNA/mL Dose-level 2 (N=3-6) - 800µg DNA/mL Treatment Cycle (SoC): 12 weeks Dose-level 3 (N=3-6) - 2,500µg DNA/mL EG-70 Dosing: -1 1
3 8 10 3 10 12 13 96 Weeks 1 and 2 of each cycle Weeks Weeks Days ✓✓ Dose Cohorts: Urine✓✓✓✓✓✓ Up to 3 additional 3-6 patients per dose level (up to 4 cohorts) dosing cycles
✓✓✓✓✓✓ Plasma (elective) if patients Endpoints: ✓✓ Biopsy have SD or CR at 12- 1° - Safety ✓✓ Cystoscopy weeks 2° - Identify RP2D; Efficacy Cytology✓✓
Exploratory - Biomarkers Unit dose-escalation is complete with excellent safety and efficacy results aggregated 26 | © 2023 | enGene

70% Complete Response rate at 3-months with EG-70 in high 1 grade NMIBC
patients with CIS that are BCG-unresponsive CR at 15* and 18* months ALL DOSE LEVELS 1 3-Months: 70% CR (14/20) DL1 2 6-Months: 39% CR (7/18) (2x250µg) 3 Patients continuing past 3-Months: 4 85% (17/20) DL2 5 (2x800µg) 6 RP2D: DL2’
4X800 UG 7 3-Months: 75% CR (6/8) DL3 8 6-Months: 50% CR (3/6) (2x2500µg) 9 Patients continuing past 3-Months: * 10 100% (8/8) DL2’ 11 (4x800µg) 12 LEGEND 13 DL2E** Currently on Treatment 14 (2x800µg) Treatment Cycle Complete 15
* 16 Complete Response 17 Progression or Relapse ,† DL2’E** 18 Stable Disease (4x800µg) * 19 Discontinued Treatment * 20 Preliminary Data, Subject to Source Data * Verification 0 3 6 9 12 Data cutoff 3.20.2023; **E means
‘expansion’; Efficacy Assessments (months) † One additional patient was dosed in this group but later deemed by the independent DSMB to be ineligible and excluded from ‘efficacy set’ . Full patient disposition info is
summarized in slide 46 1 BCG doses per patient (Mean=12); months between last BCG dose and Day 1 visit (Mean=17 ) Note: As further specified in the “Risk Factors” section, this slide may include preliminary data from our clinical trials
that may change as more patient data become available and are subject to audit and 27 | © 2023 | enGene verification procedures that could result in material changes in the final data. Dose-level and patient number

Urinalysis demonstrates EG-70 proof-of-mechanism Urine IL-12 in Phase 1
dose-escalation 1000 DL1 DL2 IL-12 quantified in all patients dosed to-date* 100 DL3 Drug is administered on Days 1 and 8 10 Urine collected prior to dosing 1 Dose-response, localized to the bladder Urine IL-12 levels in DL2 are an order of
magnitude higher than DL1 0.1 No further increases in IL-12 production with DL3 IL-12 is not detected in plasma demonstrating localized effects 0 D1 D3 D8 D10 W3 W10 st nd 1 dose 2 dose Strong localized production of IL-12 with unremarkable plasma
cytokine excursions validates EG-70 proof-of-mechanism 28 | © 2023 | enGene *Slide reflects all data available as of September 2022. DL1 is dose-level 1 (250µg/mL); DL2 is dose-level 2 (800µg/mL); DL3 is dose-level 3 (2,500µg/mL)
Human IL-12 (pg/mL)

Excellent position to optimize EG-70 RP2D based on efficacy and
biomarkers – not based on toxicity! Reported AEs to-date are largely consistent with instrumentation/intravesical administration Grade 2 AEs Grade 1 AEs Grade 3 AEs Grade 4 AEs Grade 5 AEs (mild) (moderate) (severe) (life-threatening) (result
in death) Aggregate Safety 77 25 3 0 0 (N, all dose-levels) • UTI • Renal failure* • Hematuria (9%) • Pneumonia** • Dysuria (5%)• UTI (16%) • Constipation (4%)• Enterococcal (4%)• Pneumonitis**
Most commonly • Micturition urgency (8%) • Contusion (4%) - - reported AEs (%)• Flank Pain (4%) • Micturition urgency (4%) • Arthralgia (4%) No correlation between dose and grade or number of AEs Primary endpoint of the
Phase 1 study has been met; decision-making for RP2D based on efficacy and biomarkers, not toxicity, a rare position in oncology Data cutoff: 03.30.2023; *patient had a history of renal failure – enrollment criteria for Phase 2 amended to
exclude history of renal failure; ** Occurred in the same patient, considered not related to study drug 29 | © 2023 | enGene

Summary and Future Directions; LEGEND (EG-70-101) • EG-70 is a
novel, non-viral gene therapy in development for NMIBC • In a first-in-human study, this preliminary analysis demonstrated • A safe and tolerable adverse event profile, with • A high level of efficacy, that is • driven by
demonstrated local expression of therapeutic gene cargos 30 | © 2023 | enGene

Acknowledgement We wish to thank all the patients, clinicians and staff
from all the sites that participated in the LEGEND study. enGene, Inc. Clinical & Regulatory CMC Non-Clinical • Rosemary Mazanet • Carlos Fleet • Shauna Dauphinee • Christine Tossone • Daniel Veilleux • Jose
Lora • Loraine Warner • Ximin Chen • James Sullivan • Bao Li • David Gareau • Marie-Line Goulet • Yulin Wang • David Lazure Project Management • Rajarshi Roychaudhury • Darius Bilimoria
• Sharon Tan • Natalie Tam • Sebestien Sublemontier • Pei Lian Ma • Jeremy Dupaul-Chicoine Quality Assurance • Rajesh Krishnan • Sarah Stevenson • Jamie Jamshidi • Deborah Savuto • Kristine
Sarah Louis • Tara Adams • Christine Zouki • Jean-Francois Marquis 31 | © 2023 | enGene

Important Additional Information and Where to Find It enGene Inc. and
Forbion European Acquisition Corp. (“FEAC”) (Nasdaq: FRBN) have announced they have entered into a definitive agreement respecting a business combination. In connection therewith, a newly formed entity, enGene Holdings Inc.
(“Newco”), intends to file with the U.S. Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, which will include a preliminary proxy statement/prospectus. After the registration statement has
been declared effective, the definitive proxy statement/prospectus will be mailed to shareholders of FEAC as of a record date to be established for voting on the proposed business combination and certain other proposals regarding the proposed
business combination. Before making any voting or investment decision, investors and shareholders of FEAC are urged to carefully read the entire proxy statement/prospectus, when available, because it will contain important information about the
proposed business combination. FEAC shareholders and others will also be able to obtain copies of the preliminary and definitive proxy statement/prospectuses, without charge, once available, at the SEC’s website at www.sec.gov, or by directing
a request to: Forbion European Acquisition Corp., Gooimeer 2-35, 1411 DC Naarden, The Netherlands, Attention: Cyril Lesser. Participants in the Solicitation FEAC, enGene, Newco and their respective directors, managers, executive officers, other
members of management and employees may be deemed participants in the solicitation of proxies from FEAC’s shareholders with respect to the proposed business combination under the rules of the SEC. FEAC’s investors and security holders
may obtain more detailed information regarding the names and interests in the proposed business combination of FEAC’s directors and officers, without charge, in FEAC’s filings with the SEC, including, when filed with the SEC, the
preliminary proxy statement/prospectus, the definitive proxy statement/prospectus, and other documents filed with the SEC. Such information with respect to enGene’s and Newco’s directors and executive officers will also be included in
the proxy statement/prospectus. No Offer or Solicitation This document is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed business combination and will not
constitute an offer to sell or exchange, or a solicitation of an offer to buy or exchange, any securities (including securities of enGene, FEAC, or Newco). 32 | © 2023 | enGene
Forbion European Acquisi... (NASDAQ:FRBNU)
過去 株価チャート
から 11 2024 まで 12 2024

Forbion European Acquisi... (NASDAQ:FRBNU)
過去 株価チャート
から 12 2023 まで 12 2024
